Abstract
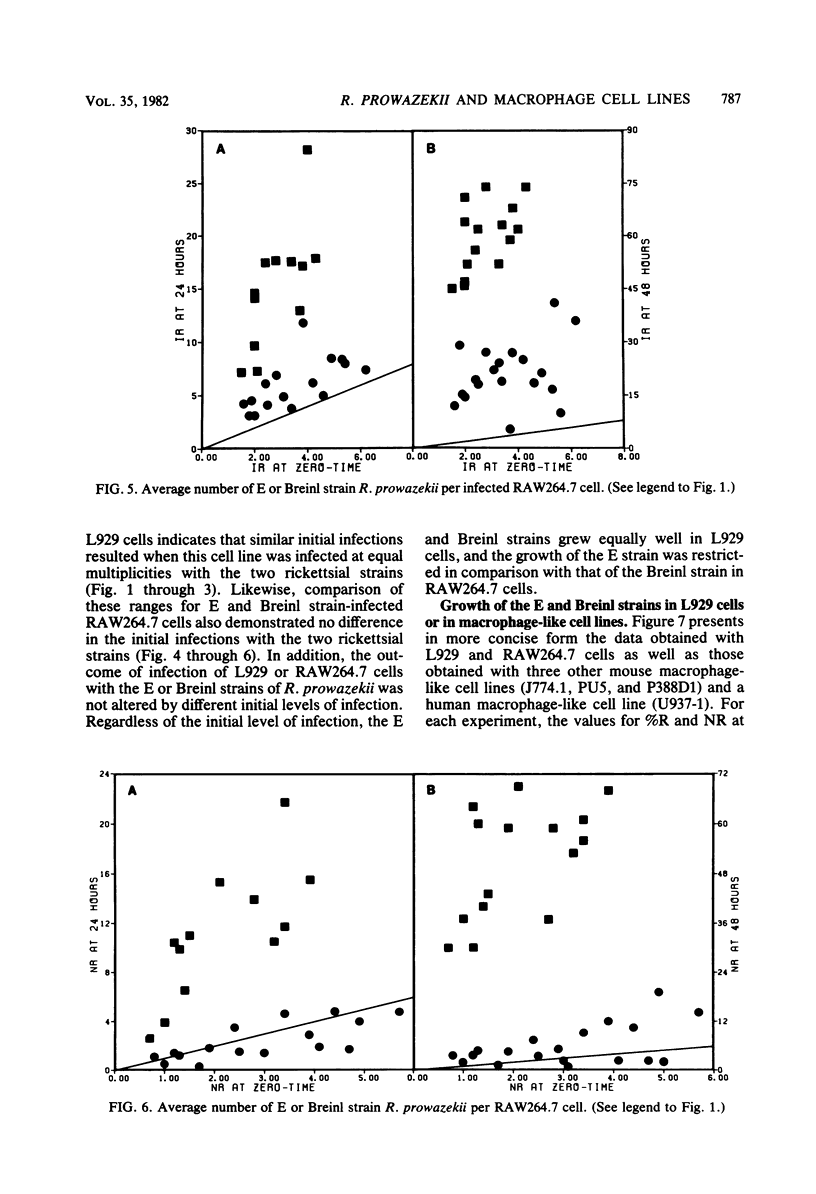
The growth of avirulent (E) and virulent (Breinl) strains of Rickettsia prowazekii was compared in four mouse macrophage-like cell lines (RAW264.7, J774.1, P388D1, and PU5), one human macrophage-like cell line (U937-1), and the mouse fibroblast line L929. The E and Breinl strains grew equally well in L929 cells. However, all of the mouse macrophage-like cell lines clearly differentiated between the two strains by restricting the growth of the E strain relative to that of the Breinl strain. A nonuniform response to infection was sometimes observed in which E strain rickettsiae were cleared from the majority of the infected cells, but multiplied in some of the remaining infected cells. The human line U937-1 was not very effective at differentiating the E and Breinl strains. Addition of rabbit antirickettsial antiserum to the Breinl or E strains of R. prowazekii immediately before infection of L929 cells caused a marked decrease in the initial infection but had no effect on the subsequent growth of the rickettsiae in the L929 cells. In contrast, addition of antiserum to Breinl or E strain rickettsiae immediately before infection of macrophage-like cell lines caused either no change or an increase in the initial infection. Most of the rickettsiae that infected the mouse macrophage-like cell lines in the presence of antiserum were destroyed in these cell lines. Thus, when the infection took place in the presence of antiserum, the mouse macrophage-like cell lines no longer differentiated between the E and Breinl strains. These data indicate that mouse macrophage-like cell lines should be a useful model system for defining the differences between the E and Breinl strains of Rickettsia prowazekii, differences which should lead to an understanding of the biochemical basis of virulence in this organism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese J. L., Wisseman C. L., Jr, Walsh W. T., Fiset P. Antibody and antibiotic action on Rickettsia prowazeki in body lice across the host-vector interface, with observations on strain virulence and retrieval mechanisms. Am J Epidemiol. 1973 Oct;98(4):262–282. doi: 10.1093/oxfordjournals.aje.a121556. [DOI] [PubMed] [Google Scholar]
- FOX J. P. Immunization against epidemic typhus; a brief general review and a description of the status of living, avirulent R. prowazeki (strain E) as an immunizing agent. Am J Trop Med Hyg. 1956 May;5(3):464–479. [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Fischer D. G., Anderson S. J., Koren H. S. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol. 1980 Jul;125(1):6–12. [PubMed] [Google Scholar]
- Morahan P. S. Macrophage nomenclature: where are we going? J Reticuloendothel Soc. 1980 Feb;27(2):223–245. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Broxmeyer H. E., Schrader S. Immunologic functions and in vitro activation of cultured macrophage tumor lines. Natl Cancer Inst Monogr. 1978 May;(48):303–310. [PubMed] [Google Scholar]
- Silverman D. J., Fiset P., Wisseman C. L., Jr Simple, differential staining technique for enumerating rickettsiae in yolk sac, tissue culture extracts, or purified suspensions. J Clin Microbiol. 1979 Mar;9(3):437–440. doi: 10.1128/jcm.9.3.437-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial hemolysis: adsorption, desorption, readsorption, and hemagglutination. Infect Immun. 1977 Sep;17(3):607–612. doi: 10.1128/iai.17.3.607-612.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. In vitro studies of the action of antibiotics on Rickettsia prowazeki by two basic methods of cell culture. J Infect Dis. 1974 Dec;130(6):564–574. doi: 10.1093/infdis/130.6.564. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazeki. Infect Immun. 1974 Mar;9(3):571–575. doi: 10.1128/iai.9.3.571-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


