Abstract
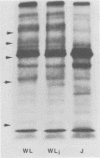
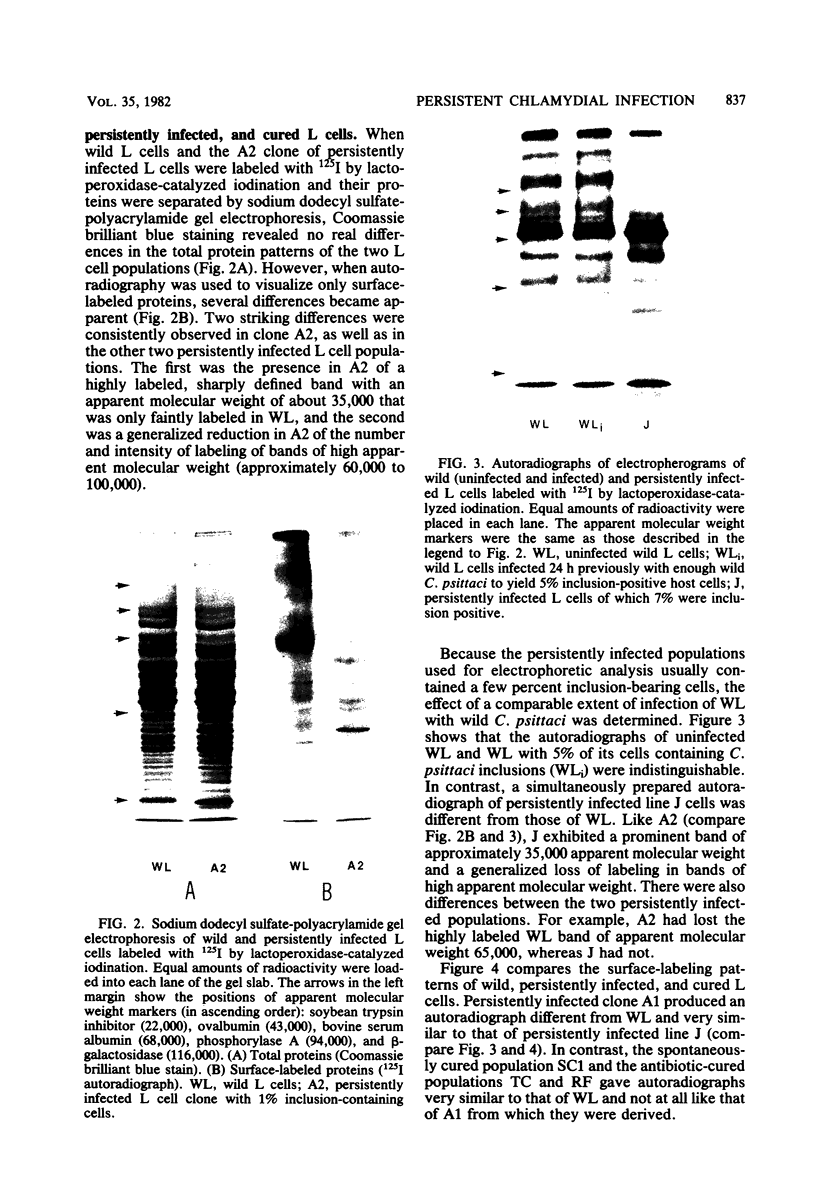
When mouse fibroblasts (L cells) were persistently infected with Chlamydia psittaci strain 6BC, they became immune to superinfection because they no longer associated with exogenous C. psittaci in a way that led to ingestion and intracellular multiplication. At the same time, the persistently infected L cells also exhibited changes in surface structure as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiographic visualization of the surface-exposed plasma membrane proteins that had been labeled with 125I by lactoperoxidase-catalyzed iodination. The most prominent changes were the appearance of a highly labeled band with an apparent molecular weight of 35,000 and the generalized reduction in intensity of labeling of proteins migrating in the apparent molecular weight range of 60,000 to 100,000. Neither resistance to superinfection nor alteration in cell surface structure depended on the presence of visible chlamydial inclusions. When L cells were cured of persistent infection, either spontaneously or by treatment with chlortetracycline or rifampin, immunity to superinfection disappeared, and the patterns of surface-labeled proteins of the cured cells once again resembled the patterns of wild L cells. It was suggested that resistance to superinfection is the result of reversible changes in the structure of the putative host cell receptor for chlamydiae that are produced in some unknown way by the persistent chlamydial infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Zeichner S. L., Lee C. K. Attachment defect in mouse fibroblasts (L cells) persistently infected with Chlamydia psittaci. Infect Immun. 1981 Oct;34(1):285–291. doi: 10.1128/iai.34.1.285-291.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]