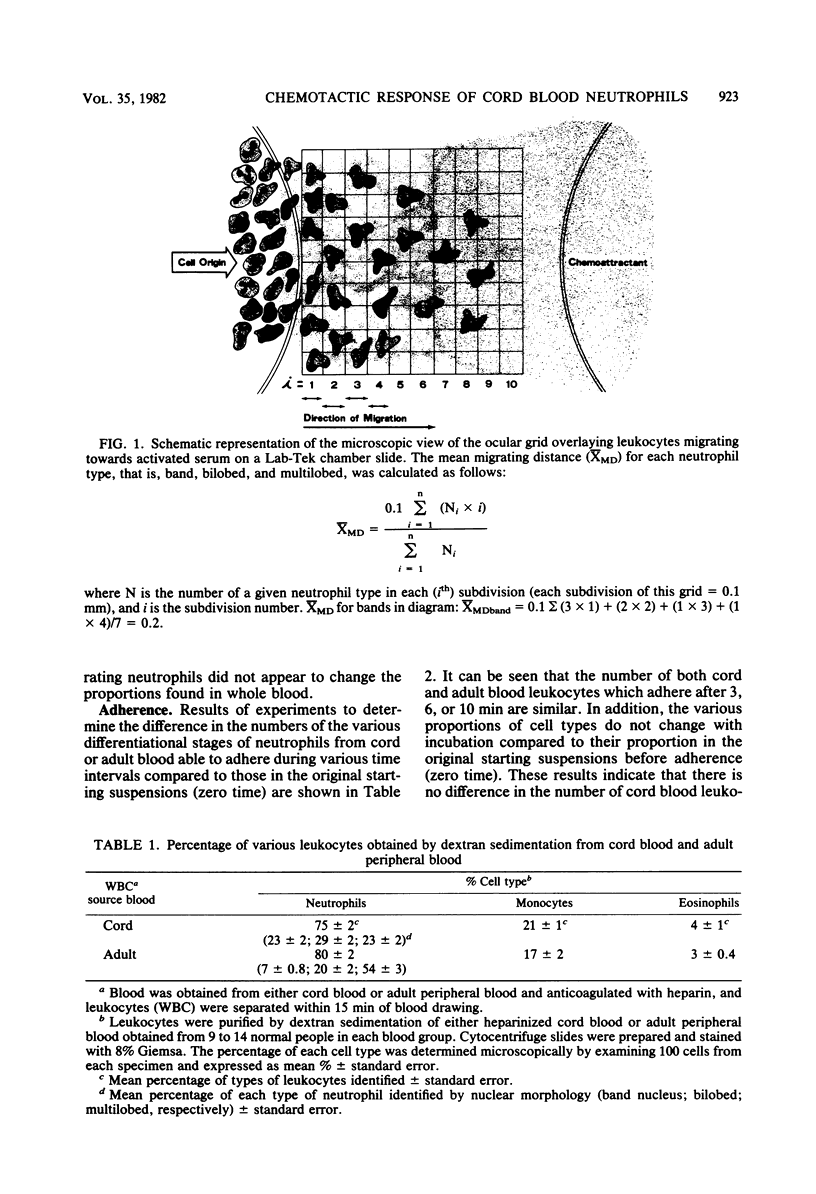
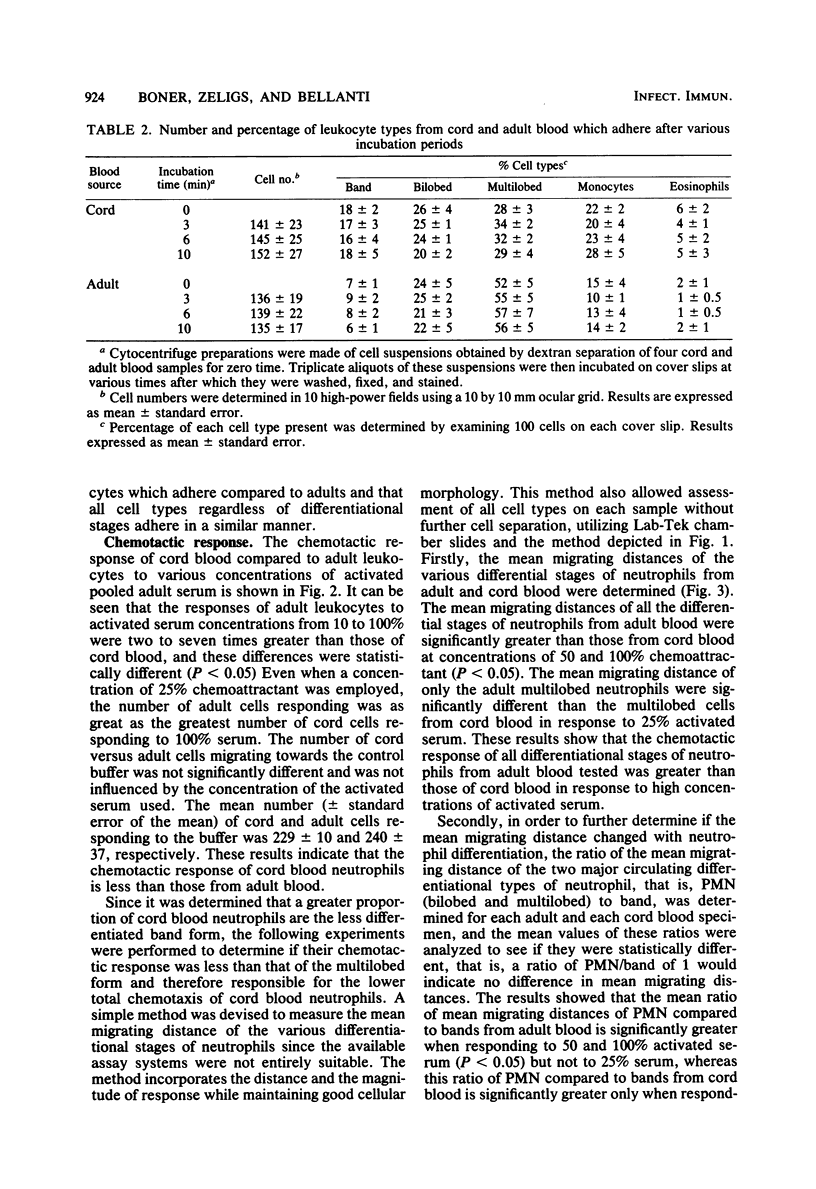
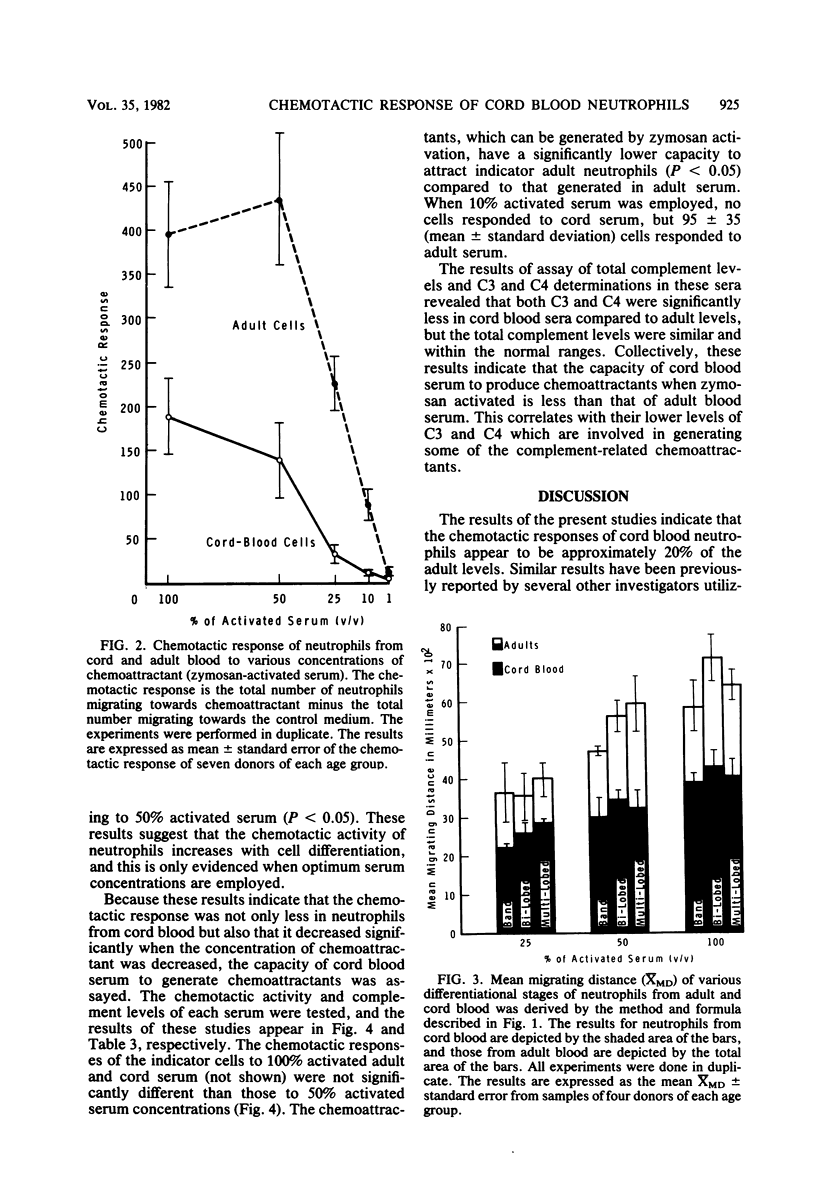
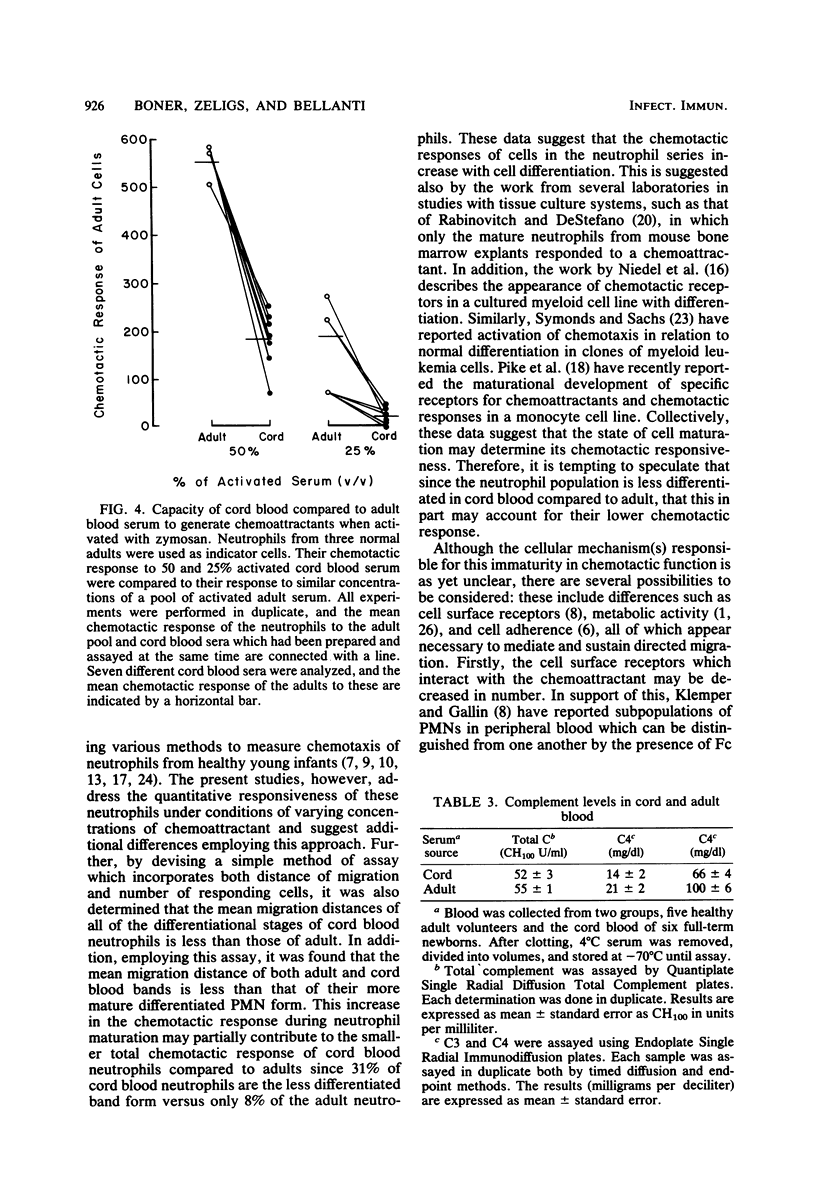
Abstract
Chemotactic response and responsiveness, that is, the ability to respond to various concentrations of the chemoattractant zymosan-activated serum was measured for cord and adult blood neutrophils. Chemotactic response of neutrophils from adult blood was two to seven times greater than that of neutrophils from cord blood. In addition, neutrophils from cord blood showed poor response to concentrations of 25% chemoattractant or less, compared to those from adults, which responded to concentrations of 10%. Further, it was found that approximately 30% of neutrophils in cord blood were the band form compared to only 9% in adult. Based upon this, a simple method was devised to assay mean migrating distance of various differentiational stages of neutrophils incorporating both distance and magnitude of their responses. The results of these assays showed that all differentiational stages of cord blood neutrophils have a mean migrating distance less than those of adults. In addition, the band form from both cord and adult blood had a mean migrating distance less than the polymorphonuclear form. Adherence studies revealed that all differentiational stages of neutrophils adhered in a similar manner, and no difference was detected between cells from cord and adult blood. Assays to test the ability of cord blood to produce chemotactic activity when activated revealed 50% less activity than that obtained from adult serum which further decreased with dilution of attractant. These data suggest that both the cellular and humoral systems involved in chemotactic responses are less in cord blood compared to adult blood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carruthers B. M. Leukocyte motility. II. Effect of absence of glucose in medium; effect of presence of deoxyglucose, dinitrophenol, puromycin, actinomycin D, and trypsin on the response to chemotactic substance; effect of segregation of cells from chemotactic substance. Can J Physiol Pharmacol. 1967 Mar;45(2):269–280. doi: 10.1139/y67-029. [DOI] [PubMed] [Google Scholar]
- Christensen R. D., Rothstein G. Efficiency of neutrophil migration in the neonate. Pediatr Res. 1980 Oct;14(10):1147–1149. doi: 10.1203/00006450-198010000-00013. [DOI] [PubMed] [Google Scholar]
- Fireman P., Zuchowski D. A., Taylor P. M. Development of human complement system. J Immunol. 1969 Jul;103(1):25–31. [PubMed] [Google Scholar]
- Fischer G. W., Podgore J. K., Bass J. W., Kelley J. L., Kobayaski G. Y. Enhanced host defense mechanisms with levamisole in suckling rats. J Infect Dis. 1975 Nov;132(5):578–581. doi: 10.1093/infdis/132.5.578. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wolff S. M. Leucocyte chemotaxis: physiological considerations and abnormalities. Clin Haematol. 1975 Oct;4(3):567–607. [PubMed] [Google Scholar]
- Klein R. B., Fischer T. J., Gard S. E., Biberstein M., Rich K. C., Stiehm E. R. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Separation and functional characterization of human neutrophil subpopulations. Blood. 1978 Apr;51(4):659–669. [PubMed] [Google Scholar]
- Laurenti F., Ferro R., Marzetti G., Rossini M., Bucci G. Neutrophil chemotaxis in preterm infants with infections. J Pediatr. 1980 Mar;96(3 Pt 1):468–470. doi: 10.1016/s0022-3476(80)80700-1. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A. Cellular deformability during maturation of the myeloblast. Possible role in marrow egress. N Engl J Med. 1970 Oct 29;283(18):943–948. doi: 10.1056/NEJM197010292831801. [DOI] [PubMed] [Google Scholar]
- Mease A. D., Fischer G. W., Hunter K. W., Ruymann F. B. Decreased phytohemagglutinin-induced aggregation and C5a-induced chemotaxis of human newborn neutrophils. Pediatr Res. 1980 Feb;14(2):142–146. doi: 10.1203/00006450-198002000-00015. [DOI] [PubMed] [Google Scholar]
- Mills E. L., Thompson T., Björkstén B., Filipovich D., Quie P. G. The chemiluminescence response and bactericidal activity of polymorphonuclear neutrophils from newborns and their mothers. Pediatrics. 1979 Mar;63(3):429–434. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Niedel J., Kahane I., Lachman L., Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R., Grimes E., Smithwick E. Cellular and humoral components of monocyte and neutrophil chemotaxis in cord blood. Pediatr Res. 1977 May;11(5):677–680. doi: 10.1203/00006450-197705000-00010. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Fischer D. G., Koren H. S., Snyderman R. Development of specific receptors for N-formylated chemotactic peptides in a human monocyte cell line stimulated with lymphokines. J Exp Med. 1980 Jul 1;152(1):31–40. doi: 10.1084/jem.152.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E. M., Ross G. D., Trang H. T., Williams N., Metcalf D. Membrane receptors of mouse leukocytes. II. Sequential expression of membrane receptors and phagocytic capacity during leukocyte differentiation. J Exp Med. 1978 Feb 1;147(2):434–445. doi: 10.1084/jem.147.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. In vitro chemotaxis of mouse bone marrow neutrophils. Proc Soc Exp Biol Med. 1978 Jun;158(2):170–173. doi: 10.3181/00379727-158-40164. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Jarowski C. I., Rabellino E. M., Winchester R. J. The sequential appearance of Ia-like antigens and two different complement receptors during the maturation of human neutrophils. J Exp Med. 1978 Mar 1;147(3):730–744. doi: 10.1084/jem.147.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F., Goetzl E. J. Chemotactic activity derived from interaction of factors D and B of the properdin pathway with cobra venom factor or C3B. J Clin Invest. 1975 Mar;55(3):587–592. doi: 10.1172/JCI107966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds G., Sachs L. Activation of normal genes in malignant cells: activation of chemotaxis in relation to other stages of normal differentiation in myeloid leukemia. Somatic Cell Genet. 1979 Nov;5(6):931–944. doi: 10.1007/BF01542652. [DOI] [PubMed] [Google Scholar]
- Tono-Oka T., Nakayama M., Uehara H., Matsumoto S. Characteristics of impaired chemotactic function in cord blood leukocytes. Pediatr Res. 1979 Mar;13(3):148–151. doi: 10.1203/00006450-197903000-00002. [DOI] [PubMed] [Google Scholar]
- Valone F. H. Modulation of human neutrophil and eosinophil polymorphonuclear leukocyte chemotaxis: an analytical review. Clin Immunol Immunopathol. 1980 Jan;15(1):52–65. doi: 10.1016/0090-1229(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Ward P. A. Leukotaxis and leukotactic disorders. A review. Am J Pathol. 1974 Dec;77(3):520–538. [PMC free article] [PubMed] [Google Scholar]


