Abstract
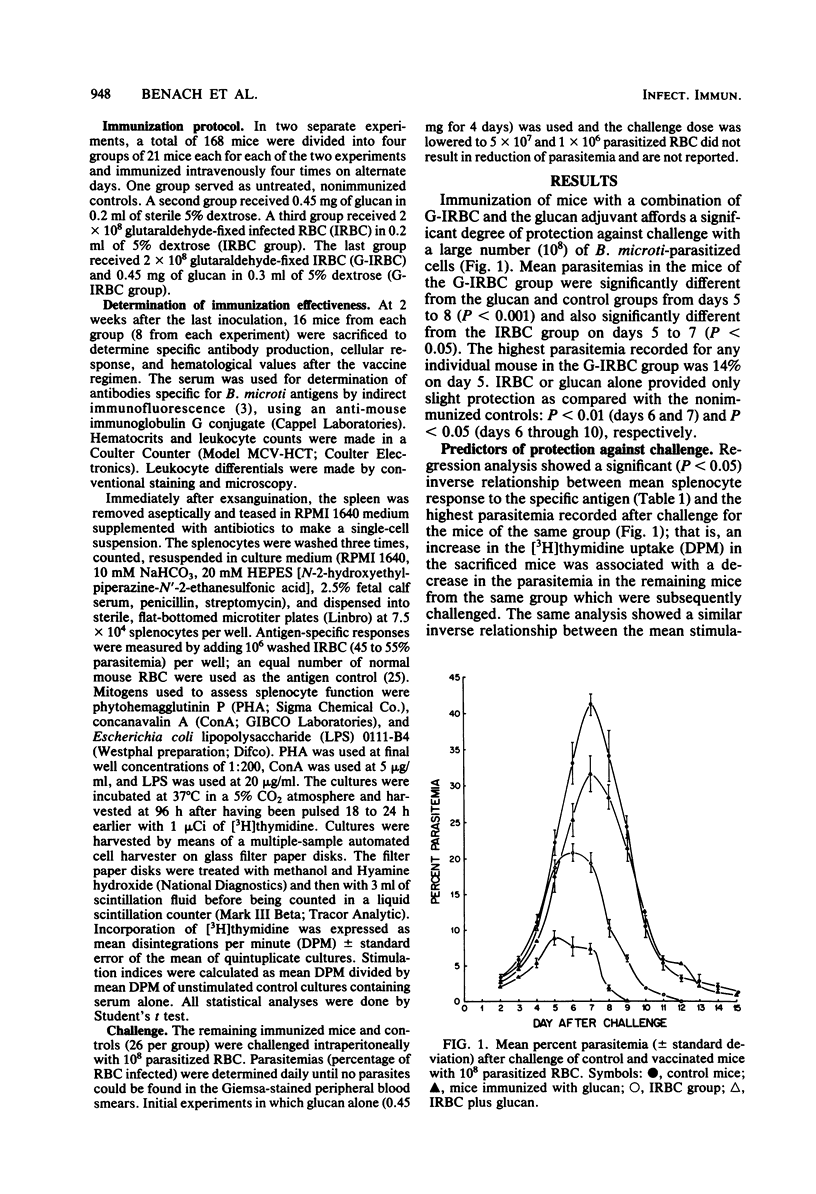
A vaccination protocol against murine Babesia microti infection, using glucan, a beta-1,3-glucopyranose derivative of yeast cell walls, and glutaraldehyde-fixed infected erythrocytes was evaluated. BALB/c mice were immunized intravenously four times at 2-day intervals with 2 X 10(8) fixed infected erythrocytes with and without glucan (0.45 mg). The mice were challenged 2 weeks after the last immunization with 10(8) viable infected erythrocytes. Peak parasitemia was significantly reduced (8.9 +/- 1.0%; P less than 0.001) in glucan-immunized mice as compared with nonimmunized controls (41.2 +/- 1.4%), glucan-treated controls (31.7 +/- 2.5%; P less than 0.05), or mice which received fixed infected erythrocytes without glucan (21.0 +/- 1.2%; P less than 0.01). Humoral and cellular immunity to B. microti was evaluated before challenge by measuring antibody titers and splenocyte blastogenic responses to B. microti antigens. The in vitro cellular response was inversely correlated with mean peak parasitemia (P less than 0.05). These observations demonstrate that glucan is an effective adjuvant in enhancing immunity to murine babesiosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgaleta C., Golde D. W. Effect of glucan on granulopoiesis and macrophage genesis in mice. Cancer Res. 1977 Jun;37(6):1739–1742. [PubMed] [Google Scholar]
- Chisholm E. S., Ruebush T. K., 2nd, Sulzer A. J., Healy G. R. Babesia microti infection in man: evaluation of an indirect immunofluorescent antibody test. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):14–19. doi: 10.4269/ajtmh.1978.27.14. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Holbrook T. W., Parker B. W. Visceral leishmaniasis in mice: protective effect of glucan. J Reticuloendothel Soc. 1980 Jun;27(6):567–573. [PubMed] [Google Scholar]
- Cook J. A., Taylor D., Cohen C., Hoffmann E. O., Rodrigue J., Malshet V., Di Luzio N. R. Evaluation of effector cells mediating the antitumor action of glucan. J Reticuloendothel Soc. 1977 Jul;22(1):21–34. [PubMed] [Google Scholar]
- Dammin G. J., Spielman A., Benach J. L., Piesman J. The rising incidence of clinical Babesia microti infection. Hum Pathol. 1981 May;12(5):398–400. doi: 10.1016/s0046-8177(81)80020-2. [DOI] [PubMed] [Google Scholar]
- Hamuro J., Hadding U., Bitter-Suermann D. Solid phase activation of alternative pathway of complement by beta-1,3-glucans and its possible role for tumour regressing activity. Immunology. 1978 Apr;34(4):695–705. [PMC free article] [PubMed] [Google Scholar]
- Hamuro J., Röllinghoff M., Wagner H. beta(1 leads to 3) Glucan-mediated augmentation of alloreactive murine cytotoxic T-lymphocytes in vivo. Cancer Res. 1978 Sep;38(9):3080–3085. [PubMed] [Google Scholar]
- Hamuro J., Wagner H., Röllinghoff M. Beta (1-3) glucans as a probe for T cell specific immune adjuvants. II. Enhanced in vitro generation of cytotoxic T lymphocytes. Cell Immunol. 1978 Jul;38(2):328–335. doi: 10.1016/0008-8749(78)90064-3. [DOI] [PubMed] [Google Scholar]
- Holbrook T. W., Cook J. A., Parker B. W. Glucan-enhanced immunogenicity of killed erythrocyte stages of Plasmodium berghei. Infect Immun. 1981 May;32(2):542–546. doi: 10.1128/iai.32.2.542-546.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Smith P. M., Mitchell G. F. Characterization of surface proteins and glycoproteins on red blood cells from mice infected with haemosporidia: Babesia rodhaini infections of BALB/c mice. Parasitology. 1980 Oct;81(2):251–271. doi: 10.1017/s003118200005602x. [DOI] [PubMed] [Google Scholar]
- Jack R. M., Ward P. A. The role in vivo of C3 and the C3b receptor in babesial infection in the rat. J Immunol. 1980 Apr;124(4):1574–1578. [PubMed] [Google Scholar]
- Kokoshis P. L., Williams D. L., Cook J. A., Di Luzio N. R. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science. 1978 Mar 24;199(4335):1340–1342. doi: 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- Levy M. G., Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980 Mar 14;207(4436):1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- Mansell P. W., Ichinose H., Reed R. J., Krementz E. T., McNamee R., Di Luzio N. R. Macrophage-mediated destruction of human malignant cells in vivo. J Natl Cancer Inst. 1975 Mar;54(3):571–580. [PubMed] [Google Scholar]
- Reynolds J. A., Kastello M. D., Harrington D. G., Crabbs C. L., Peters C. J., Jemski J. V., Scott G. H., Di Luzio N. R. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980 Oct;30(1):51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Y. J. Autoimmune and polyclonal B cell responses during murine malaria. Nature. 1978 Jul 13;274(5667):170–172. doi: 10.1038/274170a0. [DOI] [PubMed] [Google Scholar]
- Ruebush M. J., Hanson W. L. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979 Jun;65(3):430–433. [PubMed] [Google Scholar]
- Smith R. D., James M. A., Ristic M., Aikawa M., Vega y Murguia C. A. Bovine babesiosis: protection of cattle with culture-derived soluble Babesia bovis antigen. Science. 1981 Apr 17;212(4492):335–338. doi: 10.1126/science.7209532. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Sterzel R. B., Lucia H. L., Campbell G. H., Jack R. M. Complement does not facilitate plasmodial infections. J Immunol. 1981 May;126(5):1826–1828. [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]
- Williams D. L., Cook J. A., Hoffmann E. O., Di Luzio N. R. Protective effect of glucan in experimentally induced candidiasis. J Reticuloendothel Soc. 1978 Jun;23(6):479–490. [PubMed] [Google Scholar]
- Williams D. L., Di Luzio N. R. Glucan-induced modification of murine viral hepatitis. Science. 1980 Apr 4;208(4439):67–69. doi: 10.1126/science.7361108. [DOI] [PubMed] [Google Scholar]


