Abstract
Saccharomyces cerevisiae is one of the premier model systems for studying the genomics and evolution of transposable elements. The availability of the S. cerevisiae genome led to unprecedented insights into its five known transposable element families (the LTR retrotransposons Ty1-Ty5) in the years shortly after its completion. However, subsequent advances in bioinformatics tools for analysing transposable elements and the recent availability of genome sequences for multiple strains and species of yeast motivates new investigations into Ty evolution in S. cerevisiae. Here we provide a comprehensive phylogenetic and population genetic analysis of all Ty families in S. cerevisiae based on a systematic re-annotation of Ty elements in the S288c reference genome. We show that previous annotation efforts have underestimated the total copy number of Ty elements for all known families. In addition, we identify a new family of Ty3-like elements related to the S. paradoxus Ty3p which is composed entirely of degenerate solo LTRs. Phylogenetic analyses of LTR sequences identified three families with short-branch, recently active clades nested among long branch, inactive insertions (Ty1, Ty3, Ty4), one family with essentially all recently active elements (Ty2) and two families with only inactive elements (Ty3p and Ty5). Population genomic data from 38 additional strains of S. cerevisiae show that the majority of Ty insertions in the S288c reference genome are fixed in the species, with insertions in active clades being predominantly polymorphic and insertions in inactive clades being predominantly fixed. Finally, we use comparative genomic data to provide evidence that the Ty2 and Ty3p families have arisen in the S. cerevisiae genome by horizontal transfer. Our results demonstrate that the genome of a single individual contains important information about the state of TE population dynamics within a species and suggest that horizontal transfer may play an important role in shaping the genomic diversity of transposable elements in unicellular eukaryotes.
Introduction
Transposable elements (TEs) have been shown to be a component of most, although not all, studied eukaryotic genomes [1]. Empirical and theoretical work from a broad range of host organisms suggests that TE insertions are generally deleterious and that natural selection acts to suppress proliferation in host populations [2]–[7]. Classically, it has been thought that a balance between increases in copy number by transposition and selection against deleterious insertions govern the dynamics of TE evolution [8]. However, more recent work has shown that some proportion of TE insertions may also evolve under positive selection (e.g. [9]–[13]), and that TE families undergo horizontal transfer at a higher rate than previously anticipated (e.g. [14]–[17]). In the genomics era, many unresolved questions about the factors that control TE evolution can now be addressed with the growing volumes of available sequence data.
The first eukaryote to have its genome completely sequenced was the yeast Saccharomyces cerevisiae [18]. This landmark achievement allowed unprecedented insights into the impact that TEs have on genome structure and evolution. The 12.2 Mb yeast genome is known to harbour five families of TEs, Ty1 through Ty5, all of which are long terminal repeat (LTR) retrotransposons [19]. Ty1 and Ty2 have been studied intensively and are known to be active families [20] that together make up ∼75% of the Ty insertions in the reference genome [21], [22]. These two closely-related families can be differentiated by sequence divergence in their gag open-reading frame (ORF) and a hypervariable region of the pol ORF [22]. In contrast, the only fixed difference between their LTR sequences is a one base pair deletion present in all copies of Ty2 [22]. The high level of nucleotide identity between Ty1 and Ty2 LTRs permits interfamily recombination, and elements with hybrid Ty1/2 LTRs are present in the S. cerevisiae genome [23]. The smaller families Ty3-Ty5 have been characterised to a more limited extent: Ty3 is thought to be an active family [24] with full-length elements in the genome; full-length copies of Ty4 exist in the genome but transposition of this family has not been observed [25]; and there are no longer any functionally-intact copies of Ty5 in the yeast genome [19].
The abundance of Ty elements varies considerably between populations of S. cerevisiae, with lab strains tending to possess greater abundance than wild populations [26], [27]. The relatively low copy numbers observed in wild populations, despite ongoing transposition, is consistent with the proliferation of elements being held in check by natural selection. Work by Blanc et al. [28] has suggested that the presence of a single Ty element insert results in a <2% loss of fitness. Not all Ty element insertions result in deleterious mutations, however, with some Ty insertions being proposed to play adaptive roles in the evolution of gene regulation [29]. As with other eukaryotes, the evolutionary and genomic forces that control TE abundance and variation in yeast remain a matter of debate, necessitating detailed investigation of the copy number, allele frequency and sequence variation of Ty elements within and among S. cerevisiae genomes.
Kim et al. [22] conducted the first systematic survey of Ty insertions in the S. cerevisiae reference genome, determining both the copy number and genomic distribution of all five known Ty families and performing a phylogenetic analysis of Ty1 and Ty2 LTR sequences. Jordan and McDonald [30] used the element copies identified by Kim et al. [22] to show that all five Ty families exhibit low levels of sequence variation in their ORFs and appear to be evolving under purifying selection. Comparative analysis involving other species of Saccharomyces sensu stricto clade [31]–[34] provides evidence that Ty families have been inherited both vertically and horizontally within the Saccharomyces genus, as well as undergoing occasional loss from their host’s genome. These insights into the evolution of Ty families in Saccharomyces species have been based primarily on single reference genomes. Genomic analysis of Ty elements in multiple individuals within species has, until very recently, required sophisticated microarray techniques to be devised [35]–[37]. However, with the advent of high-throughput re-sequencing approaches, it is now possible to add a population genomic perspective to the evolution of Ty elements in yeast species [27], [38]–[42].
Here we investigate the evolutionary genomics of all Ty families in S. cerevisiae using a combination of bioinformatic, phylogenetic and population genomic approaches. We first provide a re-annotation of the S. cerevisiae reference genome, showing a considerably larger copy number than that originally reported by Kim et al. [22]. The majority of newly discovered Ty insertions are small, degenerate fragments that provide important data for inferring the evolutionary history of these families. In addition to finding new insertions of all five known Ty families, we also find insertions for a previously unannotated family, Ty3p. We then conduct phylogenetic and molecular evolutionary analyses on LTR sequences of all Ty families, which provide insight into the transpositional activity and evolutionary history of these families. For all Ty copies present in the reference genome, we also determine their presence or absence in a large sample of genomes from the Saccharomyces Genome Resequencing Project (SGRP) [27] and map this population genomic data onto Ty element phylogenies. In general, we find strong concordance between inferences of insertion history based on phylogenetic analysis with those based on population genomic evidence, but also identify a small subset of young Ty insertions at high frequency that may be under the influence of natural selection. Finally, we investigate the origins of Ty2 and Ty3p in S. cerevisiae by phylogenetic analysis of the Ty1/Ty2 and Ty3/Ty3p super-families in the Saccharomyces sensu stricto clade, and provide evidence that both Ty2 and Ty3p in S. cerevisiae arose by horizontal transfer at different times in the past. Together our results demonstrate that the reference genome of a single individual contains important information about the state of TE population dynamics within a species, but that full insight into TE evolutionary genomics requires complete genome sequences from multiple individuals and species.
Materials and Methods
Re-annotation of Ty Elements in S. cerevisiae
The June 2008 (sacCer2) version of the S. cerevisiae genome was downloaded from the UCSC Genome database and screened for TE sequences using RepeatMasker open-3.1.6 using a custom yeast TE library that includes Ty1-Ty5 from S. cerevisiae as well as Ty3p from S. paradoxus (File S1). Our custom TE library was constructed by exporting “saccharomyces” from the 20061006 RepeatMasker library, removing the redundant TY sequence, replacing TY4 with the first LTR element (positions 262–632) and internal sequence (positions 633–6116) from GenBank accession X67284 [43], adding the Ty3p LTR (Genbank: AY198187) and internal sequences (positions 306–4980 of Genbank: AY198186) [32], and renaming fasta headers for consistency. The decision to include Ty3p in our library was based on preliminary analyses that revealed additional Ty3-like sequences in the S. cerevisiae genome that were not fully identified using the Ty3 element as a query.
RepeatMasker output was then processed with REannotate version 26.11.2007 (options: -g -f -d 10000) [44] in order to join LTRs to internal sequences and defragment nested and degenerate insertions (see Figure 1 for an example screenshot of the REannotate output). LTR sequences from Ty1 and Ty2 were treated as equivalent in the defragmentation process. We then compared individual Ty copies from the REannotate output to Ty annotations in sacCer2 taken from SGD and a small number were manually edited to correct any obvious errors. In total, 10 Ty annotations from the REannotate output were manually split or joined to correct defragmentation problems, and coordinate spans of an additional 19 REannotate fragments were updated to remove redundant overlaps among features.
Figure 1. Example re-annotation of Ty elements in the S. cerevisiae genome.
Re-annotation of Ty elements using RepeatMasker and REannotate is shown in black above, and the original annotation of Ty elements and tRNA genes are shown in blue below. The red arrow indicates a newly annotated Ty1 fragment 5′ to a full-length Ty2 element that joins with a pre-existing Ty1 fragment 3′ to the Ty2 element, revealing a new TE nesting event.
LTR sequences of Ty1 and Ty2 are distinguished by a single base pair deletion in Ty2 elements [22]. We labelled LTRs identified by REannotate from either of these families as Ty2 when this deletion could clearly be identified. We also re-evaluated the identity of all Ty1 and Ty2 LTRs after a preliminary phylogenetic analysis of LTRs from both families. All LTRs nested within the Ty2 clade, including those that had secondary deletions spanning the diagnostic one bp Ty2 deletion, were then relabelled as Ty2 inserts. In total, 85 LTR fragments were relabelled from Ty1 to Ty2 or vice versa. The final dataset of curated Ty annotations can be found in File S2 and the RepeatMasker/REannotate fragments supporting these annotations can be found in File S3.
Phylogenetic Analysis of Paralogous LTR Sequences
Multiple alignments of paralogous LTRs were created individually for all five families (Ty1, Ty2, Ty3+Ty3p, Ty4 and Ty5) using MUSCLE 3.7 [45]. Phylogenetic trees were generated from each alignment using maximum likelihood and Bayesian inference analyses. The maximum likelihood analyses were performed using RAxML 7.2.6 [46] on raxmlGUI 0.9.3. The analyses were initiated with 100 parsimony trees, created using the recommended GTRCAT model incorporating 25 site-rate categories, and bootstrapped with 1,000 replicates. Parameters for each analysis were determined by RAxML. Bayesian phylogenies were created using MrBayes 3.1.2 [47]. MrBayes analyses were run using a GTR+I+Γ model and a four-category gamma distribution. Searches consisted of two parallel chain sets run at default temperatures, with a sample frequency of 10, until they had reached convergence (i.e. average standard deviation of split frequencies equal to 0.01) or had ran for 5,000,000 generations. Runs consisted of a minimum of 500,000 generations. The first 25% of sampled trees were discarded as burnin before calculating posterior probabilities.
Phylogenies of the Ty3/Ty3p and Ty1/Ty2 super-families were created using LTR sequences from multiple Saccharomyces sensu stricto species. Sequence similarity searches were performed on the genomes of S. bayanus, S. castellii, S. kluyveri, S. kudriavzevii, S. mikatae, S. paradoxus, S. servazii and S. unisporus. LTR sequences were extracted using WU-BLAST2 at SGD using the 3′ LTRs of u11 (Ty3) and u23 (Ty2) as the query sequences. With the exception of the highly degenerate Ty3p LTR sequences in S. cerevisiae, hits were filtered to a minimum length of 280 bp and an E-value of 1×10−10 for inclusion in these alignments. For each super-family, LTR sequences from each species were first aligned in MUSCLE and preliminary phylogenies were created from the unedited alignments using RAxML with 100 rapid bootstrap replicates using the GTRCAT model. These preliminary phylogenies were used to identify short branch (presumably recently active) sequences that spanned the diversity of active elements from each species for inclusion in the multi-species alignment. The final multispecies datasets were aligned using MUSCLE and maximum likelihood phylogenies were created following the same protocol as used for the individual S. cerevisiae Ty families. MrBayes analyses were also performed, running for 5,000,000 generations, a sampling frequency of 100 and with a burnin of 12,500, using the same protocol as for the individual Ty families. Nexus files for the Ty3/Ty3p and Ty1/Ty2 super-family alignments can be found in File S4 and S5, respectively.
For the Ty1/Ty2 superfamily the total number of hits from S. mikatae was 145, of which 44 were excluded on the basis of their long-branch length. LTR sequences of full-length Ty1 elements from S. cerevisiae (excluding the recombinant Ty1/Ty2 hybrid elements) were added to the dataset, as were LTR sequences of Ty2 from S. cerevisiae (excluding the long branch elements s304, st39, st3, st97, st98 and st107). For the Ty3 super-family, the Ty3p LTRs from S. cerevisiae were restricted to the seven longest sequences (which had a minimum length of 175 bp).
Molecular Evolutionary Analyses of Paralogous LTR Sequences
Phylogenetic trees were inspected and used to classify individual elements in the reference genome manually as being derived from ‘active’ or ‘inactive’ clades. Short-branch LTRs from clades with full-length elements were classified as active irrespective of whether the insert was itself still capable of transposition (e.g. a short-branch solo LTR from a recent intra-element recombination event would be classified as being in an active clade). LTRs present on long branches were classified as being in inactive clades. Separate alignments were created for all, inactive and active paralogous LTR sequences using MUSCLE, with only the 5′ LTR included from full-length elements containing both LTRs. An archive of all the paralogous LTR alignments used for molecular evolutionary analysis can be found in File S6.
Nucleotide diversity between paralogous LTRs in the three datasets (all, inactive and active) for each family was determined in DnaSP 5.1 [48] using π [49] and θ [50]. Tajima’s D [51] was calculated from the alignments of active elements to detect departures from expected patterns of nucleotide diversity under a standard neutral model of sequence evolution with families that have a constant rate of insertion that are at copy number and coalescent equilibrium [52]–[54].
Recombination events between recently active elements were identified with two datasets using Hudson and Kaplan’s RM [55]. First, the minimum number of recombination events (RM) was determined for all full-length elements for Ty1, Ty2 and Ty4. Second, we determined RM for all LTR sequences of active elements for Ty1, Ty2, Ty3 and Ty4. This second dataset was constructed to increase the number of paralogous elements included in this analysis, albeit at the expense of sequence length. Because of limited data, Ty3, Ty3p and Ty5 were excluded from analyses of recombination in full-length elements, and Ty3p and Ty5 were excluded from analyses of recombination among recently active LTRs.
Population Genomic Analyses of Orthologous Ty Insertions
S. cerevisiae resequencing data was downloaded from the SGRP ftp site: ftp://ftp.sanger.ac.uk/pub/dmc/yeast/latest/misc.tgz (last modified on 20/9/08). Coordinates of re-annotated Ty elements in the sacCer2 reference genome were transferred to the coordinate system of the SGRP reference genome from the by extracting the entire insertion including ±200 bp of flanking sequence from the sacCer2 genome and searching for an exact match in the SGRP reference genome. SGRP based coordinates were then used to extract multiple alignments of sequenced nucleotides prior to imputation using alicat.pl (option: q40). The S. cerevisiae strains involved were: 273614N, 322134S, 378604X, BC187, DBVPG1106, DBVPG1373, DBVPG1788, DBVPG1853, DBVPG6040, DBVPG6044, DBVPG6765, K11, L_1374, L_1528, NCYC110, NCYC361, RM11_1A, S288c, SK1, UWOPS03_461_4, UWOPS05_217_3, UWOPS05_227_2, UWOPS83_787_3, UWOPS87_2421, W303, Y12, Y55, Y9, YIIc17_E5, YJM789, YJM975, YJM978, YJM981, YPS128, YPS606, YS2, YS4, YS9. Resulting multiple alignments were viewed in Seaview 3.2 [56] and each Ty insertion was manually classified as present, absent or missing data for all 38 strains of S. cerevisiae. Insertion sites were then classified as fixed (scored as present or missing data in all genomes), polymorphic (scored as both present and absent in genomes with data), or S288c-only (found only in the S288c reference genome). Alignments of population genomic data in the SGRP strains for each Ty insertion can be found in File S7.
Results
Re-evaluation of TE Content and Copy Number in the S288c Reference Genome
We reannotated Ty elements in the reference S. cerevisiae genome and found a total of 483 Ty element insertions (Table 1; Files S2 and S3), which is ∼46% higher than the estimate of 331 by Kim et al. [22]. Increased copy number is observed for all five previously annotated Ty families, but is greatest for the most abundant family Ty1 (Table 1). Our reannotation uncovered 406,829 bp of DNA (3.35%) in the S. cerevisiae reference genome that was of recognisable Ty element origin. This value is only ∼30 kb greater than the estimate of Kim et al. [22], indicating that the majority of previously unannotated Ty insertions we identify are small fragments. The majority of the 152 new insertions identified here are degenerate solo LTRs, reinforcing the findings of Kim et al. [22] that full-length elements only make up a small proportion of Ty insertions in S. cerevisiae.
Table 1. Copy numbers and fixation status of Ty families within the S. cerevisiae genome.
| Family | Total | Full-length | Truncated | Solo LTR | Polymorphic | Fixed | Percentage Fixed |
| Ty1 | 313 (217) | 32 (32) | 2 (0) | 279 (185) | 48 (4) | 265 | 84.7 |
| Ty2 | 46 (34) | 13 (13) | 2 (0) | 31 (21) | 35 (1) | 11 | 23.9 |
| Ty3 | 45 (41) | 2 (2) | 0 (0) | 43 (39) | 22 (1) | 22 | 48.9 |
| Ty3p | 15 (0) | 0 (0) | 0 (0) | 15 (0) | 1 (0) | 14 | 93.3 |
| Ty4 | 49 (32) | 3 (3) | 1 (0) | 45 (29) | 18 (0) | 31 | 63.3 |
| Ty5 | 15 (7) | 1 (1) | 0 (0) | 14 (6) | 2 (0) | 13 | 86.7 |
| Total | 483 (331) | 51 (51) | 5 (0) | 427 (280) | 127 (6) | 356 | 73.7 |
Full-length elements are defined as those that possess both LTRs; truncated elements have lost one LTR and/or internal DNA through deletion other than LTR-LTR recombination; solo LTRs are generated through intra-element LTR-LTR recombination and can be complete or partial LTR sequences. Numbers in parentheses for the total, full-length, truncated and solo LTR columns are those reported in Kim et al. [22]. Fixed elements are those where a particular insertion was present in all scorable SGRP genomes. Numbers in parentheses for the polymorphic column indicate the number of insertions that could only be found in the S288c reference genome.
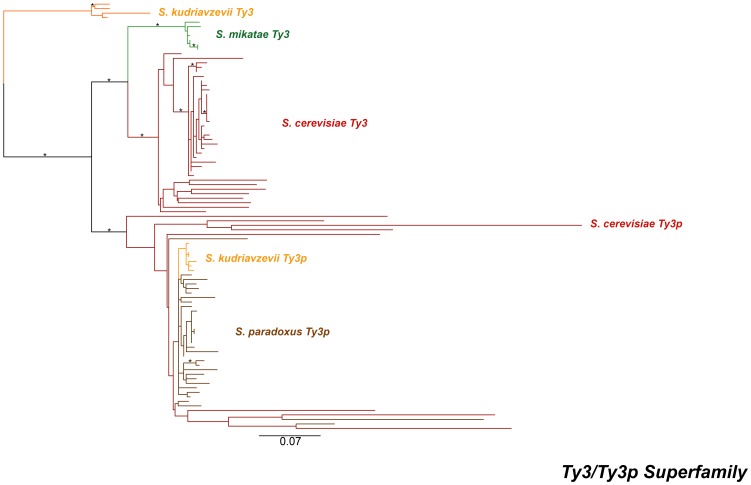
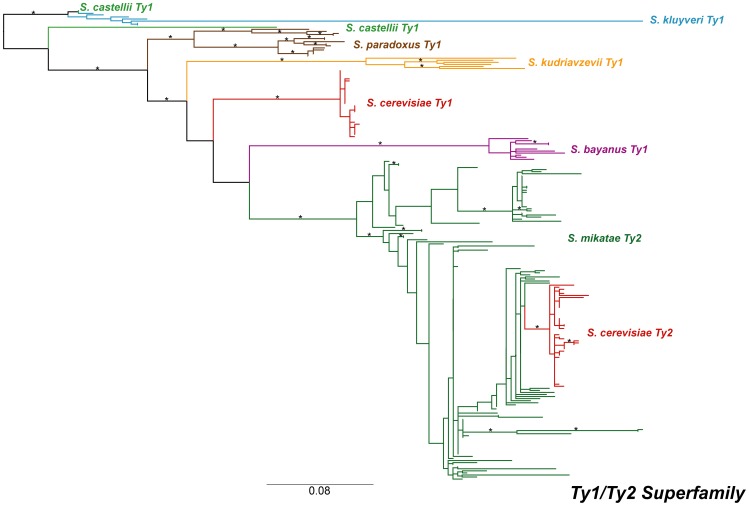
Fifteen of the newly annotated inserts were identified by including Ty3p (a Ty3 family from S. paradoxus [32]) as a query. Evidence for Ty3p sequences in the S. cerevisiae genome has not previously been reported, although a solo LTR (YNLWsigma2) previously annotated as a Ty3 insertion by Kim et al. [22] on chromosome XIV corresponds to a copy of Ty3p, s349, found here. Ty3p insertions are present only as solo LTRs in S. cerevisiae (Table 1) and thus, like Ty5, this lineage is comprised entirely of non-functional insertions in S. cerevisiae. We do not believe that the Ty3p sequences we detect in S. cerevisiae are simply ancient copies from the Ty3 lineage, since a phylogeny of the Ty3 super-family (Figure 2) across the Saccharomyces genus showed that Ty3 and Ty3p are distinct lineages that are separated by strongly supported branches (maximum likelihood bootstrap percentage, mlBP≥70%, Bayesian inference posterior probability, biPP≥0.95). The S. cerevisiae Ty3 elements form a sister-group with the Ty3 sequences of S. mikatae, while the highly degenerate S. cerevisiae Ty3p insertions a found in a well-supported clade with the Ty3p sequences of S. paradoxus and S. kudriavzevii. Furthermore, both Ty3 and Ty3p lineages are present and recently active in S. kudriavzevii suggesting that these are indeed distinct lineages. These results are consistent with the origin of the Ty3p family in S. cerevisiae by horizontal transfer (see below), and justify treatment of the S. cerevisiae Ty3p as a distinct family in the following analyses.
Figure 2. Maximum likelihood phylogeny of LTR sequences from the Ty3/Ty3p super-family within Saccharomyces sensu stricto.
The phylogeny was created from 983 aligned nucleotide positions. The branches are colour-coded based on the species labelled on the phylogeny. Individual sequence names have been omitted. Branches are drawn to scale and the bar represents the number of nucleotide substitutions per site. A “*” denotes branches with both mlBP ≥70% and biPP ≥0.95 support.
We identified only one additional full-length element, a Ty1 element (u19, on chromosome XII) relative to the SGD annotation of Ty elements on the sacCer2 genome. Examination of this region of Chromosome XII reveals a 5,926 bp element that possesses 338 bp LTRs and encodes putative TYA (Gag) and TYB (Pol) proteins. This insertion was erroneously annotated in the sacCer2 SGD annotation as two solo LTRs (YLRCdelta2 and YLRCdelta3) [22].
Kim et al. [22] classified all Ty insertions either as full-length or solo LTRs. Our re-annotation also identified a new class of five “truncated” elements generated through deletions of genomic DNA rather than LTR-LTR recombination. Three of the truncated elements (t41, t120 and t194) were identified previously as solo LTRs [22] while the other two truncated elements (t219 and t220), which both lack any LTR sequence, have not been identified previously.
Molecular Evolution of Paralogous Ty LTR Sequences
Variation among paralogous sequences of Ty elements in the S. cerevisiae reference genome allows indirect observations to be made of element activity using both phylogenetic and molecular evolutionary approaches. Recently transposed elements are unlikely to have accumulated a large number of mutations that differentiate them from their parental element; therefore recently active TEs are expected to exhibit short branches in phylogenetic trees generated from paralogous LTRs in a single genome. Likewise, active TE families that have undergone a recent increase in copy number will harbour an excess of sequence differences between paralogous copies that are at low frequency in the sample, resulting in a negative value of the Tajima’s D statistic [53], [54]. This pattern is analogous to the signature of recent population growth in population genetic analyses of an orthologous loci [51]. Finally, patterns of molecular variation that arise from genetic exchange can be used to detect gene conversion or recombination between paralogous Ty sequences in the past. Results for these three approaches are presented below to infer aspects of the evolutionary history for each Ty family.
Ty1
Ty1 has a much higher copy number (n = 313) than all other Ty families present in the S. cerevisiae genome (Table 1; see also [22]). Long branch, presumably inactive, insertions make up the majority (∼87%, n = 272; Table 2) of Ty1 insertions; these are exclusively solo LTRs (Figure 3). Short branch, presumably active Ty1 elements cluster together on the phylogeny, suggesting that all such elements have a recent common ancestry. There are a total of 41 Ty1 elements that appear to have inserted recently into the reference genome (Table 2, Figure 3). Eight of these young elements have subsequently undergone intra-element LTR-LTR recombination to become solo LTRs.
Table 2. Patterns of nucleotide diversity among paralogous LTR sequences within Ty families in the S. cerevisiae genome.
| All | Inactive | Active | Mean Terminal Branch Length(subs/site ± S.D.) | |||||||||
| Family | n | π | θ | n | π | θ | n | π | θ | Tajima’s D | Fixed | Polymorphic |
| Ty1 | 313 | 0.300 | 0.308 | 272 | 0.321 | 0.314 | 41 | 0.076 | 0.054 | 1.176 | 0.210±0.151 | 0.020±0.062 |
| Ty2 | 44 | 0.046 | 0.088 | 9 | 0.119 | 0.142 | 35 | 0.025 | 0.029 | −0.900 | 0.044±0.091 | 0.003±0.011 |
| Ty3 | 45 | 0.116 | 0.173 | 21 | 0.192 | 0.218 | 24 | 0.019 | 0.035 | −1.878 | 0.212±0.165 | 0.005±0.007 |
| Ty3p | 15 | 0.397 | 0.349 | 15 | 0.411 | 0.351 | 0 | - | - | - | 0.374±0.120 | 0.359±0.032 |
| Ty4 | 49 | 0.304 | 0.251 | 33 | 0.292 | 0.247 | 16 | 0.021 | 0.027 | −1.614 | 0.225±0.107 | 0.004±0.004 |
| Ty5 | 15 | 0.332 | 0.262 | 15 | 0.322 | 0.256 | 0 | - | - | - | 0.143±0.208 | 0.046±0.064 |
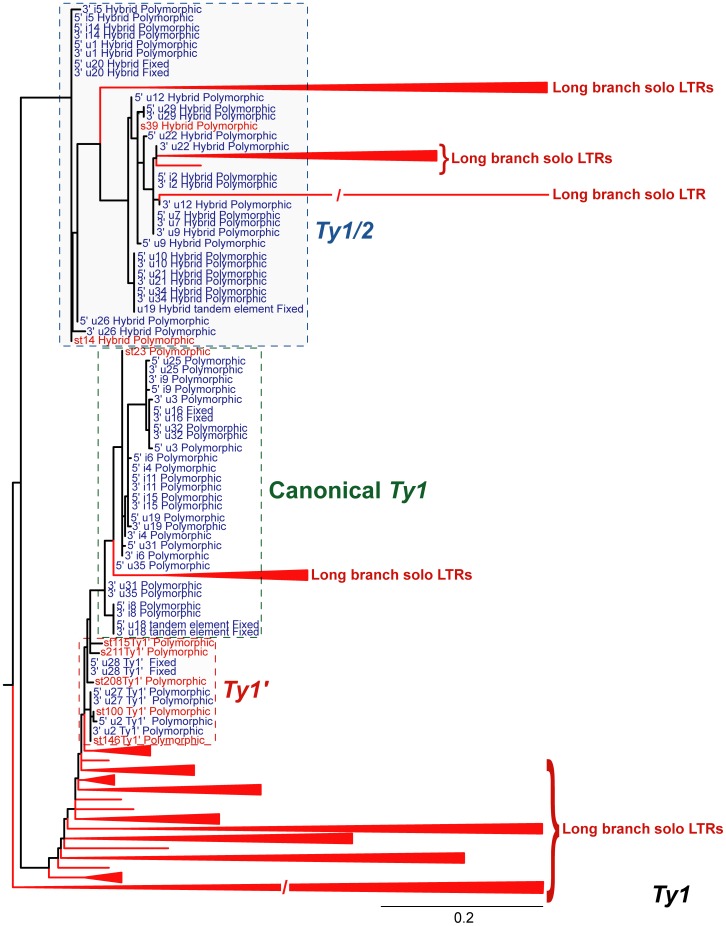
Figure 3. Maximum likelihood phylogeny of Ty1 LTR sequences from the reference genome of S. cerevisiae.
The phylogeny was created from 731 aligned nucleotide positions. LTRs in red are from partial elements and are no longer capable of autonomous transposition; LTRs in blue are from full-length elements and denoted as being from the 5′ or 3′ end of the element. Canonical, Ty1′ and hybrid Ty1/2 elements are boxed in green, red and blue, respectively. Clades composed entirely of inactive elements have been collapsed and labelled “Long branch solo LTRs”, while recently active solo-LTRs are individually named. Branches are drawn to scale and the bar represents the number of nucleotide substitutions per site. A “/” denotes arbitrarily shortened branches and a “*” denotes branches with both mlBP ≥70% and biPP ≥0.95 support.
The phylogeny also supports subdivision of the active Ty1 lineages into three previously proposed subfamilies [22], [23] that we refer to as “canonical” Ty1, Ty1′ and Ty1/Ty2 (Figure 3). The majority of Ty1 insertions are from the canonical, presumably ancestral, subfamily. In the clade containing the active canonical class, there are fourteen full-length elements and one solo LTR. The Ty1′ subfamily [22] is characterised by its highly divergent gag ORF. In addition to the three full-length elements described by Kim et al. [22], we identify a further five solo LTRs in the Ty1′ subfamily. The Ty1 phylogeny also supports a clade of 18 recombinant Ty1/2 elements [23], which fall into two distinct groups. The maximum likelihood tree places five long-branch solo LTRs within the Ty1/2 clade (shown as collapsed red clades in Figure 3), however their positions are not strongly supported and they are not recovered in this position in the Bayesian tree (data not shown). Fourteen of the Ty1/2 elements have previously been described and the approximate sites of recombination mapped [23], [57]. The newly identified hybrid elements appear to show the same recombination breakpoints (File S8).
LTR sequences of the active Ty1 elements harbour greater nucleotide diversity in comparison to the LTRs of active elements from other families (Table 2). Tajima’s D for the active Ty1 elements is positive, rather than negative (Table 2). It is unlikely that this result is due to a recent contraction in the number of elements within the genome; the phylogeny strongly suggests that Ty1 is currently transposing in S. cerevisiae, a result confirmed in numerous studies (e.g. [58], [59]). A more probable explanation is the presence of multiple active Ty1 subfamilies (see above, Figure 3). This phylogenetic sub-structure (like population subdivision) will result in intermediate frequency polymorphisms in the total population of active elements, leading to a positive value of D among paralogous Ty1 elements within the reference genome. Separate alignments of only canonical, Ty1′, or Ty1/2 elements however all exhibit a negative value for Tajima’s D (data not shown), consistent with their recent transposition.
Patterns of sequence diversity provide evidence for recombination between the full-length Ty1 copies that have integrated into the genome, with a minimum of 41 recombination events occurring between the 32 full-length elements. These results are consistent with recombination among paralogous sequences occurring at low frequency [60].
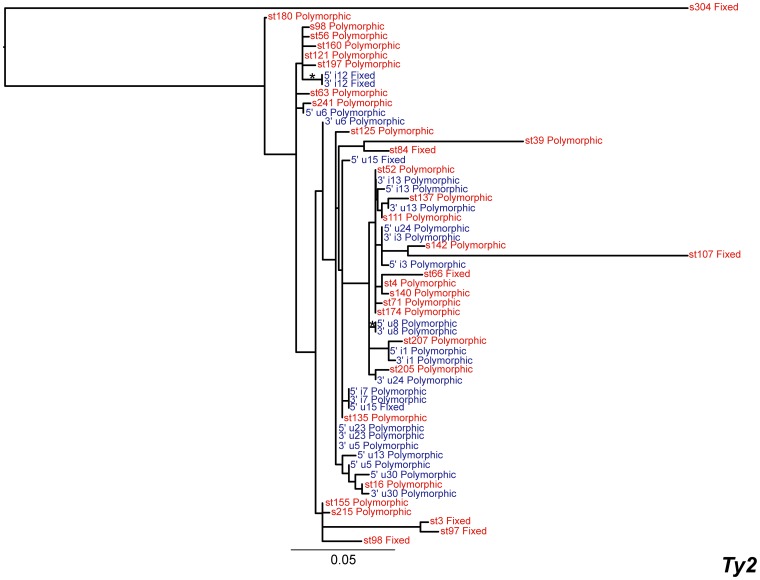
Ty2
The total copy number of Ty2 (n = 46) is similar to those of Ty3 (n = 45) and Ty4 (n = 49), but substantially lower than Ty1 (Table 1). The Ty2 phylogeny is poorly resolved under both maximum likelihood and Bayesian inference methods (Figure 4). Unlike Ty1, Ty3 and Ty4 there is no clear separation of long-branch, inactive elements and short branch, putatively active elements. There are nine long-branch solo LTRs in the phylogeny, which are presumably older inserts, however they do not cluster together. The remaining 35 inserts are present on short branches and appear to have recently integrated. Intra-element LTR-LTR recombination or truncation has converted 23 of the 35 recently integrated copies to solo LTRs.
Figure 4. Maximum likelihood phylogeny of Ty2 LTR sequences from the reference genome of S. cerevisiae.
The phylogeny was created from 362 aligned nucleotide positions. Colour codes and nomenclature for LTRs are the same as in Figure 3, with the exception that no clades are composed entirely of inactive elements and so none have been collapsed.
Consistent with a poorly resolved phylogeny, Ty2 elements have diverged less in comparison to all other families in the S. cerevisiae genome. Recent common ancestry of most Ty2 copies seems to be the most likely explanation of the poorly resolved phylogeny and relatively low nucleotide diversity in comparison to the other TE families in the genome (see below). A negative value of Tajima’s D is consistent with the recent arrival of Ty2 elements in the S. cerevisiae genome (Table 2).
In spite of their recent origin, there is evidence that paralogous copies of Ty2 are undergoing recombination, similar to Ty1. A minimum of 19 recombination events can be detected between the 13 full-length copies of Ty2 and recently integrated Ty2 LTR sequences also show evidence for three recombination events among paralogous copies.
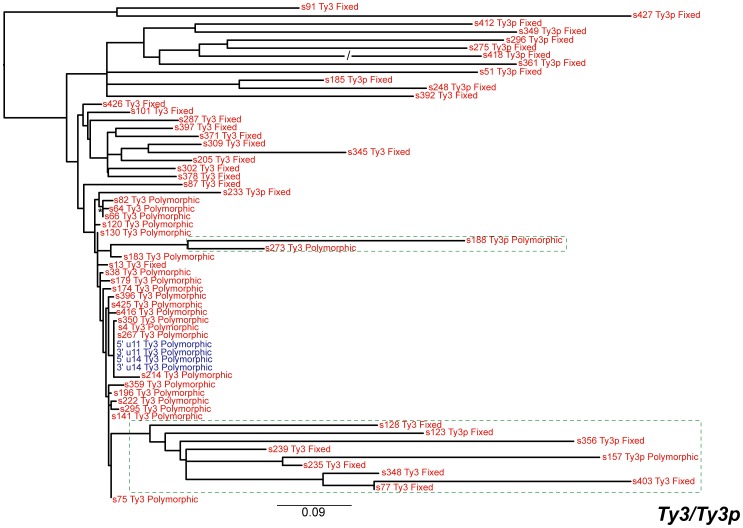
Ty3
The 45 copies of Ty3 exhibit lower levels of nucleotide diversity than most other families, with only copies of Ty2 showing less variation (Table 2). The Ty3 phylogeny exhibits long-branch, older inserts as well as a single clade containing putatively active elements (Figure 5). Nested among the short branch, younger inserts are two clades of long branch LTRs. The placements of the two long branch clades have no support (mlBP<50%, biPP<0.70), are not supported by the Bayesian phylogeny, and their placement in the tree are most likely to be phylogenetic artefacts.
Figure 5. Maximum likelihood phylogeny of Ty3 and Ty3p LTR sequences from the reference genome of S. cerevisiae.
The phylogeny was created from 625 aligned nucleotide positions. Green boxes highlight long-branch clades whose positions among the short-branch, younger elements are probably phylogenetic artefacts since they found in different positions in the Bayesian analysis. Formatting of the tree is otherwise the same as in Figure 4.
The active clade of Ty3 (excluding the long branch LTRs) is composed of 24 inserts and shows the lowest nucleotide diversity of all the active Ty lineages. Tajima’s D calculated from the alignment of LTRs from this clade is negative, consistent with this group of elements having undergone a recent expansion (Table 2). Although this clade has recently been active there are only two full-length, and therefore potentially transpositionally competent, elements in the reference genome. The remainder of the inserts within this clade are solo LTRs, suggesting a high rate of intra-element LTR-LTR recombination for this family The proportion of solo LTRs seen in the active Ty3 lineage is not significantly different to that seen in the active lineage of Ty4 elements (Fisher’s exact test; P = 0.36), however it is significantly higher than both Ty1 (P<0.0001) and Ty2 (P<0.0001).
As there are only two full-length copies of Ty3 in the reference genome, it was not possible to detect any possible recombination events between full-length paralogues. Nucleotide sequences of the recently inserted copies of Ty3 do not show any evidence of recombination between the LTR sequences at paralogous sites.
Ty3p
The 15 copies of the new Ty3p family we report here are only present as highly degenerate solo LTRs (Table 1). This family also harbours the highest level of nucleotide diversity among paralogous copies in the reference genome (Table 2). In addition, no copies of Ty3p are present on short branches in the combined Ty3 and Ty3p phylogeny (Figure 5), highlighting a lack of recent transposition for this family. Together, these features indicate that Ty3p is an ancient component of the genome that lost the ability to transpose in S. cerevisiae long ago. Because there are no LTRs from full-length elements for this family, we did not perform recombination analyses for Ty3p.
Ty4
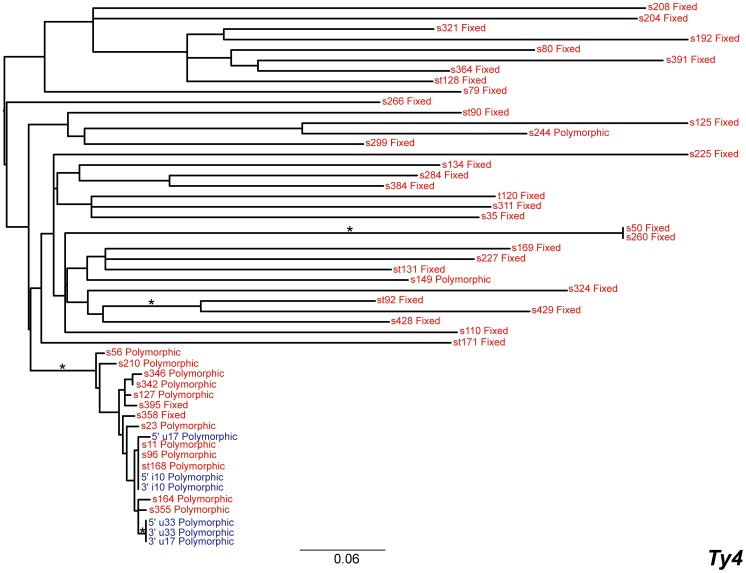
The phylogeny of the 49 Ty4 insertions shows a single, well-supported (72% mlBP, 0.77 biPP) recently active lineage of 16 insertions with the remainder of the 33 LTR sequences being present on long, poorly supported branches (Figure 6). Only three of the insertions from the recently active clade are present as full-length copies in the reference genome, since the remainder have undergone LTR-LTR recombination. Tajima’s D is negative for the active clade of Ty4 (Table 2), consistent with recent transposition for this group of elements. Hudson and Kaplan’s RM failed to find any evidence of recombination between the full-length copies of Ty4 or between the active LTR sequences of this family.
Figure 6. Maximum likelihood phylogeny of Ty4 LTR sequences from the reference genome of S. cerevisiae.
The phylogeny was created from 450 aligned nucleotide positions. Formatting of the tree and labels is the same as in Figure 4.
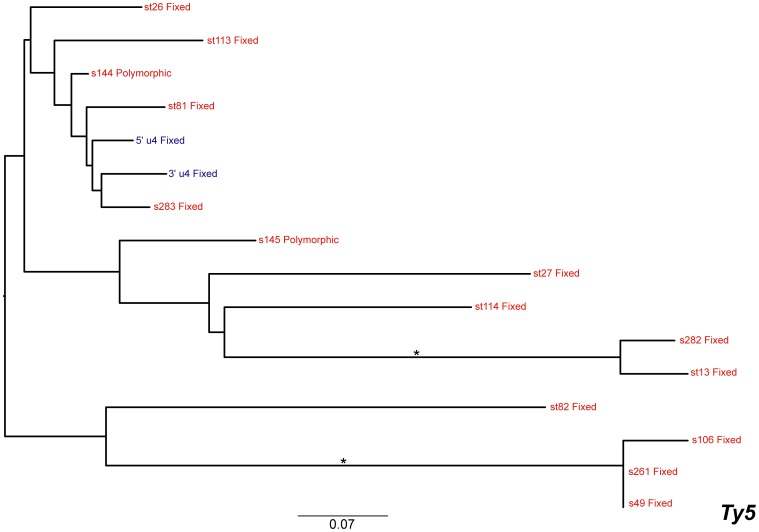
Ty5
Phylogenetic analysis of the 15 Ty5 insertions revealed the absence of a putatively active clade for this family, suggesting that there has been very little, if any, transposition of this family in the recent evolutionary history of S. cerevisiae (Figure 7). This result supports previous findings [61] and is reinforced by the fact that the only full-length copy of Ty5 in the reference genome harbours internal deletions and the LTRs share 91% identity [19]. There are two identical Ty5 solo LTRs on chromosomes IV and X (s49 and s261) that give rise to short branches in the phylogeny; however examination of their flanking DNA shows that they have arisen due to an inter-chromosomal segmental duplication rather than transposition, as has been observed previously for Ty1 [22]. The same genomic duplication event also generated additional copies of the Ty4 solo LTRs that flank the s49 and s261 Ty5 fragments, since the Ty4 inserts s50 and s260 are also present in the duplicated region. Because there are no LTRs from full-length elements for this family, we did not perform recombination analyses for Ty5.
Figure 7. Maximum likelihood phylogeny of Ty5 LTR sequences from the reference genome of S. cerevisiae.
The phylogeny was created from 268 aligned nucleotide positions. Formatting of the tree and labels is the same as in Figure 4.
Population Genomic Analyses of Ty Elements in 38 S. cerevisiae Strains
While important inferences about the history of Ty element activity such as those above can be made from a single reference genome, it remains an open question whether information in reference genomes accurately represent the state of TE population dynamics within a species. To address this issue, all 483 Ty element insertions present in the reference genome were screened in silico for their presence or absence in whole genome reference-based alignments of the 37 other S. cerevisiae strains sequenced by the SGRP. Because we only use the actual, not imputed, sequence data from the SGRP alignments, the relevant segment of an SGRP strain can be missing due to the low-coverage nature of these shotgun sequences. The number of genomes with data available to score presence/absence of an insertion ranged from 2–37 per locus and, in total, 44% of alleles were classified as missing data across all strains and loci (see File S2). Because of the substantial degree of missing data, we did not attempt to analyze allele frequencies for each insertion and instead chose to classify Ty insertions as fixed, polymorphic or S288c-only. Polymorphic Ty insertions may be misclassified as S288c-only because of missing data in other strains, and thus both classes can be considered polymorphic.
Based on presence/absence data in the SGRP alignments, we estimate that 73.7% (356/483) of the Ty elements in the reference genome are fixed (scored as present in all genomes with sequence data) in the S. cerevisiae population (Table 1). Ty2 shows the lowest proportion of fixed elements (23.9%), consistent with most insertions from this family having recently integrated into the S. cerevisiae genome. Nearly half of the Ty3 insertions are fixed (48.9%), and the majority of elements in the other Ty families are fixed. Fourteen of the fifteen Ty3p solo LTRs are fixed within the scored genomes. Only one copy appears to be polymorphic, but this is due to a post-fixation secondary deletion in the strain RM11_1A that covers the insertion site as well as the flanking DNA. Fixation of all Ty3p insertions is consistent with lack of recent transposition for this family in the S. cerevisiae genome. Previous studies have reported a lack of Ty5 in some strains of S. cerevisiae [34], [61]; our in silico screen of the SGRP genomes however shows that Ty5 copies are predominantly fixed, with only two segregating inserts present in the reference genome. The two polymorphic inserts are both solo LTRs and are adjacent to each other, albeit on different strands, on Chromosome V.
We mapped polymorphism or fixation states of each insert onto the LTR phylogenies, which showed that most of the long-branch, inactive inserts are fixed within the population. In contrast, almost all of the inserts in the putatively active clades are polymorphic (Figures 3, 4, 5, 6, 7). This result demonstrates that both terminal branch lengths estimated from phylogenies of paralogous LTRs and fixation status of insertions estimated from population genomic data provide consistent inferences about the history of individual Ty insertions in S. cerevisiae. The mean terminal branch lengths of fixed elements for Ty1, Ty2, Ty3 and Ty4 are an order of magnitude or more greater than the mean terminal branch lengths of polymorphic inserts (Table 2). The mean terminal branch length for fixed copies of Ty1, Ty3 and Ty4 are of a similar scale, suggesting that they have been components of the S. cerevisiae genome for a similar length of time. In contrast, fixed copies of Ty2 show considerably shorter terminal branch lengths, which is consistent with a more recent origin of Ty2 in S. cerevisiae.
Horizontal Transfer Explains the Existence of Closely Related Ty Families in S. cerevisiae
Two contrasting models can be put forward to explain the existence of closely related Ty families (like Ty1 and Ty2, or Ty3 and Ty3p) in the S. cerevisiae genome [22]. The first model proposes that closely-related families are sub-lineages of an ancestral super-family that evolved by vertical transmission and TE “speciation” within S. cerevisiae or its ancestor. Evidence for distinct, active subfamilies of Ty1 in S. cerevisiae support the plausibility of the TE speciation model (Figure 3).
The second model proposes that closely-related families are sub-lineages of the same super-family that arose in different Saccharomyces species and subsequently came together in S. cerevisiae or its ancestor by horizontal transfer [22], [34]. In the case of Ty1 and Ty2, support for the horizontal transfer model comes from the fact that some Saccharomyces species (e.g. S. paradoxus and S. bayanus) appear to have only Ty1 but not Ty2 elements [62], while S. mikatae appears to have only Ty2 but not Ty1 elements [63]. Furthermore, Liti et al. [34] noted that Ty2 is present in S. mikatae but not in species more closely related to S. cerevisiae, and that Ty2 from S. cerevisiae and S. mikatae share a high level of nucleotide identity. These observations led Liti et al. [34] to propose a horizontal transfer of Ty2 between S. cerevisiae and S. mikatae, but based on available data these authors could not determine the direction of any putative horizontal transfer event.
The low degree of nucleotide diversity observed here in Ty2 is consistent with a recent invasion of the S. cerevisiae genome by this family. Furthermore Ty2 has a significantly lower proportion of both long-branch and fixed copies in comparison to other families, and its fixed copies exhibit a considerably shorter mean terminal branch length (Table 2). The recent origin of virtually all Ty2 copies in S. cerevisiae and the absence of Ty2 in S. paradoxus – other than in hybrid strains [34] – point to the direction of horizontal transfer being from S. mikatae to S. cerevisiae. To test this model, we conducted a phylogenetic analysis of active Ty1 and Ty2 LTR sequences from multiple Saccharomyces sensu stricto species (Figure 8). This analysis provides direct evidence that Ty2 is not a subfamily of the S. cerevisiae Ty1 family, as the Ty2 families of S. cerevisiae and S. mikatae robustly cluster together (92% mlBP, 0.97 biPP). The phylogeny nests the monophyletic S. cerevisiae Ty2 elements within the S. mikatae Ty2 elements, with strong support (74% mlBP, 0.97 biPP), providing clear evidence that the direction of the horizontal transfer was from S. mikatae to S. cerevisiae. The observed phylogeny is not simply the result of LTR swapping through recombination, as protein phylogenies of Gag and a 300 amino acid residue region the of Pol both show that the S. cerevisiae Ty2 is more closely related to Ty2 of S. mikatae than S. cerevisiae Ty1 (data not shown).
Figure 8. Maximum likelihood phylogeny of the Ty1/Ty2 super-family within Saccharomyces sensu stricto.
The phylogeny was created from 294 aligned LTR nucleotide positions. The branches have been colour coded based on the species from the LTRs were sequenced; species clades are labelled on the phylogeny. Individual sequence names have been omitted. Formatting of the tree and labels is the same as in Figure 2.
Support for horizontal transfer as the primary explanation to understand the existence of closely related Ty families within a yeast species is also found in our Ty3/Ty3p superfamily analysis (Figure 2). However, the observation of distinct Ty3 and Ty3p lineages in S. cerevisiae and S. kudriavzevii is compatible with several possible scenarios involving horizontal transfer. Under one scenario, a single Ty3 family would have arisen in the ancestral Saccharomyces species with vertical transmission leading to a single Ty3-like family in most species except S. cerevisiae and S. kudriavzevii, which have two Ty3-like families due to horizontal transfer events: (i) one unsuccessful transfer of Ty3p from S. paradoxus to S. cerevisiae and (ii) one successful transfer of Ty3p from S. paradoxus to S. kudriavzevii. Alternatively, the presence of S. cerevisiae and S. kudriavzevii could be explained by speciation of the Ty3-like ancestor into Ty3 and Ty3p along the lineage leading to S. kudriavzevii, an ancient horizontal transfer of the S. kudriavzevii Ty3p family into the ancestor of S. cerevisiae and S. paradoxus, followed by inactivation of Ty3p in S. cerevisiae and complete loss of Ty3 in S. paradoxus. Several other plausible alternatives that require at least one horizontal transfer event can also be formulated. Evaluation of which scenario can best explain the data will require further systematic genomic analysis in multiple Saccharomyces species. We note however that, while at face value our conclusion that Ty3 has undergone horizontal transfer seems to contradict previous claims [32], our results are in fact compatible with the data of Fingerman et al. [32] which only support a long-term association of Ty3-like lineages in the Saccharomyces genus, but do not actually preclude the possibility of horizontal transfer events within the genus.
Discussion
The transposable elements of S. cerevisiae represent the most comprehensively investigated set of TEs studied at the genomic level within the eukaryotes. However our current understanding of evolutionary trends within Ty elements is predominantly based on research conducted at the end of the last century using data from a single reference genome [22], [23], [30], [64]. Furthermore, previous studies focussed almost exclusively on a subset of families (namely Ty1 and Ty2), and essentially no inferences have been drawn about the history of the smaller families (Ty3, Ty3p, Ty4 and Ty5) (but see [30]). With more sophisticated bioinformatic techniques now available to study TEs in genome sequences [65], as well as population and comparative genomic data from multiple Saccharomyces strains and species [27], [66], [67], it is an opportune time to re-evaluate the evolution of Ty elements in S. cerevisiae. The analyses performed here have shown that many of the ideas on TE evolution in S. cerevisiae require substantial revision.
Our high-resolution re-annotation of Ty elements presented here reveals that the original survey of Ty elements in the S. cerevisiae genome underestimated copy number by almost 50% [22]. Kim et al. [22] used cutoffs that allowed the identification of all full-length elements in the reference genome, but omitted a large number of additional partial elements that we have detected with improved bioinformatic methods and a refined library of Ty sequences. One of the more surprising aspects of the re-analysis is the identification of a previously un-annotated family of LTR retrotransposons, Ty3p. In addition to improving the S. cerevisiae genome annotation and providing resources to shed light on the evolution of transposable elements, the additional Ty sequences found here have practical importance for efforts to engineer synthetic yeast chromosomes that lack destabilising sequences like Ty elements and tRNA genes [68].
Our results also suggest that some conclusions about the long-term co-evolutionary relationship of Ty elements with their host should be re-interpreted. Kim et al. [22] proposed that Ty3 and Ty4 may be recent arrivals in the S. cerevisiae genome, due to the low average nucleotide diversity that they observed in both families. In contrast, our phylogenetic analysis reveals long-branch, ancient lineages in these families indicating that both families are long-term inhabitants of the S. cerevisiae genome. Ty3 and Ty4 both also exhibit short-branch clades in their phylogenies providing evidence for the recent, and probably current, activity of these families in the S. cerevisiae population. This inference is supported by the negative value of Tajima’s D for the putatively active lineages of Ty3 and Ty4, which implies recent growth in the number of elements for these families within the yeast genome.
Our work also resolves controversy about the origin of the Ty2 family, which has been proposed to have evolved either from a Ty1-like ancestor or arisen by horizontal transfer [22], [34]. Both the lack of old copies of Ty2 in the S. cerevisiae genome and the phylogenetic position within the Ty1/Ty2 super-family are consistent with the recent horizontal transfer of this family, with S. mikatae the most probable donor species. The ability of Saccharomyces sensu stricto species to form viable hybrids naturally in the wild (reviewed in [69]) provides a simple mechanism for the horizontal transfer of TEs between species without the requirement of an intermediate vector. Interspecific hybrids typically show dramatically reduced fertility (reviewed in [69]), but viable gametes can be produced by interspecific hybrids [70]–[72] that can backcross with their parental species [72]. Moreover, several studies have reported naturally-occurring introgressions between S. cerevisiae and S. paradoxus [73]–[76] demonstrating the successful transfer of genetic information between Saccharomyces species. If interspecific hybridisation is involved in the transfer of Ty families between Saccharomyces species it must be rare, since Ty element sequences are clearly not homogenized across yeast species [34].
The phylogenies presented here show that Ty families, with the exception of the inactive Ty3p and Ty5, contain clusters of short-branched elements that we consider to be the products of recent transposition events. However, the homogenizing effects of gene conversion can also lead to short branch lengths between paralogous copies of Ty elements, with branch lengths reflecting the time of the last gene conversion event rather than the last transposition event. It seems unlikely however that gene conversion, as opposed to recent transposition, is the cause of the short-branched clusters present in the LTR trees for several reasons. First, Kupiec and Petes [60] noted that gene conversion events occur at low frequency, suggesting that such a mechanism is not strong enough to homogenize the sequences within families in the genome. Second, the presence of ancient, highly divergent, elements in the genome, as well as three proliferating subfamilies of Ty1, also provides direct evidence that transposable element sequences can escape the action of gene conversion and diverge within the S. cerevisiae population. Third, the only two families that do not contain clusters of short branch elements, Ty3p and Ty5, are known to be incapable of transposition, but have no obvious impediment to gene conversion between paralogous copies. On balance, it would appear that the phylogenies mainly represent the history of transposition within each family, rather than reflecting the action of widespread gene conversion.
The availability of genomic data from multiple S. cerevisiae strains means that studies on the evolution of Ty elements in this species are no longer limited to studies on a single reference genome. Our population genomic analyses show that, despite the assumed deleterious nature of most TE insertions, a surprisingly high percentage (73.7%) of Ty copies are fixed in S. cerevisiae. These results support previous work that has shown many Ty LTR sequences in S. paradoxus are fixed in natural populations [32], [77], permitting their use as putatively unconstrained sequences in molecular evolutionary studies [77], [78]. The vast majority (98%) of fixed Ty insertions are partial elements. The dearth of fixed full-length elements may be due in part to LTR-LTR recombination being a one-way process that occurs more rapidly than the sojourn time to fixation. However it is also likely that solo LTRs are less deleterious to their hosts [7], and are therefore more likely to go to fixation by drift. Regardless of the processes that lead to fixation, the fact that most Ty insertions are fixed suggests that the catalogue of Ty insertion sites discovered using the S288c reference genome describes the core state of most Ty element locations across strains in this species.
While our results show that the majority of Ty elements are likely to be fixed in S. cerevisiae, it is worth considering how our results would be affected by using other strains besides S288c strain for Ty element discovery. Discovery of Ty element locations using reference assemblies from other strains of S. cerevisiae besides S288c is likely to be biased toward finding fewer (or more highly fragmented) Ty elements, since repetitive sequences like TEs are often collapsed or present near gaps in draft assemblies. Moreover, previous work has shown that S288c has a higher proportion of Ty sequences than any other strain in the SGRP data set [27]. Therefore, population genomic analysis based on discovery of Ty elements using S288c as a reference strain is likely to survey a greater number of potential insertion sites than those based on other S. cerevisiae strains. Furthermore, it would not be possible to use the SGRP whole genome alignments as we have here to estimate polymorphism or fixation status of Ty elements using an alternative reference strain, since the SGRP alignments are reference-based and explicitly linked to the S288c genome. Future work using unassembled reads to identify de novo Ty element locations (e.g. [38]) and estimate allele frequencies will be needed to overcome the limitation of using draft assemblies and reference-based whole genome alignments in order to determine the generality of conclusions based on Ty element discovery in S288c.
While the majority of fixed elements are old Ty insertions, we have identified 33 young insertions that are segregating at high frequencies (≥0.50, >15 scored genomes) in sequenced S. cerevisiae strains (File S2), seven of which are full-length copies and 26 are solo LTRs. These insertions may be at high frequency due to drift or hitchhiking along with advantageous host alleles, but it is also possible that a number of these elements are positively selected. The LTRs of Ty elements contain multiple modifiers of transcription [25], [79], [80], and TEs are known to be able to alter the expression of neighbouring host genes [80], [81]. However, none of the high frequency Ty insertions identified here correspond to a Ty1 insertion previously reported to confer an adaptive change in gene expression of the HAP1 gene [29], [82]. Nevertheless, the high-frequency, young insertions identified here present a candidate set for further investigation into potential advantageous Ty insertions in S. cerevisiae.
By combining phylogenetic inferences with population genomic data on the presence or absence of Ty elements, our work provides important confirmation that terminal branch lengths in phylogenetic trees provide information about the population frequency of individual insertions. Long branch inserts tend to be fixed in the S. cerevisiae genome, whereas short branch inserts mainly segregate at lower frequencies. This observation supports many previous studies (e.g. [7], [22], [83], [84]) that rely on information from a single genome to make inferences about the dynamics of TE evolution in a given species. As we enter the era of widespread population-genomic resequencing, it will be of considerable interest to see how commonly this trend holds for LTR elements and other classes of TEs in the genomes and populations of other eukaryote species.
Supporting Information
Database of Ty element reference sequences used for re-annotation of the yeast genome. Fasta file of LTR and internal sequences from Ty1-Ty5, with the inclusion of Ty3p from S. paradoxus.
(TXT)
Summary of the annotated Ty elements in the June 2008 version of S. cerevisiae genome from the UCSC Genome Database (sacCer2). Shown are the REannotate identifier, genomic coordinates of the complete span of all fragments from an individual element, strand, name(s) of corresponding Ty elements from the SGD sacCer2 annotation, structural classification, activity status as determined by phylogenetic analysis, estimated allele frequency, and polymorphism/fixation status based on SGRP alignments. More specifically the status of Ty insertions in each SGRP alignment is classified as polymorphic or fixed, but Ty elements that are present only in the reference strain are labelled as S288c in this table and classified as polymorphic in subsequent analyses. Presence (1), absence (0) or missing data (n.a.) status is shown for individuals SGRP strains in columns following the “sgrp alignment status” column. REannotate identifiers are comprised of the chromosome name, the insertion type, a numerical identifier and Ty family name. Insertion type can be full-length (i or u), truncated (t) or solo LTR (s or st). Insertions from the Ty3p family are labelled as TY3_1p according to RepBase nomeclature.
(TXT)
GFF file of Repeatmasker/REannotate Ty fragments in the June 2008 version of S. cerevisiae genome from the UCSC Genome Database (sacCer2). Individual fragments from the same element are given the same name in the ID column. The span of the union of fragments is the same as the range of coordinates given in File S2.
(TXT)
Nexus file of aligned LTR sequences used for phylogenetic analysis of Ty3/Ty3p superfamily. Alignments of paralogous LTR sequence from the Ty3/Ty3p superfamily used to create the phylogeny in Figure 2. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(TXT)
Nexus file of aligned LTR sequences used for phylogenetic analysis of Ty1/Ty2 superfamily. Alignments of paralogous LTR sequence from Ty1/Ty2 superfamily used to create the phylogeny in Figure 8. Positions enclosed in square brackets were excluded from the phylogenetic analyses. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(TXT)
ZIP archive of LTR alignments used for molecular evolutionary analyses. Alignments of paralogous LTR sequence from all, active and inactive elements are provided for Ty1, Ty2, Ty3+Ty3p, Ty4 and Ty5, respectively. For full-length element only the 5′ LTR was included. Also included are alignments of the Ty3/Ty3p superfamily and the Ty1/Ty2 superfamily, respectively. Positions enclosed in square brackets were excluded from the phylogenetic analyses. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(ZIP)
ZIP archive of SGRP strain alignments used for population genomic analyses. Alignments of the orthologous genomic region in each SGRP strain corresponding to each Ty insertion in our re-annotation of the S. cerevisiae reference genome. N’s represent low quality (<Q40 phred score) or missing data, primarily due to the low-coverage sequence of many SGRP strains. The DNA sequence of sacCer2 is included as “REF” and each alignment includes the TE locus and 200 bp of DNA sequence on either side of the Ty insertion site. A dummy sequence shows the position of each TE in the alignment with “T”s.
(ZIP)
Visualization of recombination breakpoints in Ty1, Ty1/2 and Ty2 complete LTR sequences. The two approximate recombination breakpoints are highlighted with black lines. Asterisks highlight columns in the alignment where the base is conserved across all sequences.
(PDF)
Acknowledgments
We thank Jainy Shah for preliminary analysis of Ty re-annotation, Killian McSorley for preliminary analysis of Ty4 and Ty5 phylogenies, Vini Periera for assistance interpreting REannotate output, Esther Chan for providing scripts to generate liftOver files, and Daniela Delneri and Ed Louis for helpful discussion. We also thank Daniela Delneri and Ben Blackburne for critical comments on the manuscript.
Funding Statement
This work was partly supported by a NERC Fellowship to Douda Bensasson [grant number NE/D008824/1] (http://www.nerc.ac.uk). No additional external funding was received for this study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pritham EJ (2009) Transposable elements and factors influencing their success in eukaryotes. J Hered 100: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montgomery E, Charlesworth B, Langley CH (1987) A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster . Genet Res 49: 31–41. [DOI] [PubMed] [Google Scholar]
- 3. Biemont C, Tsitrone A, Vieira C, Hoogland C (1997) Transposable element distribution in Drosophila. Genetics 147: 1997–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charlesworth B, Langley CH, Sniegowski PD (1997) Transposable element distributions in Drosophila . Genetics 147: 1993–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollister JD, Gaut BS (2007) Population and evolutionary dynamics of Helitron transposable elements in Arabidopsis thaliana. Mol Biol Evol 24: 2515–2524. [DOI] [PubMed] [Google Scholar]
- 6. Song M, Boissinot S (2007) Selection against LINE-1 retrotransposons results principally from their ability to mediate ectopic recombination. Gene 390: 206–213. [DOI] [PubMed] [Google Scholar]
- 7. Carr M, Nelson M, Leadbeater BS, Baldauf SL (2008) Three families of LTR retrotransposons are present in the genome of the choanoflagellate Monosiga brevicollis. Protist 159: 579–590. [DOI] [PubMed] [Google Scholar]
- 8. Charlesworth B, Langley CH (1989) The population genetics of Drosophila transposable elements. Annu Rev Genet 23: 251–287. [DOI] [PubMed] [Google Scholar]
- 9. Daborn PJ, Yen JL, Bogwitz MR, Le Goff G, Feil E, et al. (2002) A single p450 allele associated with insecticide resistance in Drosophila. Science 297: 2253–2256. [DOI] [PubMed] [Google Scholar]
- 10. Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D (2007) Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae). Cell Microbiol 9: 2022–2029. [DOI] [PubMed] [Google Scholar]
- 11. Schlenke TA, Begun DJ (2004) Strong selective sweep associated with a transposon insertion in Drosophila simulans. Proc Natl Acad Sci U S A 101: 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez J, Lenkov K, Lipatov M, Macpherson JM, Petrov DA (2008) High rate of recent transposable element-induced adaptation in Drosophila melanogaster. PLoS Biol 6: e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aminetzach YT, Macpherson JM, Petrov DA (2005) Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila . Science 309: 764–767. [DOI] [PubMed] [Google Scholar]
- 14. Bartolome C, Bello X, Maside X (2009) Widespread evidence for horizontal transfer of transposable elements across Drosophila genomes. Genome Biol 10: R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lerat E, Burlet N, Biemont C, Vieira C (2011) Comparative analysis of transposable elements in the melanogaster subgroup sequenced genomes. Gene 473: 100–109. [DOI] [PubMed] [Google Scholar]
- 16. Thomas J, Schaack S, Pritham EJ (2010) Pervasive horizontal transfer of rolling-circle transposons among animals. Genome Biol Evol 2: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert C, Schaack S, Pace JK 2nd, Brindley PJ, Feschotte C (2010) A role for host-parasite interactions in the horizontal transfer of transposons across phyla. Nature 464: 1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, et al.. (1996) Life with 6000 genes. Science 274: 546, 563–547. [DOI] [PubMed]
- 19. Voytas DF, Boeke JD (1992) Yeast retrotransposon revealed. Nature 358: 717. [DOI] [PubMed] [Google Scholar]
- 20. Kingsman AJ, Gimlich RL, Clarke L, Chinault AC, Carbon J (1981) Sequence variation in dispersed repetitive sequences in Saccharomyces cerevisiae. J Mol Biol 145: 619–632. [DOI] [PubMed] [Google Scholar]
- 21. Hani J, Feldmann H (1998) tRNA genes and retroelements in the yeast genome. Nucleic Acids Res 26: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JM, Vanguri S, Boeke JD, Gabriel A, Voytas DF (1998) Transposable elements and genome organization: a comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence. Genome Res 8: 464–478. [DOI] [PubMed] [Google Scholar]
- 23. Jordan IK, McDonald JF (1998) Evidence for the role of recombination in the regulatory evolution of Saccharomyces cerevisiae Ty elements. J Mol Evol 47: 14–20. [DOI] [PubMed] [Google Scholar]
- 24. Hansen LJ, Sandmeyer SB (1990) Characterization of a transpositionally active Ty3 element and identification of the Ty3 integrase protein. J Virol 64: 2599–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hug AM, Feldmann H (1996) Yeast retrotransposon Ty4: the majority of the rare transcripts lack a U3-R sequence. Nucleic Acids Res 24: 2338–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilke CM, Maimer E, Adams J (1992) The population biology and evolutionary significance of Ty elements in Saccharomyces cerevisiae. Genetica 86: 155–173. [DOI] [PubMed] [Google Scholar]
- 27. Liti G, Carter DM, Moses AM, Warringer J, Parts L, et al. (2009) Population genomics of domestic and wild yeasts. Nature 458: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blanc VM, Adams J (2004) Ty1 insertions in intergenic regions of the genome of Saccharomyces cerevisiae transcribed by RNA polymerase III have no detectable selective effect. FEMS Yeast Res 4: 487–491. [DOI] [PubMed] [Google Scholar]
- 29. Fraser HB, Moses AM, Schadt EE (2010) Evidence for widespread adaptive evolution of gene expression in budding yeast. Proc Natl Acad Sci U S A 107: 2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jordan IK, McDonald JF (1999) Tempo and mode of Ty element evolution in Saccharomyces cerevisiae. Genetics 151: 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neuveglise C, Feldmann H, Bon E, Gaillardin C, Casaregola S (2002) Genomic evolution of the long terminal repeat retrotransposons in hemiascomycetous yeasts. Genome Res 12: 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fingerman EG, Dombrowski PG, Francis CA, Sniegowski PD (2003) Distribution and sequence analysis of a novel Ty3-like element in natural Saccharomyces paradoxus isolates. Yeast 20: 761–770. [DOI] [PubMed] [Google Scholar]
- 33. Moore SP, Liti G, Stefanisko KM, Nyswaner KM, Chang C, et al. (2004) Analysis of a Ty1-less variant of Saccharomyces paradoxus: the gain and loss of Ty1 elements. Yeast 21: 649–660. [DOI] [PubMed] [Google Scholar]
- 34. Liti G, Peruffo A, James SA, Roberts IN, Louis EJ (2005) Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 22: 177–192. [DOI] [PubMed] [Google Scholar]
- 35. Wheelan SJ, Scheifele LZ, Martinez-Murillo F, Irizarry RA, Boeke JD (2006) Transposon insertion site profiling chip (TIP-chip). Proc Natl Acad Sci U S A 103: 17632–17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gabriel A, Dapprich J, Kunkel M, Gresham D, Pratt SC, et al. (2006) Global mapping of transposon location. PLoS Genet 2: e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carreto L, Eiriz MF, Gomes AC, Pereira PM, Schuller D, et al. (2008) Comparative genomics of wild type yeast strains unveils important genome diversity. BMC Genomics 9: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibata Y, Malhotra A, Bekiranov S, Dutta A (2009) Yeast genome analysis identifies chromosomal translocation, gene conversion events and several sites of Ty element insertion. Nucleic Acids Res 37: 6454–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mularoni L, Zhou Y, Bowen T, Gangadharan S, Wheelan S, et al.. (2012) Retrotransposon Ty1 integration targets specifically positioned asymmetric nucleosomal DNA segments in tRNA hotspots. Genome Res. [DOI] [PMC free article] [PubMed]
- 40.Qi X, Daily K, Nguyen K, Wang H, Mayhew D, et al.. (2012) Retrotransposon profiling of RNA polymerase III initiation sites. Genome Research. [DOI] [PMC free article] [PubMed]
- 41. Baller JA, Gao J, Voytas DF (2011) Access to DNA establishes a secondary target site bias for the yeast retrotransposon Ty5. Proc Natl Acad Sci U S A 108: 20351–20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baller JA, Gao J, Stamenova R, Curcio MJ, Voytas DF (2012) A nucleosomal surface defines an integration hotspot for the Saccharomyces cerevisiae Ty1 retrotransposon. Genome Res. [DOI] [PMC free article] [PubMed]
- 43. Janetzky B, Lehle L (1992) Ty4, a new retrotransposon from Saccharomyces cerevisiae, flanked by tau-elements. J Biol Chem 267: 19798–19805. [PubMed] [Google Scholar]
- 44. Pereira V (2008) Automated paleontology of repetitive DNA with REANNOTATE. BMC Genomics 9: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57: 758–771. [DOI] [PubMed] [Google Scholar]
- 47. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 48. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 49. Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76: 5269–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watterson GA (1975) On the number of segregating sites in genetical models without recombination. Theor Popul Biol 7: 256–276. [DOI] [PubMed] [Google Scholar]
- 51. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brookfield JF (1986) A model for DNA sequence evolution within transposable element families. Genetics 112: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maside X, Bartolome C, Charlesworth B (2003) Inferences on the evolutionary history of the S-element family of Drosophila melanogaster. Mol Biol Evol 20: 1183–1187. [DOI] [PubMed] [Google Scholar]
- 54. Sanchez-Gracia A, Maside X, Charlesworth B (2005) High rate of horizontal transfer of transposable elements in Drosophila. Trends Genet 21: 200–203. [DOI] [PubMed] [Google Scholar]
- 55. Hudson RR, Kaplan NL (1985) Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111: 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12: 543–548. [DOI] [PubMed] [Google Scholar]
- 57. Jordan IK, McDonald JF (1999) Comparative genomics and evolutionary dynamics of Saccharomyces cerevisiae Ty elements. Genetica 107: 3–13. [PubMed] [Google Scholar]
- 58. Cameron JR, Loh EY, Davis RW (1979) Evidence for transposition of dispersed repetitive DNA families in yeast. Cell 16: 739–751. [DOI] [PubMed] [Google Scholar]
- 59. Curcio MJ, Sanders NJ, Garfinkel DJ (1988) Transpositional competence and transcription of endogenous Ty elements in Saccharomyces cerevisiae: implications for regulation of transposition. Mol Cell Biol 8: 3571–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kupiec M, Petes TD (1988) Meiotic recombination between repeated transposable elements in Saccharomyces cerevisiae. Mol Cell Biol 8: 2942–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zou S, Wright DA, Voytas DF (1995) The Saccharomyces Ty5 retrotransposon family is associated with origins of DNA replication at the telomeres and the silent mating locus HMR. Proc Natl Acad Sci U S A 92: 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Naumov GI, Naumova ES, Lantto RA, Louis EJ, Korhola M (1992) Genetic homology between Saccharomyces cerevisiae and its sibling species S. paradoxus and S. bayanus: electrophoretic karyotypes. Yeast 8: 599–612. [DOI] [PubMed] [Google Scholar]
- 63. Fink GR, Boeke JD, Garfinkel DJ (1986) The mechanism and consequences of retrotransposition. Trends in Genetics 2: 118–123. [Google Scholar]
- 64. Jordan IK, McDonald JF (1998) Interelement selection in the regulatory region of the copia retrotransposon. J Mol Evol 47: 670–676. [DOI] [PubMed] [Google Scholar]
- 65. Bergman CM, Quesneville H (2007) Discovering and detecting transposable elements in genome sequences. Brief Bioinform 8: 382–392. [DOI] [PubMed] [Google Scholar]
- 66. Kellis M, Patterson N, Endrizzi M, Birren B, Lander ES (2003) Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254. [DOI] [PubMed] [Google Scholar]
- 67. Cliften P, Sudarsanam P, Desikan A, Fulton L, Fulton B, et al. (2003) Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301: 71–76. [DOI] [PubMed] [Google Scholar]
- 68. Dymond JS, Richardson SM, Coombes CE, Babatz T, Muller H, et al. (2011) Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 477: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sipiczki M (2008) Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res 8: 996–1007. [DOI] [PubMed] [Google Scholar]
- 70. Marinoni G, Manuel M, Petersen RF, Hvidtfeldt J, Sulo P, et al. (1999) Horizontal transfer of genetic material among Saccharomyces yeasts. J Bacteriol 181: 6488–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sebastiani F, Barberio C, Casalone E, Cavalieri D, Polsinelli M (2002) Crosses between Saccharomyces cerevisiae and Saccharomyces bayanus generate fertile hybrids. Res Microbiol 153: 53–58. [DOI] [PubMed] [Google Scholar]
- 72. Greig D, Louis EJ, Borts RH, Travisano M (2002) Hybrid speciation in experimental populations of yeast. Science 298: 1773–1775. [DOI] [PubMed] [Google Scholar]
- 73. Liti G, Barton DB, Louis EJ (2006) Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wei W, McCusker JH, Hyman RW, Jones T, Ning Y, et al. (2007) Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc Natl Acad Sci U S A 104: 12825–12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Doniger SW, Kim HS, Swain D, Corcuera D, Williams M, et al. (2008) A catalog of neutral and deleterious polymorphism in yeast. PLoS Genet 4: e1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Esberg A, Muller LA, McCusker JH (2011) Genomic structure of and genome-wide recombination in the Saccharomyces cerevisiae S288C progenitor isolate EM93. PLoS One 6: e25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bensasson D, Zarowiecki M, Burt A, Koufopanou V (2008) Rapid evolution of yeast centromeres in the absence of drive. Genetics 178: 2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bensasson D (2011) Evidence for a high mutation rate at rapidly evolving yeast centromeres. BMC Evol Biol 11: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boeke JD, Sandmeyer SB (1991) Yeast transposable elements. In: Broach JR, Jones EW, Prongle J, editors. The Molecular and Cdllular Biology of the Yeast Saccharomyces cerevisae. Cold Spring Harbor, N.Y.: Cold Spring Harbor Press. 193–261.
- 80. Servant G, Pennetier C, Lesage P (2008) Remodeling yeast gene transcription by activating the Ty1 long terminal repeat retrotransposon under severe adenine deficiency. Mol Cell Biol 28: 5543–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Knight SA, Labbe S, Kwon LF, Kosman DJ, Thiele DJ (1996) A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev 10: 1917–1929. [DOI] [PubMed] [Google Scholar]
- 82. Gaisne M, Becam AM, Verdiere J, Herbert CJ (1999) A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1). Curr Genet 36: 195–200. [DOI] [PubMed] [Google Scholar]
- 83. Jordan IK, McDonald JF (1999) The role of interelement selection in Saccharomyces cerevisiae Ty element evolution. J Mol Evol 49: 352–357. [DOI] [PubMed] [Google Scholar]
- 84. Bergman CM, Bensasson D (2007) Recent LTR retrotransposon insertion constrasts with waves of non-LTR insertion since speciation in Drosophila melanogaster . Proc Natl Acad Sci U S A 104: 11340–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Database of Ty element reference sequences used for re-annotation of the yeast genome. Fasta file of LTR and internal sequences from Ty1-Ty5, with the inclusion of Ty3p from S. paradoxus.
(TXT)
Summary of the annotated Ty elements in the June 2008 version of S. cerevisiae genome from the UCSC Genome Database (sacCer2). Shown are the REannotate identifier, genomic coordinates of the complete span of all fragments from an individual element, strand, name(s) of corresponding Ty elements from the SGD sacCer2 annotation, structural classification, activity status as determined by phylogenetic analysis, estimated allele frequency, and polymorphism/fixation status based on SGRP alignments. More specifically the status of Ty insertions in each SGRP alignment is classified as polymorphic or fixed, but Ty elements that are present only in the reference strain are labelled as S288c in this table and classified as polymorphic in subsequent analyses. Presence (1), absence (0) or missing data (n.a.) status is shown for individuals SGRP strains in columns following the “sgrp alignment status” column. REannotate identifiers are comprised of the chromosome name, the insertion type, a numerical identifier and Ty family name. Insertion type can be full-length (i or u), truncated (t) or solo LTR (s or st). Insertions from the Ty3p family are labelled as TY3_1p according to RepBase nomeclature.
(TXT)
GFF file of Repeatmasker/REannotate Ty fragments in the June 2008 version of S. cerevisiae genome from the UCSC Genome Database (sacCer2). Individual fragments from the same element are given the same name in the ID column. The span of the union of fragments is the same as the range of coordinates given in File S2.
(TXT)
Nexus file of aligned LTR sequences used for phylogenetic analysis of Ty3/Ty3p superfamily. Alignments of paralogous LTR sequence from the Ty3/Ty3p superfamily used to create the phylogeny in Figure 2. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(TXT)
Nexus file of aligned LTR sequences used for phylogenetic analysis of Ty1/Ty2 superfamily. Alignments of paralogous LTR sequence from Ty1/Ty2 superfamily used to create the phylogeny in Figure 8. Positions enclosed in square brackets were excluded from the phylogenetic analyses. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(TXT)
ZIP archive of LTR alignments used for molecular evolutionary analyses. Alignments of paralogous LTR sequence from all, active and inactive elements are provided for Ty1, Ty2, Ty3+Ty3p, Ty4 and Ty5, respectively. For full-length element only the 5′ LTR was included. Also included are alignments of the Ty3/Ty3p superfamily and the Ty1/Ty2 superfamily, respectively. Positions enclosed in square brackets were excluded from the phylogenetic analyses. GenBank Accession Numbers are shown for sequences from species other than S. cerevisiae.
(ZIP)
ZIP archive of SGRP strain alignments used for population genomic analyses. Alignments of the orthologous genomic region in each SGRP strain corresponding to each Ty insertion in our re-annotation of the S. cerevisiae reference genome. N’s represent low quality (<Q40 phred score) or missing data, primarily due to the low-coverage sequence of many SGRP strains. The DNA sequence of sacCer2 is included as “REF” and each alignment includes the TE locus and 200 bp of DNA sequence on either side of the Ty insertion site. A dummy sequence shows the position of each TE in the alignment with “T”s.
(ZIP)
Visualization of recombination breakpoints in Ty1, Ty1/2 and Ty2 complete LTR sequences. The two approximate recombination breakpoints are highlighted with black lines. Asterisks highlight columns in the alignment where the base is conserved across all sequences.
(PDF)