Abstract
Apoptosis-associated speck-like protein containing a CARD (ASC) was originally named because it triggered apoptosis in certain tumors. More recently, however, ASC was found to be a central adaptor protein of inflammasome which mediates the secretion of pro-tumorigenic inflammation. Here we examined the role of ASC in tumorigenesis of human melanoma. Compared with primary melanoma, ASC protein expression was generally downregulated in metastatic melanoma. While overexpressing ASC in metastatic melanoma showed no effects on cell viability, silencing ASC with short hairpin RNA induced G1 cell cycle arrest, reduced cell viability and suppressed tumorigenesis in metastatic melanoma. On the other hand, silencing ASC in primary melanoma reduced cell death, increased cell viability and enhanced tumorigenesis. In primary and metastatic melanoma cells, ASC knockdown inhibited inflammasome-mediated caspase-1 activity and IL-1β secretion. However, phosphorylated IKKα/β expression and NF-κB activity were suppressed in metastatic melanoma and enhanced in primary melanoma after ASC knockdown. These findings suggest stage-dependent dual roles of ASC in tumorigenesis. ASC expression in primary melanoma inhibits tumorigenesis, by reducing IKKα/β phosphorylation and inhibiting NF-κB activity. In metastatic melanoma, on the other hand, this inhibitory effect is diminished, and ASC induces tumorigenic pathways through enhanced NF-κB activity and inflammasome-mediated IL-1β secretion.
Keywords: ASC, inflammasome, melanoma, IL-1β, NF-κB, caspase-1
INTRODUCTION
ASC (Apoptosis-associated Speck-like protein containing a CARD, caspase recruitment domain) was originally named because it induced apoptosis when overexpressed in HL-60 human leukemia cells (Masumoto et al., 1999). ASC facilitates translocation of Bax to mitochondria, and increases mitochondrial permeability, cytochrome c release, and activation of caspase-9 and -3 (McConnell and Vertino, 2004; Ohtsuka et al., 2004). ASC is also called TMS1 (Target of Methylation-mediated Silencing) because ASC expression is suppressed in many human tumors by methylation of CpG islands in ASC gene, which likely prevents cancer cells from undergoing apoptosis (Conway et al., 2000; Das et al., 2006; Stone et al., 2004). These data suggest a tumor suppressor role of ASC.
Structurally, ASC contains an N-terminal pyrin domain (PYD) and a C-terminal caspase recruitment domain (CARD) (Martinon et al., 2002). ASC is highly expressed in immune cells, particularly in neutrophils and monocytes. As a central adaptor protein of inflammasome, ASC was recently found to mediate inflammatory signals by recruiting CARD-containing pro-caspase-1 to several PYD-containing NOD-like receptors (NLRs), including NLRP3. ASC thus plays a pivotal role in the caspase-1-dependent processing of pro-inflammatory cytokines such as IL-1β. Because IL-1β is a pleiotropic pro-inflammatory cytokine involved in cell growth, differentiation, tissue repair and regulation of immune response (Dinarello, 2009), tumor cells secreting IL-1β have a greater propensity for invasion, angiogenesis, anti-tumor suppression and metastasis (Song et al., 2005; Song et al., 2003) and IL-1β is strongly implicated in tumor progression (Dinarello, 2010; Okamoto et al., 2010). We have previously shown that the NLRP3 inflammasome (composed of NLRP3, ASC and pro-caspase-1) is constitutively assembled and activated in human melanoma cells, resulting in spontaneous IL-1β secretion in metastatic melanoma cells (Okamoto et al., 2010). These results suggest a tumor promoting role of ASC in human melanoma.
In this study, we examined the role of ASC in melanomagenesis using primary and metastatic human melanoma cell lines. We provide evidence that ASC plays stage-dependent dual roles in tumorigenesis by differentially regulating nuclear factor-κB (NF-κB) activity and IL-1β secretion in human melanoma cells.
RESULTS
ASC expression is downregulated in metastatic melanoma
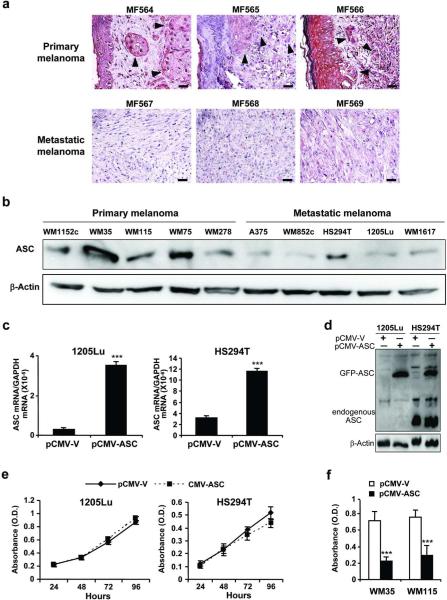
We analyzed twelve human melanoma tumor specimens (6 primary and 6 metastatic melanoma tumors) for ASC expression. Consistent with a published report (Guan et al., 2003), ASC was highly expressed in normal melanocytes (Supplementary Figure 1) and suppressed in human melanoma tissues. There was a trend of ASC to a further reduction in metastatic melanoma compared with primary melanoma (Figure 1a, Supplementary Table 1). We then analyzed ASC expression in ten human melanoma cell lines (5 primary and 5 metastatic lines). Western blot showed reduced ASC expression in most metastatic melanoma cell lines (Figure 1b). To investigate expression-dependent and/or stage-dependent roles of ASC in human melanoma, we used 2 primary melanoma cells: WM35 and WM115, expressing relatively higher (WM35) and lower (WM115) ASC among primary cells, and 2 metastatic melanoma cells: HS294T and 1205Lu, expressing higher (HS294T) and lower (1205Lu) ASC among metastatic cells, in the subsequent experiments.
Figure 1. Effects of ASC expression in melanoma cells.
(a) Representative staining of human melanoma tumors with ASC. Bar=100 μm. Arrows depict melanoma cells. (b) Western blot of ASC in human melanoma cell lines. β-Actin served as a loading control. (c) qRT-PCR of ASC after transfection with pCMV-GFP-V empty vector (pCMV-V) or pCMV-GFP-ASC vector (pCMV-ASC). Expression levels normalized to GAPDH. (d) Western blot of cells transfected with pCMV-V or pCMV-ASC. Anti-ASC antibody detects both transfected (depicted as GFP-ASC) and endogenous ASC. β-Actin served as a loading control. (e) Cell viability in 1205Lu and HS294T transfected with pCMV-V or pCMV-ASC. (f) Cell viability in WM35 and WM115 after 48 hours of transfection with pCMV-V or pCMV-ASC. Data represent mean + S.E. (n=3). ***P≤0.001 compared with transfection with pCMV-V.
ASC overexpression in metastatic melanoma has little effect on cell viability despite its killing effects on primary melanoma
Because ASC expression was higher in primary melanoma than metastatic melanoma, we hypothesized that ASC overexpression may induce growth suppression in human melanoma. 1205Lu and HS294T cells were transfected with an ASC expression plasmid. Successful transfection was confirmed by an increase in ASC mRNA (11.3-fold increase in 1205Lu cells and 3.7-fold increase in HS294T cells, Figure 1c) and protein (Figure 1d) expression. Overexpression of ASC induced no significant change in cell viability in 1205Lu and HS294T cells (Figure 1e); however, it reduced cell viability in primary melanoma cells (Figure 1f).
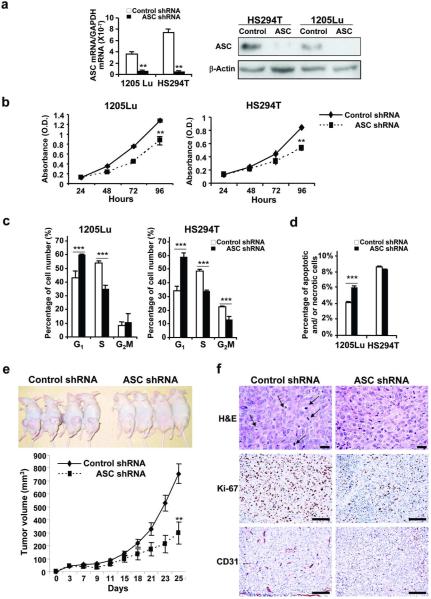
ASC knockdown reduces cell viability and inhibits tumor growth in metastatic melanoma
To investigate the role of ASC on tumor growth, we knocked down ASC expression by transducing ASC shRNA into HS294T and 1205Lu cells. Successful transduction was confirmed by a decrease in ASC mRNA (84% reduction in 1205Lu cells and 95% reduction in HS924T cells) and protein expression in 1205Lu and HS294T cells compared with their control cells (Figure 2a). Silencing ASC reduced cell viability in 1205Lu cells (32% reduction) and HS294T cells (38% reduction) (Figure 2b). G1 cell-cycle arrest was induced in both cells (Figure 2c) and increased cell death was observed in 1205Lu cells after ASC knockdown (Figure 2d). We then determined whether the same effect can be observed in vivo. Nude mice were injected with 1205Lu-control-shRNA cells or 1205Lu-ASC-shRNA cells, and tumor growth was monitored. Mice injected with 1205Lu-ASC-shRNA cells showed slower tumor growth and decreased tumor volume (60% reduction on day 25) compared to the mice injected with 1205Lu-control-shRNA cells (Figure 2e). Tumor tissues from mice injected with 1205Lu-ASC-shRNA cells exhibited decreased mitosis (arrows), proliferation (Ki-67-positive cells) and angiogenesis (CD31-positive cells) compared with control tumors (Figure 2f). These results indicate that ASC promotes tumor growth in metastatic melanoma.
Figure 2. Effects of ASC shRNA on metastatic melanoma.
(a) qRT-PCR (left panel) and Western blot (right panel) of ASC in cells transduced with control or ASC shRNA. mRNA expression levels normalized to GAPDH. β-Actin served as a loading control. (b) Cell viability in cells transduced with shRNA. (c) Cell cycle analysis in cells transduced with control and ASC shRNA. (d) Annexin V/PI staining in cells transduced with control and ASC shRNA. (e) Effects of ASC knockdown in vivo. Nude mice injected with 1205Lu-control-shRNA or 1205Lu-ASC-shRNA cells (upper panel). Tumor growth curve (lower panel). (f) Representative tumor sections stained with H&E (upper panel), Ki-67 (middle panel) and CD31 (lower panel). Bar=100 μm. Data represent mean + S.E. (n=3 except e, and n=8 in e). **P<0.01; ***P<0.001.
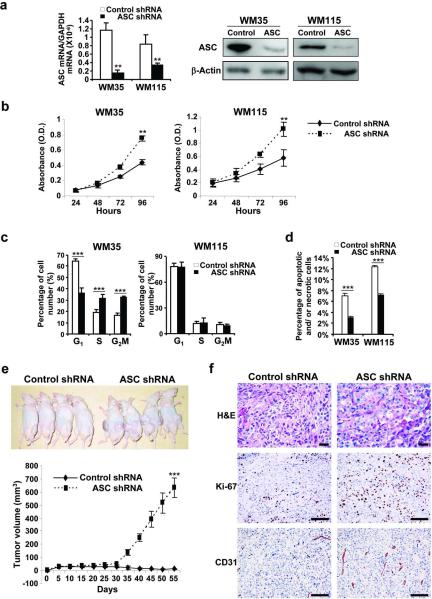
ASC knockdown increases cell viability and enhances tumor growth in primary melanoma
To investigate the role of ASC in primary melanoma, we knocked down ASC expression in WM35 and WM115 cells. Successful transduction was confirmed by a decrease in ASC mRNA (87% reduction in WM35 and 60% reduction in WM115) and protein expression (Figure 3a). In contrast with metastatic melanoma cells, however, silencing ASC in primary melanoma cells enhanced cell viability (1.7-fold in WM35 and 1.6-fold in WM115, Figure 3b). Cell proliferation was enhanced in WM35 cells (Figure 3c) and cell death was decreased in both WM35 and WM115 cells after ASC knockdown (Figure 3d). Tumors from WM35-ASC-shRNA cells grew in mice after day 30 whereas those from WM35-control-shRNA cells remained small in mice (Figure 3e). Histological analysis revealed enhanced proliferation (Ki-67-positive cells) and angiogenesis (CD31-positive cells) in tumor tissues from WM35-ASC-shRNA cells compared to those from WM35-control-shRNA cells (Figure 3f). Taken together, these results suggest that ASC has differential effects on primary and metastatic melanoma cells: ASC inhibits tumor growth in primary melanoma cells whereas it promotes tumorigenesis in metastatic melanoma cells.
Figure 3. Effects of ASC shRNA on primary melanoma.
(a) qRT-PCR (left panel) and Western blot (right panel) of ASC in cells transduced with control or ASC shRNA. mRNA expression levels normalized to GAPDH. β-Actin served as a loading control. (b) Cell viability in cells transduced with shRNA. (c) Cell cycle analysis in cells transduced with control and ASC shRNA. (d) Annexin V/PI staining in cells transduced with control and ASC shRNA. (e) Effects of ASC knockdown in vivo. Nude mice injected with WM35-control-shRNA or WM35-ASC-shRNA cells (upper panel). Tumor growth curve (lower panel). (f) Representative tumor sections stained with H&E (upper panel), Ki-67 (middle panel) and CD31 (lower panel). Bar=100 μm. Data represent mean + S.E. (n=3 except e, and n=8 in e). **P<0.01; ***P<0.001.
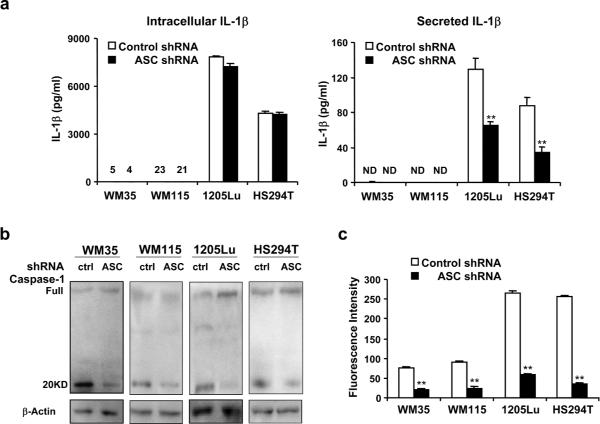
ASC knockdown impairs inflammasome-mediated caspase-1 activation in primary and metastatic melanoma
As an integral component of inflammasome, ASC plays an important role in caspase-1 activity and IL-1β secretion. To examine inflammasome function, we measured both IL-1β synthesis and secretion in primary (WM35 and WM115) and metastatic (HS294T and 1205Lu) melanoma cells after ASC knockdown (Figure 4a). Consistent with a previous report (Okamoto et al., 2010), the amount of IL-1β synthesized in WM35 and WM115 was very low (just above the limit of sensitivity) and IL-1β secretion was not detected in these primary melanoma cells (< 3 pg/ml). In all four cells transduced with ASC shRNA, IL-β synthesis was not changed relative to control cells (Figure 4a). However, IL-1β secretion was significantly decreased in metastatic melanoma cells transduced with ASC shRNA (62% reduction in HS924T-ASC-shRNA cells and 46% reduction in 1205Lu-ASC-shRNA cells, Figure 4a). Consistent with these results, activated caspase-1 (20 kD fragments) were decreased in all cells transduced with ASC shRNA (Figure 4b). Caspase-1 activity was also decreased in these cells (78%, 73%, 85% and 88% reduction in WM35-ASC-shRNA, WM115-ASC-shRNA, HS294T-ASC-shRNA and 1205Lu-ASC-shRNA cells, respectively. Figure 4c), indicating that ASC knockdown impairs inflammasome-mediated caspase-1 activation in both primary and metastatic melanoma cells.
Figure 4. Inflammasome function in melanoma cells after ASC knockdown.
(a) Intracellular and secreted IL-1β in 24 hours in WM35, WM115, HS294T and 1205Lu cells transduced with control or ASC shRNA. Levels of intracellular IL-1β from WM35 and WM115 cells transduced with control or ASC shRNA are shown above the line. N.D.; not detected. (b) Western blot of Caspase-1 in WM35, WM115, HS294T and 1205Lu cells transduced with control or ASC shRNA. β-Actin served as a loading control. (c) Caspase-1 activity in WM35, WM115, HS294T and 1205Lu cells transduced with control or ASC shRNA. Data represent mean + S.E. (n=3). *P<0.05; **P<0.01 compared with cells transduced with control shRNA.
ASC knockdown decreases NF-κB activity in metastatic melanoma but increases the activity in primary melanoma
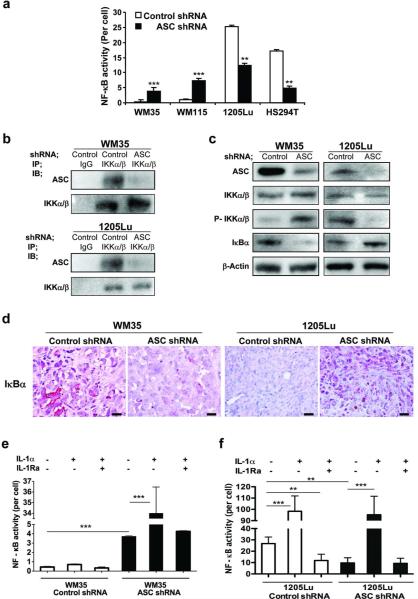
Through its PYD or CARD, ASC can associate with PYD-containing or CARD-containing proteins. In particular, ASC has been shown to mediate signals by upregulating or downregulating NF-κB (Hasegawa et al., 2005; Sarkar et al., 2006; Stehlik et al., 2002). To assess the association of ASC and NF-κB, we first measured NF-κB activity after ASC knockdown by luciferase assay. While NF-κB activity was increased in primary melanoma cells (10.9-fold in WM35 and 7.3-fold in WM115), it was decreased in metastatic melanoma cells (72% reduction in HS294T and 64% reduction in 1205Lu) after ASC knockdown (Figure 5a). To further decipher the role of ASC on NF-κB activity in melanoma cells, we examined proteins involved in the NF-κB activation pathway. Immunoprecipitation revealed interaction of ASC with IKKα/β in both primary (WM35) and metastatic (1205Lu) cells (Figure 5b, 1st and 3rd panels immunoblotted with ASC). Whereas interaction of ASC and IKKα/β was reduced after ASC knockdown (Figure 5b, 1st and 3rd panels), the expression of IKKα/b was not changed in the melanoma cells transduced with ASC shRNA (Figure 5b, 2nd and 4th panels; Figure 5c, 2nd panels). Phosphorylated IKKα/β expression, on the other hand, was increased in WM35 cells and decreased in 1205Lu cells after ASC knockdown (Figure 5c, 3rd panels). Consistent with these results, IκBα expression was decreased in WM35 cells and increased in 1205Lu cells after ASC knockdown (Figure 5c, 4th panels). The findings in IκBa expression were further confirmed in xenografted tumor tissues derived from these cells (Figure 5d). The results indicate that ASC inhibits NF-κB activity in primary melanoma cells by suppressing IKKα/β phosphorylation and inhibiting IκB degradation. On the contrary, ASC increases IKKα/β phosphorylation and IκB degradation and enhances NF-κB activity in metastatic melanoma cells.
Figure 5. NF-κB pathway in melanoma cells after ASC knockdown.
(a) NF-κB activity in melanoma cells transduced with control or ASC shRNA. (b) Immunoprecipitation interaction of IKKα/β with ASC in cells transduced with control or ASC shRNA. Lysates were immunoprecipitated (IP) and immunoblotted (IB) with antibodies. (c) Western blot of ASC, IKKα/β, phosphor-IKKα/β and IκBα in cells transduced with control or ASC shRNA. β-Actin served as a loading control. (d) Representative xenografted tumor sections stained with IκBα. Bar=100 μm. (e, f) NF-κB activity in cells transduced with control or ASC shRNA treated with IL-1α (10 ng/ml) or IL-1Ra (10 μg/ml) for 24 hours. Data represent mean + S.E. (n=3). **P<0.01; ***P<0.001 compared with cells transduced with control shRNA.
ASC knockdown decreases IL-1 receptor signaling-dependent activation of NF-κB in metastatic melanoma
Previously, we have shown a positive feedback loop of IL-1 receptor (IL-1R) signaling through secreted IL-1β in metastatic melanoma cells (Okamoto et al., 2010). IL-1R signaling activates the NF-κB pathway. Therefore, to examine the role of ASC on NF-κB in human melanoma cells, we investigated IL-1R signaling. IL-1α was used to stimulate IL-1R signaling whereas a receptor antagonist, IL-1Ra, was used to inhibit a positive feedback loop. Primary melanoma cells (WM35) did not respond to the stimulation by IL-1α unless ASC is silenced, (Figure 5e, 1st versus 2nd bars and 4th versus 5th bars), indicating that IL-1R is functional but IL-1R signaling is impaired by ASC in primary melanoma. In contrast, in metastatic melanoma cells (1205Lu) with ASC knockdown, IL-1α treatment rescued the suppressed NF-κB activity to the same level as that in 1205Lu-control-shRNA cells (Figure 5f, 2nd versus 5th bars), suggesting that IL-1R signaling in metastatic melanoma is functional despite the ASC knockdown. However, treatment with IL-1Ra did not reduce NF-κB activity in 1205Lu-ASC-shRNA cells relative to their controls (Figure 5f, 4th versus 6th bars in 1205Lu-ASC-shRNA cells and 1st versus 3rd bars in 1205Lu-control-shRNA cells), suggesting that IL-1-mediated IL-1R stimulation is already inhibited by ASC knockdown in metastatic melanoma. Taken together, these results indicate that ASC suppresses IL-1R signaling by inhibiting NF-κB activation in primary melanoma cells whereas it contributes to an inflammasome-mediated positive feedback loop of IL-1R signaling in metastatic melanoma cells.
DISCUSSION
Previous studies have indicated ASC as a tumor suppressor gene that induces apoptosis in certain tumor cell lines. The current study demonstrates, however, that while ASC suppresses tumor growth in primary melanoma, ASC promotes the growth of metastatic melanoma.
ASC contains PYD and CARD, and is thus a central adaptor protein to recruit PYD-containing NLRP3 to CARD-containing pro-caspase-1 for inflammasome activation and subsequent processing of IL-1β. We have previously shown the coexpression of ASC and NLRP3 in human melanoma cells and demonstrated that constitutively activated inflammasomes are present in both primary and metastatic melanoma (Okamoto et al., 2010). Congruent with our previous findings, ASC knockdown was shown here to decrease caspase-1 activity and impair IL-1β processing/secretion in human melanoma cells.
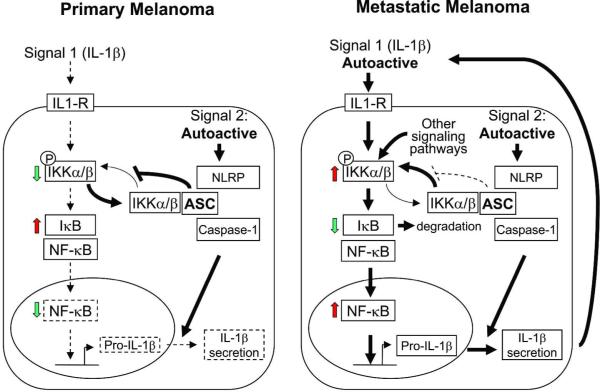
Only metastatic melanoma cells, however, spontaneously produce and secrete high levels of IL-1β, resulting in angiogenesis and tumor promotion (Okamoto et al., 2010). A previous report has shown that ASC can either inhibit or activate NF-κB, depending on the cellular context (Stehlik et al., 2002). Consistent with this, we found dual roles of ASC in human melanoma cells. While ASC is necessary for inflammasome function and maturation of IL-1β in human melanoma, ASC differentially regulates NF-κB activity in primary and metastatic melanoma. NF-κB is pivotal for transactivation of cell-cycle regulation, cytokine production and expression of adhesion molecules and is dysregulated in many cancers (Van Waes, 2007). We show here that ASC downregulates NF-κB activity in primary melanoma cells, despite the co-expression of ASC and NLRP3. In metastatic melanoma cells, however, NF-κB activity is enhanced by ASC. These seemingly contradictory functions of ASC may depend on cellular context (primary versus metastatic) (Figure 6). For example, ASC expression in primary melanoma cells inhibits NF-κB activity by inhibiting IKK-mediated degradation of IκB. This may explain why primary melanoma cells do not secrete IL-1β despite constitutively assembled and activated inflammasomes. On the other hand, the inhibitory effect of ASC on NF-κB is diminished in metastatic melanoma. Since ASC associates with multiple PYD- and CARD-containing proteins, it is tempting to speculate that decreased ASC expression in metastatic melanoma cells may result in competition among various pathways for a limited supply of ASC protein. However, overexpressing ASC in metastatic melanoma cells did not alter NF-κB activity (Supplementary Figure S2a), suggesting that constitutively active IL-1R signaling and NF-κB activity in metastatic melanoma (autoactive Signal 1) make cells less vulnerable to the inhibitory effect of ASC on NF-κB. Of note, overexpressing ASC in metastatic melanoma cells did not affect autocrine IL-1β secretion (Supplementary Figure S2b) or caspase-1 activity (Supplementary Figure S2c), suggesting that there is enough biologically functional ASC to mediate caspase-1-dependent maturation and secretion of IL-1β and the inflammasome is constitutively active in metastatic melanoma (autoactive Signal 2), resulting in a positive feedback mechanism of IL-1 signaling to further upregulate NF-κB activity in the absence of inhibitory effect of ASC on IκB. ASC may thus contribute to a positive feedback loop of autoinflammation in metastatic melanoma in which upregulated NF-κB induces transcription of pro-IL-1β, which is subsequently processed and secreted by constitutively active inflammasome, leading to further NF-κB activation through autocrine IL-1 signaling. Alterations in ASC and associated protein interactions may thus result in varying tumor-dependent responses, implying ASC could be a promising therapeutic target in metastatic cancers.
Figure 6. Hypothetical roles of ASC in melanoma tumor progression.
Solid lines indicate constitutive/autonomous pathways. Dashed lines indicate signal-dependent pathways. Arrow thickness indicates relative strength of signaling pathways. ASC inhibits phosphorylation of IKKα/β and decreases NF-κB in primary melanoma cells. Synthesis of pro-IL-1β is thus low and IL-1β secretion is minimal despite autoactive inflammasome in primary melanoma cells. In metastatic melanoma, however, autoactive IL-1R signaling and other signaling pathways result in reduced inhibition of NF-κB from ASC in the presence of sustained autoactive inflammasome, leading to the spontaneous synthesis and secretion of IL-1β from melanoma cells. IL-1β secreted from melanoma cells further augments autoinflammatory loop of metastatic melanoma cells to activate NF-κB.
In conclusion, ASC appears to have cell-dependent roles in tumor pathogenesis by differentially regulating NF-κB activity and IL-1β processing. In metastatic melanoma, ASC induces tumorigenic pathways, most likely by activating caspase-1-dependent IL-1β secretion and enhancing autoinflammatory NF-κB activity. On the other hand, higher levels of ASC expression in primary melanoma reduce IKKα/β phosphorylation and inhibit NF-κB activity. In fact, it appears that ASC transforms from a protein with anti-tumor properties in primary melanoma to a pro-tumorigenic factor in metastatic melanoma. To our knowledge, no previous studies have examined stage-dependent roles of ASC in cancer cells. Further studies of ASC and its upstream and downstream intermediaries may enhance our understanding of the molecular mechanisms governing tumorigenesis and reveal new molecular targets for designing anticancer drugs.
MATERIALS AND METHODS
Cell culture
Human melanoma cell lines were obtained from the American Type Culture Collection (Manassas, VA): WM1152c and WM35 are from radial growth phase primary melanoma; WM115, WM75 and WM278 are from vertical growth phase primary melanoma; and A375, WM852c, HS294T and WM1617 are from metastatic melanoma. 1205Lu is from a lung metastasis after subcutaneous injection of mice with a primary melanoma line, WM793. Cells were maintained in RPMI 1640 (Mediatech, Inc., Manassas, VA) (except HS294T) or Dulbecco's modified Eagle's medium (Mediatech, Inc.)(HS294T), supplemented with 10% fetal bovine serum (Mediatech, Inc.), 100 μg/ml streptomycin and 100 Units/ml penicillin (Mediatech Inc.).
Western blotting analysis
Western blotting analysis was carried out as previously described (Okamoto et al., 2010). Anti-caspase-1, IκKα/IκKβ, phospho-IκKα/IκKβ, IκBα antibodies were from Cell Signaling Technology (Boston, MA). Anti-ASC antibody was from Alexis Biochemicals (San Diego, CA). Horseradish peroxidase-conjugated anti-mouse IgG was from Jackson Immuno-Research Laboratories, Inc. (West Grove, PA) and horseradish peroxidase-conjugated anti-rabbit IgG and anti-goat IgG were from Sigma-Aldrich (St Louis, MO). Signals were detected by SuperSignal West Femto maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL), and analyzed using GelDoc 200 (Bio-Rad, Hercules, CA).
DNA transfection
Plasmid pCMV-GFP-ASC or pCMV-GFP empty Vector (OriGene Technologies, Rockville, MD) was transfected at a concentration of 0.5 μg/ml by using Lipofectamine 2000 (Invitrogen Co., Carlsbad, CA). After 48 hours, cells were collected and analyzed.
RNA extraction, quantitative RT-PCR analysis
RNA was extracted using the RNAqueous-Micro kit (Ambion Inc., Austin, TX) and reverse transcribed. Quantitative PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on the MX3000P PCR system (Stratagene, La Jolla, CA). Primers were designed to generate a PCR product of 50 to 150 bp. Primer sequences were: human ASC, forward: 5'-CATGAACTGATCGACAGGATG-3', reverse: 5'-GGACCTCCTCCAAATGTTTC-3'; human GAPDH, forward: 5'-CAGGGCTGCTTTTAACTCTGG13', reverse, 5'-TGGGTGGAATCATATTGGAACA-3'.
Cell viability assay
Cell viability was measured using CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega Corporation, Madison, WI). 1–5 × 103 cells in 100 μl of media were plated per well in 96-well plates and cultured. Viability was determined using ELX808 Ultra Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT).
Short hairpin RNA (shRNA) transduction
Cells were transducted with shRNA Lentiviral Particles against control or ASC (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in cell culture medium containing 5 μg/ml Polybrene (Santa Cruz Biotechnology, Inc.). Following overnight transduction, cells were incubated in medium with 1 μg/ml puromycin (Santa Cruz Biotechnology, Inc.) to select for stable clones expressing transduced shRNA, and maintained in medium with 1 μg/ml puromycin at 37°C.
Cell cycle analysis
Cells were stained at 4°C with Krishan's staining buffer containing 70 μM propidium iodide (Sigma-Aldrich), 3.8 mM trisodium citrate (Sigma-Aldrich), 0.01% nonidet P-40 (Sigma-Aldrich) and 0.01 mg of RNase A (Roche Diagnostics, Indianapolis, IN), and analyzed by flow cytometer FC500 (Beckman-Coulter, Inc, Brea, CA). Modfit LT program (Verity Software House, Inc., Topsham, ME) was used for data analysis.
Dead cell apoptosis assay
Cells were stained with Annexin-V and propidium iodide (PI) (Invitrogen Co.) following the manufacturer's instructions for flow cytometric analysis. Cell death was measured from apoptotic (annexin-V-positive) and/or necrotic (PI-positive) cells.
Immunohistochemistry
Human melanoma tissues were obtained from surgical specimens with patient written consent under institutional review board-approved protocols, adhering to Health Insurance Portability and Accountability Act regulations and to the declaration of Helsinki Guidelines. Paraffin sections were treated with Citrate buffer Low pH6 (Leica Microsystems, Inc., Baffalo Grove, IL). ASC and IκBα were stained with rabbit anti-human ASC (Proteintech Group, Inc., Chicago, IL) and rabbit anti-human IκBα (Abcam, Cambridge, MA), respectively, followed by rat anti-rabbit IgG link (DAKO, Inc., Carpinteria, CA) and Bond Polymer Refine Red Detection kit (Leica Microsystems, Inc.). CD31 was stained with rat anti-mouse CD31 (Dianova Inc., Rodeo, CA) and rabbit anti-rat IgG link (DAKO, Inc., Carpinteria, CA), followed by detection with Bond Polymer Refine Red Detection kit. Ki-67 was stained with rabbit anti-human Ki-67 (Thermo Scientific) and rat anti-rabbit IgG link, followed by detection with Bond Polymer Refine Detection kit (Leica Microsystems, Inc.). Sections were counterstained with hematoxylin (Leica Microsystems, Inc.), and reviewed by 2 observers.
Tumor formation in nude mice
Six-week-old female athymic nu/nu mice (Jackson Laboratory, Bar Harbor, ME) were used. Animals were kept under specific pathogen-free conditions, according to National Institutes of Health Animal Care Guidelines. Experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Colorado Denver. 1 × 106 1205Lu-control-shRNA or 1205Lu-ASC-shRNA cells or 2.5 × 106 WM35-control-shRNA or WM35-ASC-shRNA were resuspended in 0.1 ml of Matrixgel Basement Membrane Matrix (BD Biosciences, San Jose, CA) 1:1 diluted with PBS, and injected intradermally into the flank of mice. Tumor growth was monitored biweekly with electronic digital caliper (Control Company, Friendswood, TX). Tumor volume was calculated by the formula: tumor volume = (longest diameter) × (shortest diameter)2 / 2.
Enzyme-linked immunosorbent assay
Human IL-1β enzyme-linked immunosorbent assay kit was obtained from R&D Systems (Minneapolis, MN) (Okamoto et al., 2010). 1 × 105 /ml of cells were seeded in 6 well plates, and medium was changed to OptiMEM (Invitrogen Co.) when cells reached 80% confluence. After 24 hours, supernatants were collected to assess secreted IL-1β. Intracellular cytokines were assessed by lysing cells with 0.5% Triton-X-100 in PBS followed by freeze-thaw cycle.
Caspase-1 activity assay
Caspase-1 Flica™ kit (ImmunoChemistry Technologies, LLC., Bloomington, MN) was used. Cells (1.5 x 105/100 μl per well in 96 well plates) were trypsinized, washed in ice-cold PBS and incubated with caspase-1 inhibitor probe (FAM-YVAD-FMK) for one hour at 37°C, 5%CO2. The fluorescence was measured using the fluorescence plate reader (Promega Corporation). A recombinant caspase-1 was used as a positive control.
NF-κB activity assay
Ready-to-Glow™ Secreted Luciferase pNFκB-MetLuc Vector Kit (Clontech Laboratories, Inc. San Francisco, CA) was used. 5 × 103 /ml of melanoma cells were seed in 24 well plate, transfected with a control vector (pMetLuc2-Reporter) or an NF-κB vector (pNFκB-MetLuc2 Reporter) in OptiMEM, and treated with IL-1α (10 ng/ml) and/or IL-1Ra (10 μg/ml). Cell culture supernatant was collected 24 hours later and luciferase activity was measured using luminometer (Promega Corporation).
Immunoprecipitation and immunoblot analysis
Immunoprecipitation was carried out as previously described (Okamoto et al., 2010). Briefly, cell lysates were incubated with primary antibodies or pre-immune serum and immunoprecipitated with protein A/G plus agarose (Santa Cruz). The precipitates were washed, separated on SDS–polyacrylamide gel electrophoresis and analyzed with western blotting.
Statistical analysis
The values in the figures are expressed as the means ± SE. The figures in this study are representatives of more than two different experiments. Statistical analysis of the data between two groups was performed by a Student's t-test. Values of P<0.05 were considered as statistically significant.
Supplementary Material
Acknowledgements
We thank the University of Colorado Denver (UCD) melanoma tissue bank for providing human melanoma samples and the University of Colorado Cancer Center (UCCC), the Skin Disease Research Center (SDRC) flow core (Alistaire S. Acosta and Karen Helm) for helping with FACS and IHCtech LLC for helping immunohistochemical staining. We also thank Xianzhong Meng, MD, PhD (Department of Surgery, UCD) and Advance Light Microscopy Core Facility of UCD for technical support of microscopic analysis, and Manjinder Kaur, PhD (Department of Dermatology, UCD) for critically reviewing the manuscript. This work was supported, in whole or in part, by a Veterans Affairs Merit Review Award (to M.F.), US National Institutes of Health grants P30CA046934 (Flow Core of the UCCC), P30AR057212 (Flow Core of the SDRC), Dermatology Foundation (to M.F.), Wendy Will Case Cancer Fund grant (to M.F.), Cancer League of Colorado (to M.F.) and Tadamitsu Cancer Research Fund (to M.F.). The funders had no role in study design, data collection, analysis and interpretation, manuscript preparation and decision to submit the manuscript.
Abbreviations
- ASC
apoptosis-associated speck-like protein containing a CARD
- CARD
caspase recruitment domain
- IKK
IκB kinase
- IL-1
Interleukin-1
- IL-1R
IL-1 receptor
- IL-1Ra
IL-1 receptor antagonist
- NF-κB
nuclear factor-κB
- NLR
NOD-like receptor
- NLRP
NLR family containing PYD
- PYD
pyrin domain
- shRNA
short hairpin RNA
- TMS1
Target of Methylation-mediated Silencing
Footnotes
Conflict of interest The authors declare that no competing interests exist.
REFERENCES
- Conway KE, McConnell BB, Bowring CE, Donald CD, Warren ST, Vertino PM. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 2000;60:6236–42. [PubMed] [Google Scholar]
- Das PM, Ramachandran K, Vanwert J, Ferdinand L, Gopisetty G, Reis IM, et al. Methylation mediated silencing of TMS1/ASC gene in prostate cancer. Mol Cancer. 2006;5:28. doi: 10.1186/1476-4598-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–29. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Sagara J, Yokoyama T, Koganehira Y, Oguchi M, Saida T, et al. ASC/TMS1, a caspase-1 activating adaptor, is downregulated by aberrant methylation in human melanoma. Int J Cancer. 2003;107:202–8. doi: 10.1002/ijc.11376. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Imamura R, Kinoshita T, Matsumoto N, Masumoto J, Inohara N, et al. ASC-mediated NF-kappaB activation leading to interleukin-8 production requires caspase-8 and is inhibited by CLARP. J Biol Chem. 2005;280:15122–30. doi: 10.1074/jbc.M412284200. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–8. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Vertino PM. TMS1/ASC: the cancer connection. Apoptosis. 2004;9:5–18. doi: 10.1023/B:APPT.0000012117.32430.0c. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–8. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem. 2010;285:6477–88. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD. ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol. 2006;176:4979–86. doi: 10.4049/jimmunol.176.8.4979. [DOI] [PubMed] [Google Scholar]
- Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, et al. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:6448–56. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- Stehlik C, Fiorentino L, Dorfleutner A, Bruey JM, Ariza EM, Sagara J, et al. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J Exp Med. 2002;196:1605–15. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AR, Bobo W, Brat DJ, Devi NS, Van Meir EG, Vertino PM. Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol. 2004;165:1151–61. doi: 10.1016/S0002-9440(10)63376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–82. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.