Abstract
There is considerable evidence that phase variation among transparent and opaque colony phenotypes of Streptococcus pneumoniae (Spn) plays an important role in the pneumococcal adherence and invasion. The current study was designed to investigate the interactions of the opacity phenotype variants of Spn with specific complement pathway activation in a mouse model of acute otitis media (AOM). Although the opaque colony phenotype was expected to be more resistant to complement mediated killing compared to the transparent Spn variant, we discovered that C3b deposition on the transparent Spn is, in large part, dependent on the alternative pathway activation. There were no significant differences in resistance to complement mediated opsonophagocytosis between the two variants in factor B deficient mice. In addition, an in vitro study demonstrated that significantly more C4b-binding protein (C4BP) (the classical pathway inhibitor) and factor H (FH) (the alternative pathway inhibitor) bound to the transparent strain compared with the opaque one. Our data suggest that the difference in the relative virulence of Spn opacity phenotypes is associated with its ability to evade complement-mediated opsonophagocytosis in a mouse model of pneumococcal AOM.
Keywords: Streptococcus pneumoniae, colony opacity variants, complement, otitis media
1. Introduction
Streptococcus pneumoniae (Spn) is one of the most frequent causes of otitis media (OM) in children. It is estimated that 7 million cases of pneumococcal OM occur annually in the United States among children under the age five [1]. The process whereby Spn evades the host innate immune response and establishes in the middle ear remains the focus of intense investigation.
Spn undergoes spontaneous opaque/transparent phase variation in colony morphology switching at frequencies from 10−3 to 10−6 per generation [2]. The relationship between Spn opacity phase variation (variation in colony morphology) and adherence has been described by Weiser et al [2]. This has added a new dimension to our understanding of pneumococcal adherence and invasion [3–7]. Transparent Spn bacteria have thick cell wall teichoic acid with relative low levels of capsular polysaccharide. They have an increased ability to adhere to human lung epithelial cells and are selectively expanded during nasopharyngeal colonization in rodent models [4, 6]. Opaque Spn bacteria have thinner walls with relative increased amounts of capsular polysaccharide. They tend to be characteristically more virulent and are associated with invasive infections in mouse models [7].
We and others have reported the role of Spn opacity variants in the pathogenesis of OM [8–10]. In a previous study we found, in a chinchilla OM model, that there were no significant differences in the level of nasopharyngeal colonization and the induction of OM between the opaque and transparent variants unless there was a prior challenge with influenza A virus [10]. In a rat model of AOM induced by direct transbullar inoculation, the opaque phenotype variant was more efficient at survival and multiplication within the middle ear space, resulting in the accumulation of more inflammatory cells and the enhanced expression and production of inflammatory mediators [9]. Other investigators reported that in an in vitro model of simulated OM, the transparent variant was more highly adapted to a variety of middle ear environments than the opaque variant [8]. A recent clinical study demonstrated that the proportion of the opaque Spn colonies was significantly higher in middle ear isolates than in nasopharyngeal isolates in children with AOM [11]. The molecular mechanisms responsible for differences in bacterial adherence and multiplication of Spn opacity variants during OM have not as yet been fully elucidated.
The complement system is a major component of the host innate immune defense system against infection. We previously found that both of the classical and alternative complement pathways are critical for the otoimmune defense against Spn in a mouse model of AOM. The reduced capacity of complement mediated opsonization and phagocytosis in complement-deficient mice appear to be responsible for the impaired clearance of Spn from the middle ear and dissemination to the blood stream during AOM [12, 13]. However, Spn can evade the complement system by several mechanisms, including recruitment of the host complement regulators C4b binding protein (C4BP) or factor H (FH) to the bacterial surface to inhibit the classical and alternative complement pathways respectively [14,15]. Little is known about the ability of the complement system to enable killing of Spn opacity variants during the course of pneumococcal OM. In the current study, we used mice deficient in C1qa (unable to activate complement through the classical pathway), factor B (unable to activate complement through the alternative pathway) or factor B/C2, to investigated how the Spn opacity variants interact with specific complement pathway activation during the early stage of OM. We also investigated the capabilities of the opacity variants to induce complement activation in the middle ear, and in vitro binding of FH and C4BP by the opacity variants. Our findings suggest that differential resistance to complement mediated killing of the opacity variants of Spn is associated with its virulence during acute pneumococcal OM in mice.
2. Materials and methods
2.1. Bacteria
Spn serotype 6A (EF3114; kindly provided by B. Andersson, Department of Clinical Immunology, University of Gotebörg, Gotebörg, Sweden) was used for these experiments and has been previously described in detail [16]. The isogenic opaque or transparent variants of Spn 6A were isolated by Dr. Jeffrey Weiser, Children’s Hospital of Philadelphia, and have been used for OM research in our laboratories and have been previously described in detail [9, 10]. The growth conditions and inocula were prepared as previously described [9, 10]. The opacity phenotypes were confirmed prior to inoculation according to the method established by Weiser et al [2].
2.2. Mice
Eight to 12-week-old male or female C57BL/6 mice were used in this study. C57BL/6 mice homozygous for combined gene deficiencies of factor B and C2 (Bf/C2−/−), C1qa (C1qa−/−) and factor B (Bf−/−) were generated as previously described [17–19], and were backcrossed at least 9 generations onto the C57BL/6 background. Age and sex matched C57BL/6 mice, used as controls, were obtained from Taconic Farms and the Jackson Laboratories. All study procedures were approved by The Institutional Animal Care and Use Committee at the Ohio State University.
2.3. Mouse model of AOM
Mice were anesthetized by intraperitoneal injection with ketamine hydrochloride (20 mg/kg of body weight) and xylazine (5 mg/kg). AOM was then produced by direct bilateral transtympanical inoculation with approximately 1 × 103 CFU of opaque or transparent Spn in sterile pyrogen-free saline as previously described [20]. Cohorts of seven to eleven mice were used at each time point. At 24, 48 and 72 h post inoculation, mice were anesthetized and then sacrificed. The middle ear spaces were lavaged to quantitatively determine titers of Spn as previously described [13]. Blood was collected via cardiac puncture and quantitatively cultured to determine bacterial dissemination. The middle ear lavage and blood samples were cultured overnight at 37°C on Columbia CNA agar plates in an incubator supplemented with humidity and 5% CO2. The number of CFU per milliliter was determined by a standard dilution assay and plate counting.
2.4. Histology
Six temporal bones from each cohort were removed immediately after sacrifice at 24, 48 and 72 h post-challenge. The samples were processed for hematoxylin and eosin staining as described previously [13]. Mucosal thicknesses of digital micrographs were semi-quantitatively measured at a similar region of the middle ear of each sample.
2.5. Quantitation of complement component transcripts from the middle ear epithelium by real-time PCR
Total RNA from the middle ear lysate samples was purified, and real-time PCR assays were performed to quantitate C1q, C3, C4, factor B, factor H and factor I transcripts as previously described [12].
2.6. Complement factor B and C3 activation
For comparison of complement activation in the middle ears induced by Spn opacity variants, equal amounts of protein (30μg) from mouse middle ear lavage fluid samples pooled from 4–5 mice were loaded on an SDS-PAGE gel. C3 and factor B cleavage fragments were evaluated by western blot as previously described [12].
2.7. Analysis of C3b deposition on S. pneumoniae opacity variants in vivo by flow cytometry
C3b deposition on Spn opacity variants recovered from middle ear lavage fluid samples at 24 h post challenge was evaluated by flow cytometry as previously described [13]. Briefly, the middle ear lavage samples pooled from three mice in each cohort were centrifuged at 800 × g for 5 min at 4° C. The supernatants were centrifuged at 15,500 × g for 10 min at 4°C, and the bacterial pellets were washed once in DPBS and incubated with FITC-conjugated anti-mouse C3 antibody (MP Biomedicals, Aurora, OH). C3b deposition was detected using a FACS Calibur (BD Biosciences, San Jose, CA. Bacteria incubated with DPBS were used as negative controls to set the threshold and fluorescence intensity. A minimum of 20,000 cells per sample were analyzed. The data are expressed as the mean ± standard deviation of a fluorescence index (FI, the proportion of bacteria showing fluorescence expressed as a percentage multiplied by the geometric mean fluorescence intensity). The experiment was repeated three times.
2.8. In vivo phagocytosis of S. pneumoniae opacity variants by neutrophils in the middle ear
The inflammatory cells of the middle ear lavage fluid samples from five wild type or complement deficient mice were collected at 24 h post infection and pooled. The Immunostained cytospin preparations for Spn and C3/iC3b/C3c were evaluated with an Olympus Flowview 1000 laser scanning confocal microscope as previously described [12]. A quantitative analysis of Spn phagocytosis by neutrophils in vivo was determined by standard dilution and plate count as previously described [12].
2.9. C4BP or Factor H binding to bacterial cells
Bacteria were grown overnight on blood agar plates. To test binding of FH to the Pneumococci, 2 × 108 cells were incubated in 30% heat-inactivated normal human serum (NHS) (CompTech, Tyler, Texas). In another set of experiments pneumococci were incubated with purified complement FH (Quidel, San Diego, CA) at a concentration of 90 μg/ml, roughly corresponding to 90 μg/ml of FH in 30% NHS. The FH was diluted in VBS-1% bovine serum albumin (BSA) and cells were incubated for 40 min at 37°C. For C4BP binding, Spn bacteria were incubated with 50% NHS or purified human C4BP (CompTech) at a concentration of 100 μg/ml, roughly corresponding to the C4BP concentration in 50% NHS human serum, diluted in PBS -1% bovine serum albumin (BSA) for 60 min at 37°C. After three washe s, the samples were incubated for 1 h either with a monoclonal murine anti-human factor H antibody (Quidel) or a monoclonal mouse anti-human C4BP antibody (Quidel). The secondary antibody for both experiments was Alexa Fluor 488-labeled goat anti-mouse IgG (Molecular Probes, Invitrogen). Binding of C4BP or FH to bacteria was detected by flow cytometry, collecting data from 20,000 gated events. The data are expressed as the mean ± standard deviation of FIs. Each bacterial strain was analyzed in four separate experiments.
2.10. Statistical analysis
Data are presented as the mean ± the standard error of the mean (S.E.M.) or standard deviation of the mean (S.D.) as indicated. Data were analyzed using SigmaStat (SPSS Inc., Chicago, IL). Results for all comparisons between Spn opaque and transparent variants were analyzed using the student t tests or Chi-square test. One-way ANOVA and the Holm-Sidak or Dunn’s methods were used for the statistical analysis of multiple cohort and pair-wise comparisons. In all cases, a P value of <0.05 was set as the measure of significance.
3. Results
3.1. The opaque phenotype variant of Spn persists in the middle ear in complement sufficient and deficient mice
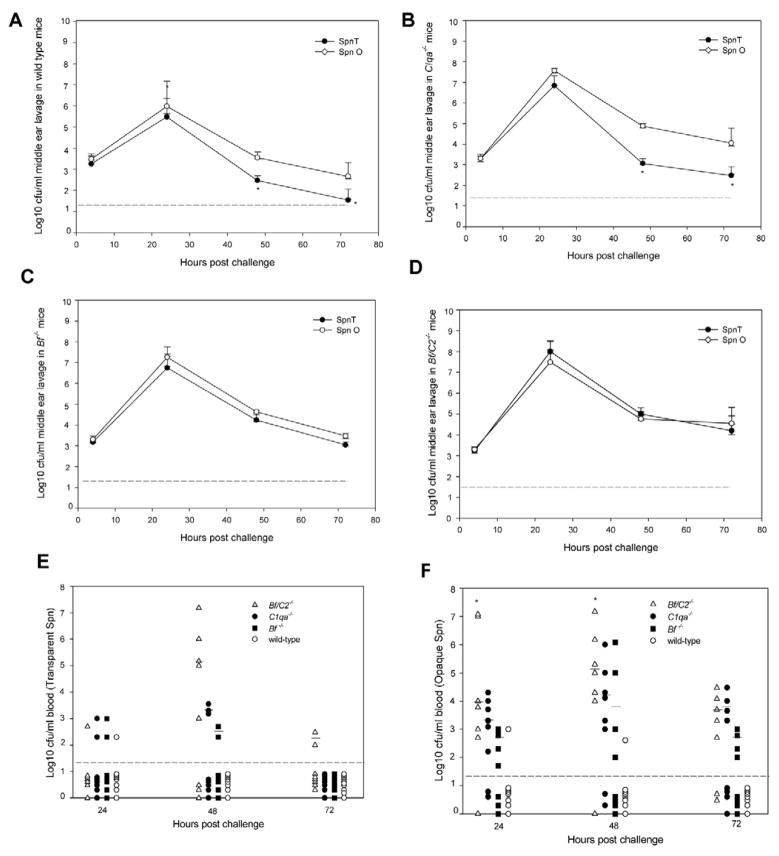
To define whether the opacity variants influence Spn survival in the middle ear and to ascertain the ability of specific complement pathways to protect the host against Spn opacity variants during AOM, the concentrations of Spn opaque/transparent variants were determined for the middle ear lavage samples from wild-type and complement deficient mice (Fig. 1A, 1B, 1C, 1D). At 4 and 24 h post challenge, there were no significant differences in concentrations between the opaque and transparent variants of Spn in the middle ear lavage samples from all the mouse strains. However, as expected from our previous report [13], complement deficient mice demonstrated a significant 1–1.5 –log10-unit increase for both the opaque and transparent variants (P < 0.05 for all compared to wild-type mice) at 24 h post infection. At 48 and 72 h post challenge, although the titers for both opacity variants remained significantly higher for complement deficient mice than wild-type mice (P < 0.05), the clearance kinetics for the transparent and opaque phenotype variants were markedly different in wild type and C1qa−/− mice. The concentration of the transparent phenotype in the middle ear-lavage fluid declined steadily, so that by 48 and 72 h post inoculation a statistically significant decrease (P < 0.05) was evident in the log10 CFU of the transparent phenotype per milliliter of middle ear lavage fluid compared with that of the opaque phenotype (Fig. 1A, 1B). The concentration of the opaque variant remained detectable at 72 h post inoculation while the transparent variant was eliminated from the middle ear in 6 out 8 wild -type mice (Fig. 1A). However, there were no significant differences in the bacterial titers between the opaque and transparent variants in the middle ear lavage samples from Bf−/− and Bf/C2−/− mice. Neither phenotype was completely cleared from the middle ear in the complement deficient mice during the 72 h experiment, which suggested the importance of the complement alternative pathway in host defense against both Spn opacity variants. There were no significant differences in the bacterial titers between C1qa−/− and Bf−/− mice at the time points examined. Ninety-six to ninety-nine percent of the middle ear lavage isolates cultured at each sampling time were of the same opacity phenotype as the respective original inoculums.
Fig. 1.
Survival of Spn type 6A opacity phenotype variants in wild type mice (A), C1qa−/− (B), Bf−/− (C) and Bf/C2−/− mice (D) in the middle ears of mice following transtympanical inoculation with approximately 1 × 103 CFU of Spn opaque or transparent variant bacteria. Each data point represents the mean of CFU of Spn (± SEM) per milliliter of the middle ear lavage fluid samples from a total of 7 to 11 animals combined from two separate experiments. *, P < 0.05 for the comparison with the opaque variant. The dashed line represents the detection limit of the assay. (E and F) Bacterial dissemination in blood. Blood samples were collected at 24, 28, and 72 h post transtympanical challenge with Spn type 6A opaque variant (E) or the transparent variant (F), then cultured on Columbia CNA agar plates. The horizontal bar shows the mean numbers of bacterial titers after 24 h post infection. *, P < 0.05 for the comparison of Bf/C2−/− mice with C1qa−/− or Bf−/− cohort. The dashed line represents the detection limit of the assay. The mice with negative blood culture results were shown below the dashed line.
3.2. Deficiencies in C1q, Bf and Bf/C2 enhance invasiveness of opaque Spn into blood
Blood culture titers of Spn opacity phenotypes for each cohort are shown in Fig. 1E and 1F. Persistent bacteremia was noted for all the cohorts of the complement-deficient mice across the 24-, 48-, and 72-h time periods. Transparent Spn bacteremia occurred in 33% of Bf/C2−/−, 17% of Bf−/−, 21% of C1qa−/− and 4% of the wild-type mice (Fig. 1E). In contrast, opaque Spn bacteremia occurred in 81% of Bf/C2−/−, 54% of Bf−/−, 67% of C1qa−/− and 8% in the wild-type mice (1F). (P < 0.05 for opaque versus transparent in C1qa−/−, Bf−/− and Bf/C2−/−mice). The frequency of positive blood cultures of the transparent phenotype was significantly higher in Bf/C2−/− compared to wild -type mice, whereas positive cultures of the opaque phenotype were significantly higher in all three strains of complement deficient mice compared to wild-type mice ( P < 0.02.) Bf/C2−/− mice had significantly higher blood titers of the opaque variant at 24 h and higher titers of both phenotypes than either C1qa−/− or Bf−/− mice at 48 h post challenge (P < 0.05). Our findings are in accord with a previous report that demonstrated the classical pathway is more important than the alternative pathway against Spn septicemia [21]. However, the alternative pathway plays a significant role against Spn opacity variants in the infected middle ear. Our data suggest that differential complement activity and effects of Spn colony opacity variation are dependent on the anatomical niche that is infected.
3.3 Role of Spn opacity variants and complement deficiencies in middle ear inflammation
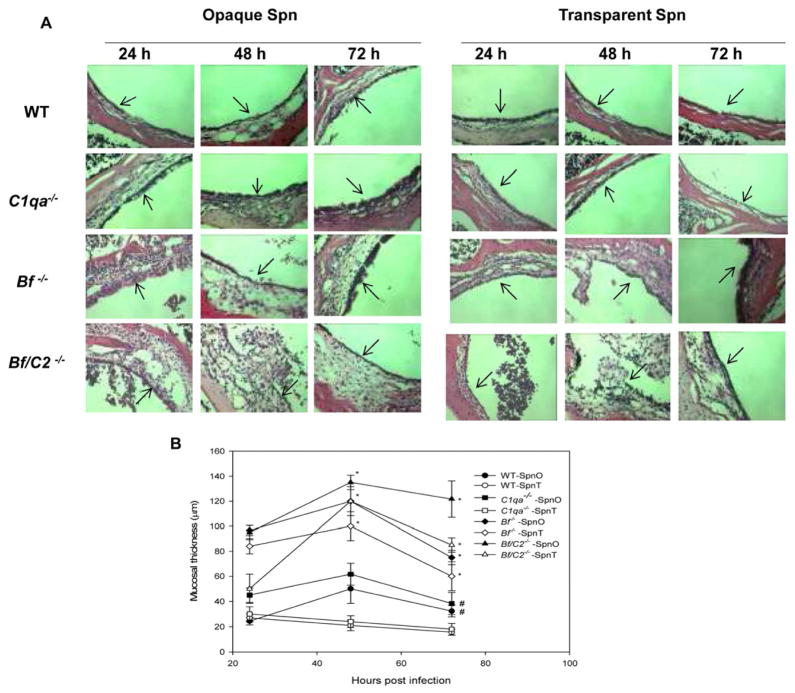
We previously found extensive inflammatory infiltrates within the epithelium or subepithelium of Bf/C2−/− mice compared to wild-type mice infected with Spn type 6A [13]. Here we compared the middle ear histology of complement deficient and wild-type mice infected with Spn opacity variants at the early stage of AOM. By 48 and 72h post infection, histological analysis revealed greater mucosa epithelium thickness and inflammatory infiltrate in opaque Spn induced AOM in wild type and C1qa−/− mice when compared with the mice infected with transparent Spn (Fig. 2, A, B). However, the middle ear mucosa of Bf−/− and Bf/C2−/− mice inoculated with either opaque or transparent variants were severely inflamed and significantly thickened with more extensive inflammatory cell infiltration than wild-type mice at 48 h and 72h post inoculation as shown in representative H&E stained specimens in Fig. 2A and 2B. These findings demonstrate that impaired bacterial clearance of both opaque Spn opacity phenotypes in the absence of the alternative pathway contributes, in large part, to the middle ear mucosa inflammation.
Fig. 2.
(A) Representative H&E-stained middle ear sections of the mice at 24, 48 and 72 h after transtympanical inoculation with Spn opacity phenotype variants in the following cohorts: wild-type, C1qa−/−, Bf−/− and Bf/C2−/−. The inflammatory infiltrates in the middle ear epithelium were evident in the Bf−/− and Bf/C2−/− mice infected with both opacity phenotype variants. Arrows indicate the middle ear mucosa (magnification, × 200). (B) Mucosal thickness of the middle ear of wild-type, C1qa−/−, Bf−/− and Bf/C2−/− mice infected with opaque or transparent Spn. Each data point represents the mean of mucosal thickness of the middle ears from a total of 4 to 6 animals.
#, P < 0.05 for the comparison with the same mouse strain infected with the transparent Spn. *, P < 0.01 for the comparison with the wild-type mouse strain infected with either transparent or opaque Spn.
3.4. Effect of Spn Opacity phenotype variants on complement activation in middle ear
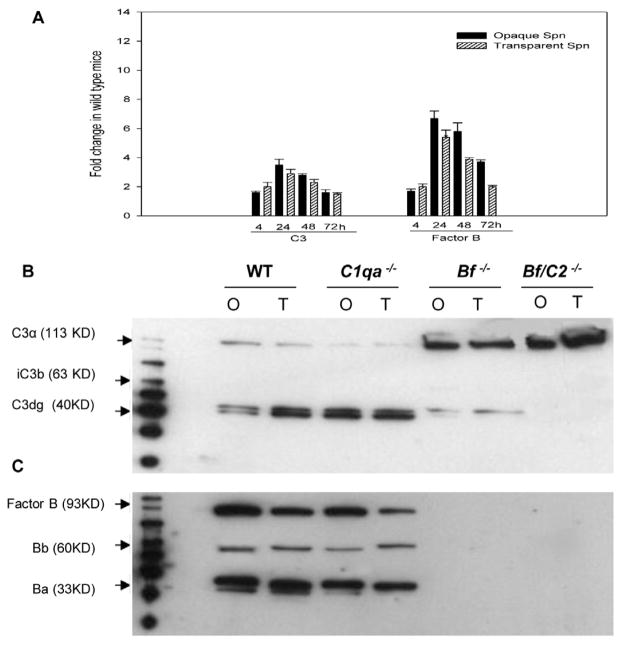
Our previous report showed that the complement activation in the middle ear is a local phenomenon during the early stage of pneumococcal OM [12]. To determine whether complement gene expression by middle ear epithelium and complement activation were differentially induced by the opacity variants during OM, real-time PCR was used to examine the expression of complement genes, and data revealed that there were no significant differences in expression of factor B and C3 genes between opaque and transparent Spn variants in wild type mice (Fig. 3A). Western blots demonstrated that both opacity variants induced C3 and factor B cleavage fragments (Fig. 3B, 3C) in the middle ear lavage fluid in both the wild-type and C1qa−/− mice. However, both opacity variants induced lower levels of C3 cleavage fragments in the middle ear lavage samples of Bf−/− mice compared to wild type and C1qa−/− mice respectively (Fig. 3B). Bf/C2−/− mice, which lack both the classical and alternative pathways, were found to have uncleaved form of C3. Factor B, Bb and Ba were not detected by western blot in Bf−/− and Bf/C2−/− mice (Fig. 3C). Our results suggest that the isogenic opacity variants have a similar capacity of inducing factor B and C3 activation in the middle ear during OM.
Fig 3.
Gene expression and activation of complement factor B and C3. (A) Induction of gene expression as measured by real-time PCR on total RNA samples by direct in situ lysis of middle ear mucosa subsequent to lavage at 4, 24, 48 and 72 h following transtympanic inoculation with Spn type 6A opacity phenotype variants in wild-type mice. Results are the mean fold changes in C3 and factor B transcript levels (± SEM) from duplicate samples pooled from five mice from two separate experiments. (B) Complement C3 activation in wild type and complement deficient mice induced by the Opaque (O) or transparent (T) variant at 24 h post infection. C3 cleavage fragments were analyzed by western blot. Approximately 30 μg of protein from the middle ear lavage fluid samples collected and pooled from five mice was subjected to SDS-PAG. The immunoreactive bands corresponding to C3α, iC3b and C3d are denoted. Prominent C3d band was found in the middle ear lavage fluid samples in wild-type and C1qa−/− mice, and both opacity variants induced C3 activation at a similar level. Weak C3d bands were found in Bf−/− and no C3d bands were found in Bf/C2−/− mice. (C) Factor B activation. Approximately 30 μg of protein from the middle ear lavage fluid samples collected and pooled from five mice at 24 h post infection was subjected to SDS-PAGE. Similar levels of Bb and Ba were detected in wild-type and C1qa−/− mice infected with the opaque or transparent variant, whereas not in the factor B deficient mice. A representative example from three independent experiments is shown.
3.5. Decreased C3b deposition on both of Spn opacity variants in factor B deficient mice impedes opsonophagocytosis of Spn by neutrophils in the middle ear
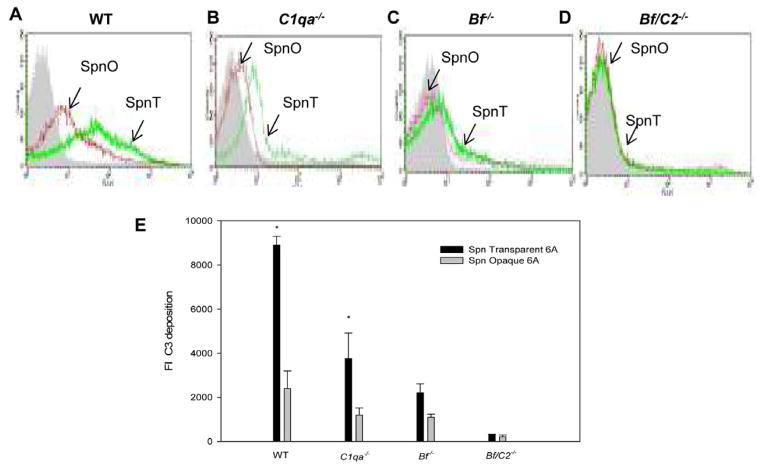
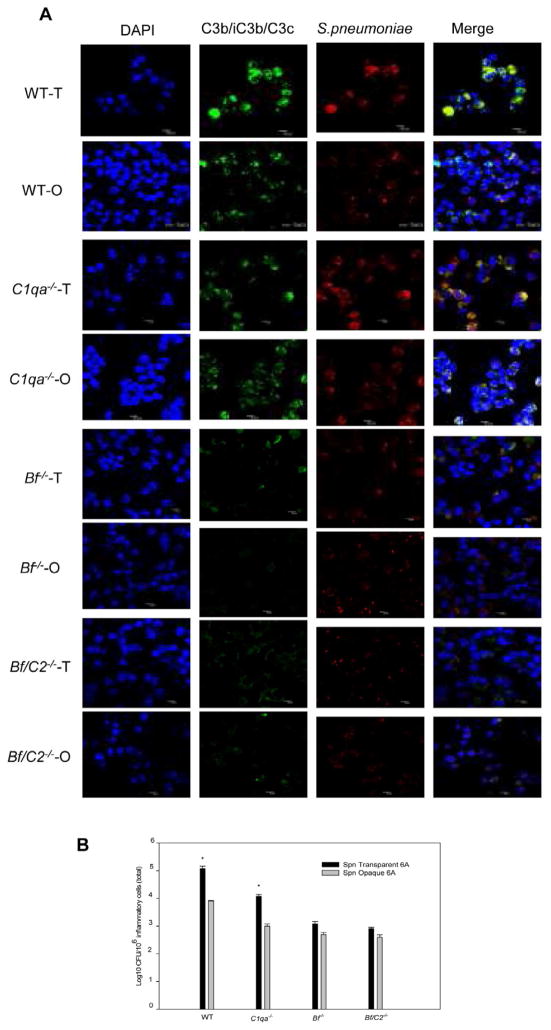
The effects of classical and/or alternative pathway activation on opsonization, attachment and phagocytosis of Spn opacity variants by neutrophils in the mouse middle ear were further evaluated by flow cytometry, colony plate count and confocal fluorescence microscopy. Deposition of C3b on Spn opaque and transparent variants recovered from the middle ear lavage samples at 24 h post-challenge obtained from the complement deficient and wild-type mice is shown in Fig. 4A, 4B, 4C and 4D. There were significantly higher FIs of transparent Spn that fixed C3b on its surfaces compared to the opaque variant In wild-type and C1qa−/− mice(Fig. 4E) (P < 0.05 for transparent versus opaque variants in wild-type and C1qa−/− mice ). There were no significant differences in the amount of C3b bound to the Spn surface between the two opacity variants recovered from the middle ear lavage samples of Bf/C2−/− and Bf−/− mice. In addition, a much lower level C3b deposition on both opacity variants was found in Bf/C2−/− and Bf−/− mice compared to wild-type mice (P < 0.05 for complement deficient versus wild-type mice in all cases). These findings indicate that complement alternative pathway activation accounts for far more deposition of C3b on Spn transparent variants. Confocal fluorescence microscopy studies confirmed greater attachment of C3b opsonized transparent Spn to neutrophils than the opaque variants in wild-type and C1qa−/− mice. In contrast, very few C3b opsonized Spn opaque or transparent variants attached to Bf−/− and Bf/C2−/− neutrophils (Fig. 5A). In addition, colony plate counts revealed that there were significantly more transparent Spn cells attached to and internalized in neutrophils in the middle ears in wild-type and C1qa−/− mice compared to opaque Spn in the same mouse strain. However, there were no significant differences in the colony counts between the two Spn opacity phenotypes in Bf −/− and Bf/C2−/− mice (Fig. 5B). Our data confirm that the absence of classical and/or alternative pathway activation greatly diminishes C3b/iC3b/C3c deposition on both opacity variants and bacterial attachment to neutrophils in the middle ear. Furthermore, our data indicate that increased complement opsonins deposited on transparent Spn via alternative pathway activation contribute to enhanced phagocytosis by neutrophils during the early stage of OM.
Fig. 4.
Representative histograms of C3b deposition on Spn opaque (SpnO) or transparent variants (SpnT) recovered from the middle ear lavage fluid samples from wild- type mice (A), C1qa−/− (B), Bf−/− (C) and Bf/C2−/− mice (D) at 24 h post challenge. C3b fragments on the bacterial surfaces were detected in a flow cytometric assay with anti-mouse C3 antibody. (E) C3b deposition on Spn. The results collected from three different experiments are expressed as means ± standard deviations. *, P < 0.05 compared with values determined for the opaque variants.
Fig. 5.
Opsonophagocytosis of Spn opacity phenotype variants by the neutrophils in the middle ear at 24 h post infection. (A) Confocal microscope images of opsonophagocytosis of Spn type 6A opacity phenotype variants by neutrophils in the middle ear of wild- type and complement deficient mice. These middle ear lavage fluid samples were immunofluorescently labeled to visualize Spn (red), C3b/iC3b/C3c (green), and nucleic acids (blue). Bacteria in the merged images may appear as pink yellow (merge of red and green), or white (merge of red, green and blue). Images are representative of three independent studies. (B) Quantitative assay for binding and ingestion of Spn type 6A opacity phenotype variants by the neutrophils. These results are expressed as the mean CFU/106 neutrophils (± SEM) bacteria binding to the neutrophils and ingested by the neutrophils from three different experiments. *, P < 0.05, compared with values determined for the opaque variant.
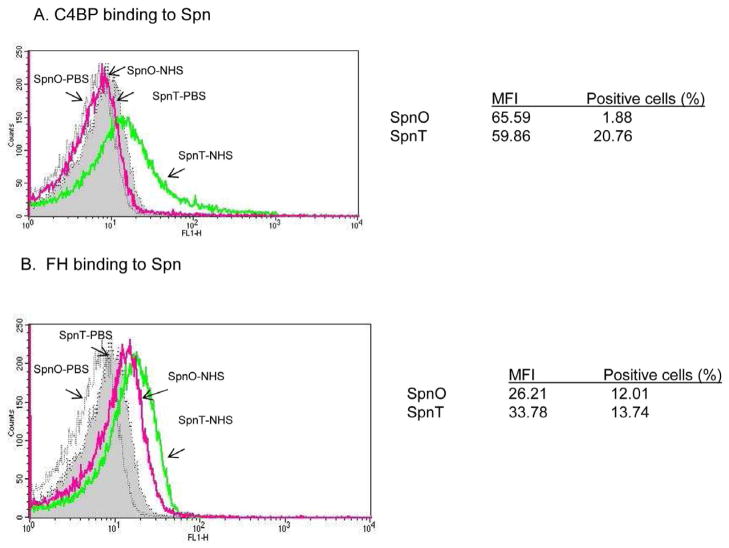
3.6 Increased binding of C4BPin transparent Spn
Flow cytometry assays demonstrated that binding of the classical pathway inhibitor C4BP to the transparent variant was greater with FI of 1,243 ±21 than to the opaque variant (FI, 123± 7) in 50% NHS (P<0.01) (Fig. 6A). This would be predicted to reduce classical pathway activation on bacterial surface of the transparent variant. Binding of purified C4BP gave similar results as that with 50% of NHS (data not shown). Binding of the alternative pathway inhibitor FH to the transparent variants was significantly higher (FI of 464 ± 14) than to the opaque variants (FI, 315±13) (P<0.01) (Fig. 6B).
Fig. 6.
Representative histograms of binding of the classical pathway inhibitor C4BP (A) and the classical pathway inhibitor FH (B) to Spn opacity phenocyte variants detected in a flow cytometric assay. Results are the mean of MFI (mean fluorescence intensity) and percentage of positive cells from three different experiments.
4. Discussion
There is considerable evidence from clinical and experimental studies indicating that the complement system plays an important role in the host defense against pneumococcal infection [13] [22, 23]. In the current study, we assessed the role of the complement system in the killing of Spn opacity variants and OM pathogenesis during AOM. Our data demonstrate that while both opacity variants persisted at an increased level in the middle ear in the complement deficient mice compared to wild -type mice, different clearance kinetics and invasiveness for the two phenotypes were evident. The opaque Spn variant was not only able to survive longer in the middle ear of wild-type and C1qa deficient mice than the transparent phenotype did, but it could also produce persistent bacteremia in complement-deficient mice.).
We found that both opacity variants are capable of inducing gene expression of complement components of C3 and factor B, as well as activation of factor B and C3 in the middle ear during the early stage of AOM in wild-type and C1qa−/− strains. Our data indicate that the capacity of Spn opacity variants inactivating local complement system has little impact on bacterial opsonization and survival during AOM. However, there was significantly more C3b deposition on the transparent variant than on the opaque variant. The difference in C3b deposition on Spn opacity variants could be attributed to cell wall structural differences between the two opacity variants. Our findings confirmed earlier observations [6] that opaque Spn is highly resistant to complement-mediated killing compared to the transparent variant.
Interestingly, although Bf−/− mice possess intact classical and lectin pathways, we showed that without complement activation amplification through the alternative pathway, the level of C3 activation was decreased in the middle ear. In addition, C3b deposition on the transparent opacity variant was almost completely blocked in the middle ear during the early stage of AOM in factor B deficient mice. There were no significant differences in bacterial clearance from the middle ear and middle ear mucosa inflammation between the two opacity variants. Similar results were also found in Bf/C2 −/− mice that lack the classical and alternative pathways. These findings suggest that the virulence of Spn transparent opacity variant in the early stage of AOM is mainly dependent on the complement alternative pathway activation. These results are consistent with a previous report showing increased virulence of high C3 binding Spn strains in the development of OM in chinchillas with the depletion of C3 [22]. Given that all components of the alternative pathway have been found to be present in varying amounts in middle ear effusions from patients with OM [24,25], further studies are needed to explore the mechanisms by which C3b deposition on the transparent Spn is so heavily dependent on the alternative pathway in the middle ear during AOM.
Another important factor determining complement activation by Spn opacity phenotype variants is their relative ability to bind the classical and/or alternative pathways inhibitors. Previous reports have observed that the loss of the polysaccharide capsule significantly increased C4BP and FH binding, demonstrating that the capsule hinders rather than promotes the binding [14, 26, 27]. Bacteria-bound C4BP or FH down-regulates the activation of the classical or alternative pathway respectively and thus reduce C3b deposition on bacterial surface. [14, 26, 27]. We showed that there was more C4BP or FH bound to the transparent variant compared to the opaque variant. However, increased C3b deposition on the transparent Spn in the middle ear in wild type and C1qa−/− mice was evident compared to the opaque variant. These findings suggest that the different cell wall structure particular the thick capsule of the opaque variant may prevent the alternative pathway amplification role or directly inhibit the alternative pathway activation on the opaque variant in this OM model. Decreased C3b deposition on the transparent variant in Bf−/− mice may be attributed to increased binding of C4BP. The mechanisms responsible for the differential binding abilities of C4BP and FH between the two opacity variants are unknown. Pneumococcal surface protein PspC can bind FH and C4BP and allelic variation of PspC will affect such bindings [14, 26]. It would be interesting to learn if there is a structural difference in specific allele of PspC between the two variants.
In the present study, we also demonstrated that opaque Spn bacteria were more invasive than transparent Spn bacteria. The opaque bacteria readily entered bloodstream of Bf/C2−/− mice, indicating that complement activation is critical for preventing dissemination of this strain of Spn. A previous report demonstrated that the Spn capsule inhibits complement activity and neutrophil phagocytosis via multiple mechanisms [27]. In addition, numerous genes and proteins are subject to differential regulation in pneumococcal phase variants [28]. A recent report also showed that opaque Spn bacteria produce an extracellular matrix (biofilm), whereas the transparent variant does not. This is believed to enhance the invasiveness of the opaque biofilm-derived Spn (27). The differential gene expression of lic operon and ciaRH between the opaque and transparent variants contributes to this process (29). It is possible that one or more of these mechanisms may act in concert to confer resistance of opaque Spn. Further understanding of the complexity of interactions between these different molecules is needed in order to fully determine how Spn opacity variants evade host defense.
In conclusion, the current study demonstrates that the complement classical and alternative pathways play a critical role in host defense against Spn opacity variants during the early stage of AOM. Transparent Spn bacteria were killed by an alternative pathway-dependent process. The ability of the opaque Spn variants to evade complement-mediated opsonophagocytosis is associated with increased virulence in a mouse model of acute pneumococcal OM. Interactions with other capsule mediated and complement dependent and independent host defense mechanisms may also contribute to the pathogenesis of pneumococcal OM and warrant further investigation.
Acknowledgments
This study was supported by Award Number R01DC009235 from the National Institute on Deafness and Other Communication Disorders (HHT), National Institutes of Health, R01 DK076690 from the National Institute of Diabetes and Digestive and Kidney Diseases (JMT), National Institutes of Health, and HL52886, HL56086, HL099130, AI089781, HL092469 (GLS) from the National Institutes of Health.
We thank Dr. Jeffery N Weiser for providing the Spn 6A colony opacity variants, Drs. Thomas F. DeMaria and Calvin M. Kunin for their critical review of this manuscript, Dr. Marina Botto, Imperial College, London for her agreement to use C1qa−/− and Bf/C2−/− mice, assistance from the Flow Cytometry Core Lab at Davis Heart & Lung Research Institute, and the Campus Microscopy and Imaging Facility at The Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overturf GD American Academy of Pediatrics Committee on Infectious Diseases. Technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000;106:367–376. doi: 10.1542/peds.106.2.367. [DOI] [PubMed] [Google Scholar]
- 2.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles DE, Novak L, Hotomi M, van Ginkel FW, King J. Nasal, colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect Immun. 2005;73:6945–6951. doi: 10.1128/IAI.73.10.6945-6951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cundell DR, Weiser JN, Shen J, Young A, Tuomanen EI. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiser JN, Bae D, Epino H, Gordon SB, Kapoor M, Zenewicz LA, Shchepetov M. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect Immun. 2001;69:5430–5439. doi: 10.1128/IAI.69.9.5430-5439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae, J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 7.Kim JO, Romero-Steiner S, Skov Sorensen UB, Blom J, Carvalho M, Barnard S, Carlone G, Weiser JN. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li-Korotky HS, Banks JM, Lo CY, Zeng FR, Stolz DB, Swarts JD, Doyle WJ. Interaction of pneumococcal phase variation and middle ear pressure/gas composition: an in vitro model of simulated otitis media. Microb Pathog. 2008;45:201–206. doi: 10.1016/j.micpath.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long JP, Tong HH, Shannon PA, DeMaria TF. Differential expression of cytokine genes and inducible nitric oxide synthase induced by opacity phenotype variants of Streptococcus pneumoniae during acute otitis media in the rat. Infect Immun. 2003;71:5531–5540. doi: 10.1128/IAI.71.10.5531-5540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong HH, Weiser JN, James MA, DeMaria TF. Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect Immun. 2001;69:602–606. doi: 10.1128/IAI.69.1.602-606.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai J, Hotomi M, Hollingshead SK, Ueno Y, Briles DE, Yamanaka N. Streptococcus pneumoniae Isolates from Middle Ear Fluid and Nasopharynx of Children with Acute Otitis Media Exhibit Phase Variation. J Clin Microbiol. 2011;49:1646–16499. doi: 10.1128/JCM.01990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Li YX, Stahl GL, Thurman JM, He Y, Tong HH. Essential role of factor B of the alternative complement pathway in complement activation and opsonophagocytosis during acute pneumococcal otitis media in mice. Infect Immun. 2011;79:2578–85. doi: 10.1128/IAI.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong HH, Li YX, Stahl GL, Thurman JM. Enhanced susceptibility to acute pneumococcal otitis media in mice deficient in complement C1qa, factor B, and factor B/C2. Infect Immun. 2010;78:976–83. doi: 10.1128/IAI.01012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieudonné-Vatran A, Krentz S, Blom AM, Meri S, Henriques-Normark B, Riesbeck K, Albiger B. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J Immunol. 2009;182:7865–7877. doi: 10.4049/jimmunol.0802376. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Ma Z, Jokiranta TS, Whitney AR, DeLeo FR, Zhang JR. Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J Immunol. 2008;181:7138–7146. doi: 10.4049/jimmunol.181.10.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson B, Eriksson B, Falsen E, Fogh A, Hanson LA, Nylen O, Peterson H, Svanborg Eden C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: Differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981;32:311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor PR, Nash JT, Theodoridis E, Bygrave AE, Walport MJ, Botto M. A targeted disruption of the murine complement factor B gene resulting in loss of expression of three genes in close proximity, factor B, C2, and D17H6S45. J Biol Chem. 1998;273:1699–1704. doi: 10.1074/jbc.273.3.1699. [DOI] [PubMed] [Google Scholar]
- 20.MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. 2006;219:12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA. 2002;99:16969–74. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabharwal V, Ram S, Figueira M, Park IH, Pelton SI. Role of complement in host defense against pneumococcal otitis media. Infect Immun. 2009;77:1121–1127. doi: 10.1128/IAI.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuste J, Sen A, Truedsson L, Jönsson G, Tay LS, Hyams C, Baxendale HE, Goldblatt F, Botto M, Brown JS. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect Immun. 2008;76:3761–3770. doi: 10.1128/IAI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovitch RA, Koethe SM, Kalbfleisch JH, Preheim LC, Rytel MW. Relationships between alternative complement pathway activation, C-reactive protein, and pneumococcal infection. J Clin Microbiol. 1986;23:56–61. doi: 10.1128/jcm.23.1.56-61.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker MJ, Leopold DA, Stitzel AE, Welch TR, Weiner LB, Spitzer RE. Components of the alternative pathway of complement in otitis media with effusion. Otolaryngol Head Neck Surg. 1985;93:607–611. doi: 10.1177/019459988509300507. [DOI] [PubMed] [Google Scholar]
- 26.Yuste J, Khandavilli S, Ansari N, Muttardi K, Ismail L, Hyams C, Weiser J, Mitchell T, Brown JS. The effects of PspC on complement-mediated immunity to Streptococcus pneumoniae vary with strain background and capsular serotype. Infect Immun. 2010;78:283–92. doi: 10.1128/IAI.00541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overweg K, Pericone CD, Verhoef GG, Weiser JN, Meiring HD, De Jong AP, De Groot R, Hermans PW. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect Immun. 2000;68:4604–4610. doi: 10.1128/iai.68.8.4604-4610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trappetti C, Ogunniyi AD, Oggioni MR, Paton JC. Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS One. 2011;6:e19844. doi: 10.1371/journal.pone.0019844. [DOI] [PMC free article] [PubMed] [Google Scholar]