Abstract
Objective
Determine the extent to which self-efficacy mediates the relations between social support and childhood cancer survivors’ physical activity (PA).
Methods
A structured telephone survey was conducted with 105 childhood cancer survivor ages 8–16. Participants completed measures assessing their physical activity as well as proposed predictors of PA including various demographic, medical, cognitive, and social influences. Multiple mediation analyses were utilized to evaluate the relations between social support, cognitive influences, and survivor PA.
Results
Cognitive influences, including perceived benefits, barriers, and self-efficacy for PA, partially mediated the influence of family and peer support on survivor PA. Self-efficacy emerged as a significant unique mediator, indicating that higher levels of family and peer support are associated with higher levels of survivor PA via increases in survivor self-efficacy.
Conclusions
Social support has both direct and indirect influences on survivor PA. Indirectly, social support influences PA via survivor self-efficacy. Interventions should target family and peer support as well as self-efficacy to increase survivor PA.
Keywords: Cancer, childhood, physical activity, survivors, mediation
As many as two-thirds of the 328,000 childhood cancer survivors living in the United States [1] will experience at least one late effect related to their diagnosis and treatment including second cancers, obesity, and heart and lung problems [2,3]. Additionally, childhood cancer survivors are over eight times more likely to die prematurely compared to age- and gender-matched peers [4].
Given the significant health threat experienced by survivors of childhood cancer, it is important to identify behaviors that may reduce risk. Physical activity (PA) has been targeted as a leading health promotion priority in cancer survivorship research [5]. Pediatric survivors of cancer who engage in regular PA have increased cardiorespiratory fitness, muscle and bone strength, and more favorable cardiovascular and metabolic disease risk profiles [6,7].
Despite research suggesting PA has protective health benefits, a large proportion of childhood cancer survivors do not meet PA recommendations [8–10]. Survivor PA is generally classified according to the American Cancer Society (ACS) guidelines recommending that individuals over age 18 engage in 30 minutes, and children/adolescents 60 minutes, of moderate to vigorous activity at least 5 days a week [6]. Recent reviews evaluating PA in childhood cancer suggest survivors generally report low levels of PA and are less active than healthy peers [8–10].
Inadequate PA in child and adolescent survivors has led to increased focus on interventions to improve PA in this population. To date, empirically-tested interventions have resulted in weak to modest short-term success [7,9]. Most, but not all, published studies that include follow-up PA evaluations generally report a return to low baseline PA levels [7,9,11]. Additionally, these interventions have been largely atheoretical and have not incorporated empirically-supported constructs underlying survivor PA behaviors.
Development of effective interventions to increase PA among pediatric cancer survivors requires an understanding of the processes that determine survivors’ PA. Research on the determinants of PA in child and adolescent survivors is sparse, with most research focusing on late adolescent and adult survivors of childhood cancer [12–16]. While one might expect overlap between predictors of physical activity in child and adult survivors, important differences also may exist, largely due to development-driven differences in child and adult functioning, behavior, and decision-making.
PA behavior in youth is driven by a complex set of influences, including intrapersonal, interpersonal, and environmental factors [17–19]. The influences on pediatric survivors are likely similar, but complicated by the psychological and physical effects of surviving a major disease, plus the psychological effects on family members. Using Bronfenbrenner’s ecological theory as a framework, Gilliam and Schwebel [20] recently presented a model to conceptualize the behavioral processes through which PA might be promoted and maintained in child and adolescent survivors. Within the model, cognitive self-beliefs are proposed to mediate the relations between social influences from parents and from peers, and PA [20]. Specifically, individuals might choose to engage in PA behaviors based on cognitive processes that support self-regulation including self-efficacy (beliefs about capacity to perform behaviors) and expectancies (perceived benefits and barriers to engaging in the activity) [20]. These cognitive processes may be developed and reinforced by social interactions with family and peers.
Beyond theoretical support, the proposed mediational model has empirical support from a few perspectives. First, similar mediational models have been proposed and tested among healthy youth [21–23]. Second, the direct relations between social influences, self-beliefs and PA behavior are supported among adolescent survivors. Both greater family support [24–27] and greater peer support [19,24,28,29] for PA are associated with higher levels of PA among healthy youth and adolescents who have survived cancer. Direct associations between self-beliefs and PA behavior are similarly established. Adolescent and young adult survivors who endorse greater self-efficacy for PA are more likely to be active compared to peers who report lower self-efficacy [14,16,30]. Environmental expectancies and outcome expectations, including beliefs about the barriers and benefits to PA, are also associated with PA such that greater perceived barriers to PA predict lower PA [14,31] while greater perceived benefits of PA predict greater PA [26,33].
To date, little empirical evidence exists to support the role of self-beliefs as a mediator between social influences and PA behavior among child and adolescent survivors of cancer. The aim of the present study was to determine the extent to which self-efficacy for PA, perceived benefits of PA, and perceived barriers to PA mediate the associations between family and peer support and child and adolescent cancer survivors’ PA. We hypothesized that self beliefs, comprised of self-efficacy, perceived benefits, and perceived barriers, would mediate relations between family and peer support and survivors’ PA.
Methods
Participants
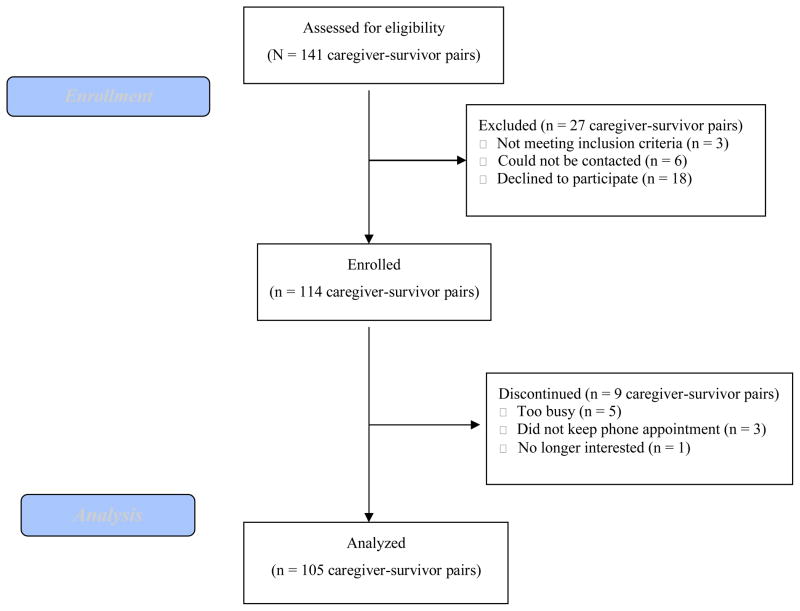
Participants were recruited from the University of Alabama at Birmingham (UAB) Division of Pediatric Hematology and Oncology at Children’s of Alabama. Inclusion criteria were children currently aged 8–16 years who had survived any type of cancer and were at least one year post-completion of treatment without evidence of disease recurrence. Exclusion criteria included child residence outside of primary caregiver’s home, disabilities that prohibited child or primary caregiver from completing measures orally by telephone, and inability of child or primary caregiver to speak English. The study flow is summarized in Figure 1. The final sample included 105 pediatric cancer survivors.
Figure 1.
CONSORT Flow Diagram
Survivors had a variety of cancer diagnoses, including leukemia (36%), CNS tumor (19%), soft tissue sarcoma (11%), non-Hodgkin’s lymphoma (11%), kidney tumor (9%), neuroblastoma (5%), osteosarcoma (4%), Hodgkin’s lymphoma (3%), and other cancers (2%). There were no significant differences (p>.05) between survivors who completed the phone survey and those who did not based on age, gender, race, cancer type, age at diagnosis, or time since treatment. The sample was representative of the population of cancer survivors treated though the Division of Pediatric Hematology and Oncology at Children’s of Alabama in regards to age, gender, race, and cancer type.
Procedure
A telephone survey assessed predictors of physical activity in childhood cancer survivors between October 2010 and March 2011. Eligible survivors were identified through patient databases of children and adolescents treated by the UAB Division of Pediatric Hematology and Oncology at Children’s of Alabama since 1994. Patient records and monthly clinic lists were reviewed to verify eligibility. Eligible participants were mailed a study description approximately two weeks prior to contact. Following the mailing, families were approached either in the clinic (n = 60; 78% enrollment) or via telephone (n = 81; 72% enrollment) to obtain consent and arrange a time to complete the telephone survey. The university’s Institutional Review Board approved all procedures.
The caregiver provided verbal consent and completed a brief demographic questionnaire. Next, verbal assent was obtained from the survivor who completed the 20-minute telephone survey assessing PA and proposed predictors of PA including family and peer support, perceived benefits, perceived barriers, and self-efficacy. Following completion of the survey, the caregiver and survivor were each mailed a $10 check to compensate them for their time.
Measures
Demographics
Caregivers completed a brief demographic questionnaire assessing survivor and caregiver characteristics including survivor and caregiver age, gender, height, and weight; survivor ethnicity; caregiver education and employment; and family income.
Medical Variables
Diagnosis, age at diagnosis, number of relapses, time since treatment, treatment modalities, and treatment-related effects were abstracted from the patient’s medical chart. Scoring for treatment-related effects was based on the Common Terminology Criteria for Adverse Events – Version 3.0 (CTCAE) developed through the National Cancer Institute for classifying both acute and chronic conditions in patients with cancer and survivors of all ages [34]. Each medical late effect was scored on a 4-point scale with scores reflecting the severity of the condition. Scores were defined as follows: Grade 1, mild adverse event (AE); Grade 2, moderate AE; Grade 3, severe AE, and Grade 4, life-threatening or disabling AE. As this study evaluated living survivors of cancer, the “Grade 5, death related to AE” scoring criteria was not applicable. Total treatment-related effects scores were obtained by summing the severity ratings for each documented adverse event. To establish interrater reliability, 25% of participants’ medical charts were independently coded by a second, independent researcher. Cohen’s kappa (categorical variables) and intraclass correlation (continuous variables) coefficients ranged from .88 to 1.00. Rare disagreements were resolved through discussion.
Physical Activity
Survivors reported current PA behavior using a modified Leisure Score Index (LSI) from the Godin Leisure Time Exercise Questionnaire [GLTEQ: 35] assessing frequency and duration of mild, moderate, and strenuous PA over a typical week. Intensity ratings for each activity were based on the updated Compendium of Physical Activities [36]. The GLTEQ has demonstrated reliability and concurrent validity based on comparisons against objective activity monitors and fitness indices in children and adults [16,37–39]. To calculate the primary outcome of total moderate and vigorous PA (MVPA), the typical frequency of PA reported per week within the moderate and strenuous intensity categories were multiplied by the average reported duration.
Family Support for Physical Activity
Family influences on PA were measured using a 19-item scale assessing frequency that adults or other children in the household encouraged, supported, or engaged in PA with the survivor during a typical week [40]. Items were answered on a 5-point Likert scale and responses were summed to create a household support score. Scale reliability is adequate, with an internal consistency Cronbach’s alpha of .78 and intraclass correlation for test-retest reliability of r = .81 [41]. Cronbach’s alpha in the current sample was .84.
Peer Support for Physical Activity
Peer support for survivors’ PA was assessed using 3 items asking about frequency with which the survivor and peers encourage each other to be physically active (2 items) and the frequency that peers are active with the child (1 item). Items were answered on a 5-point scale, and responses summed to create a peer support score. Reliability was adequate, with internal consistency Cronbach’s alpha of .74 and intraclass correlation for test-retest reliability of r = .70 [40,41]. In the current sample, Cronbach’s alpha was good, α = .88.
Perceived Benefits of Physical Activity
Survivors’ perceived benefits to PA (e.g. improved health, more energy, better body) were measured using 13 items rated on a 5-point scale ranging from “strongly disagree” to “strongly agree” [40]. Items endorsed as “somewhat agree” or “agree” were summed to create a total perceived benefits score, with higher scores indicating greater perceived benefits. Reliability is adequate, with an internal consistency Cronbach’s alpha of .92 and intraclass correlation for test-retest reliability of r = .65 [40]. Cronbach’s alpha in the present sample was acceptable. α= .71.
Perceived Barriers to Physical Activity
Survivors’ perceived barriers to PA (e.g. too tired, lack of time, PA is boring) were measured using 23 items rated on a 5-point scale ranging from “never” to “very often” [40]. Items endorsed as barriers to PA “often” or “very often” were summed to create a total barriers score, with higher scores indicating greater perceived barriers. Reliability is adequate, with an internal consistency Cronbach’s alpha of .88 and intraclass correlation for test-retest reliability of r = .90 [40]. In the current sample, Cronbach’s alpha was good, α = .86.
Self-efficacy for Physical Activity
Survivors’ confidence to engage in PA in certain situations (e.g. when they have too much homework; when family or friends want them to do something else) was assessed using 5 items rated on a 5-point scale ranging from “I’m sure I can’t” to “I’m sure I can” [40]. Survivors’ responses were summed to create a total self-efficacy score with higher scores indicating greater self-efficacy. Reliability is adequate, with an internal consistency Cronbach’s alpha of .85 and intraclass correlation for test-retest reliability of r = .89 [40]. Cronbach’s alpha in the present sample was acceptable, α = .70.
Statistical Analyses
Analyses were conducted using SPSS version 19. Correlational analyses were performed to evaluate bivariate relations between survivor MVPA and the proposed influences on MVPA including demographic (age, gender, ethnicity, and family income), medical (age at cancer diagnosis, time since treatment, treatment-related effects), social (family and peer support), and cognitive influences (perceived benefits, perceived barriers, self-efficacy). Variables significantly associated with survivor MVPA in bivariate associations were included in the main analyses.
For the main analyses, multiple mediation models were tested to determine the extent to which cognitive influences explained the association between social support and survivor MVPA. Models were evaluated separately for family and peer support. Demographic and medical variables that were significantly associated with survivor MVPA were included as covariates in both models. According to Preacher and Hayes [42], multiple mediation involves an analysis of the total indirect effect (the aggregate mediating effect of all potential mediators being examined) and an analysis of the specific indirect effect (the mediating effect of an individual variable). To test these models, bootstrapping, a nonparametric resampling procedure, was performed using an SPSS macro developed by Preacher and Hayes [43]. Bootstrapping is the preferred analytical strategy when testing for mediation given its greater statistical power without the assumption of multivariate normality in the sampling distribution [42,44,45]. Following the recommended procedures [42], all variables were standardized prior to being entered in the model. Parameter estimates and confidence intervals of the total and specific indirect effects were generated based on 5,000 random samples and a confidence level of 95%. Specific indirect effects were analyzed regardless of the significance of the total effect given that the total effect is not necessary for mediation to occur [42, 46, 47]. Mediation was demonstrated if the 95% bias-corrected confidence interval for the parameter estimate did not contain zero. The magnitude of each significant specific indirect effect was compared via contrast tests.
Results
Table 1 presents descriptive statistics. While the average survivor engaged in over 6 hours of MVPA per week, 39% of survivors reported that they did not meet the ACS recommendations for weekly PA, rates similar to those observed in healthy youth by other researchers [34%; 48]. There were no differences in the distribution of survivors who met the recommendations versus those who did not based on cancer diagnosis. Developmental differences were noted, however, with older survivors (aged 12–16) less likely to report meeting ACS recommendations (p = .02) compared to younger survivors (aged 8–11).
Table 1.
Descriptive Statistics (N=105)
| Variable | M (SD) or % |
|---|---|
| Caregiver-report | |
| Caregiver Age (years) | 40.1 (7.5) |
| Caregiver Status | |
| Mother | 84% |
| Father | 10% |
| Survivor Age (years) | 12.3 (2.4) |
| Survivor Gender | 49% Male |
| Family Income (median range) | $40,000 – 59,000 |
| Survivor Ethnicity | 72% Caucasian |
| Survivor-report | |
| Survivor MVPA (hours per week) | 6.6 (4.5) |
| Family Support (0–76 scale) | 24.2 (10.7) |
| Peer Support (0–12 scale) | 5.0 (2.9) |
| Perceived Benefits (0–13 scale) | 8.6 (2.7) |
| Perceived Barriers (0–23 scale) | 3.1 (3.4) |
| Self-efficacy (0–25 scale) | 17.0 (4.1) |
| Medical Chart Abstraction | |
| Age at Diagnosis (years) | 5.7 (3.7) |
| Time since Treatment (years) | 4.9 (3.0) |
| Treatment Modalities | |
| Chemotherapy only | 41% |
| Radiation only | 0% |
| Surgery only | 5% |
| Chemotherapy and Radiation | 19% |
| Chemotherapy and Surgery | 20% |
| Radiation and Surgery | 4% |
| Chemotherapy, Radiation, and Surgery | 12% |
| Treatment-related Effectsa | 2.6 (3.0) |
Note. MVPA: Moderate-Vigorous Physical Activity.
Treatment-related Effects is a continuous measure with scores reflecting the sum of the severity ratings for each documented adverse event.
Table 2 presents correlations between the study variables. Higher survivor MVPA was associated with male gender, greater family income, fewer treatment-related effects, greater family and peer support, more perceived benefits, fewer perceived barriers, and higher self-efficacy. Greater family support was associated with younger age, younger age at diagnosis, fewer treatment-related effects, greater peer support, greater perceived benefits, fewer perceived barriers, and higher self-efficacy. Similar associations were found for peer support, except peer support was not significantly related to perceived benefits and barriers.
Table 2.
Correlation Matrix, All Variables of Interest (N = 105)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MVPA | -- | ||||||||||||
| 2. Age | −.12 | -- | |||||||||||
| 3. Gendera | .21* | .04 | -- | ||||||||||
| 4. Income | .23* | .03 | −.17 | -- | |||||||||
| 5. Ethnicityb | −.17 | −.05 | .03 | −.26** | -- | ||||||||
| 6. Age at Diagnosis | −.15 | .59** | .02 | −.01 | −.03 | -- | |||||||
| 7. Time since Treatment | −.17 | .14 | −.15 | .15 | −.11 | −.01 | -- | ||||||
| 8. Treatment-related Effects | −.21* | .02 | −.12 | .15 | −.11 | −.01 | .12 | -- | |||||
| 9. Family Support | .55** | −.23* | .11 | .17 | −.01 | −.27** | .10 | −.28** | -- | ||||
| 10. Peer Support | .51** | −.21* | .16 | .01 | −.02 | −.25** | .12 | −.32** | .61** | -- | |||
| 11. Perceived Benefits | .24* | .11 | .15 | .04 | .03 | .11 | .01 | .06 | .31** | .13 | -- | ||
| 12. Perceived Barriers | −.30** | .02 | .05 | −.30** | .17 | .09 | −.19* | .17 | −.33** | −.13 | −.23* | -- | |
| 13. Self-efficacy | .45** | .08 | .08 | .16 | −.22* | −.06 | .17 | −.30** | .45** | .30** | .19 | −.39** | -- |
Note. MVPA: Moderate-Vigorous Physical Activity.
Female = 0, Male = 1;
White = 0, Non-white = 1.
p< .05.
p< .01
Multiple Mediation Analyses
Two multiple mediation models were tested, one each for family support and peer support. Given the significant correlations between survivor MVPA and gender, income, and treatment-related effects in the bivariate analyses, these variables were included as covariates in both models.
Family Support
Figure 2 presents the direct effects of family support on survivor MVPA and the indirect effects of family support on MVPA via the proposed mediators of perceived benefits, perceived barriers, and self-efficacy. After controlling for survivor gender, family income, and total treatment-related effects, the total effect of family support on survivor MVPA was significant (c = 0.48, p< .001; overall R2= 0.41). After adjusting for the indirect effects of the mediators, the direct effect of family support remained significant (c′= .38, p< .001) suggesting partial mediation, with family support contributing both directly and indirectly to survivor MVPA. Additionally, survivor gender demonstrated a partial effect on survivor MVPA (p = .04) indicating that after controlling for all other variables in the model, male survivors were more physically active than female survivors.
Figure 2.
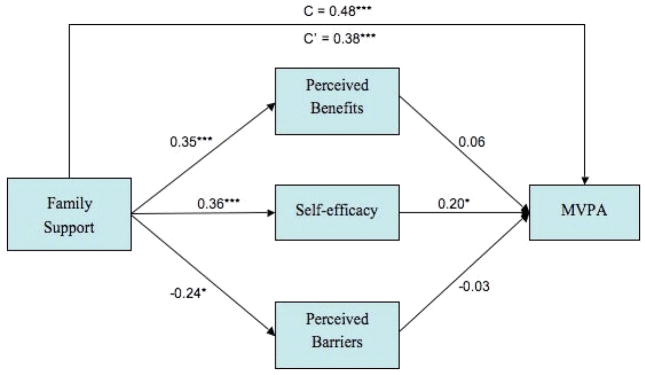
A multiple mediation model of the associations between family support and survivor moderate-vigorous physical activity via survivor’s self-efficacy for PA. Standardized regression coefficients from a bootstrap procedure are provided along the paths. * p <0.05, ** p < 0.01, ***p < 0.001.
To further examine the indirect effects of family support on survivor MVPA, mediators were analyzed both together and separately. Table 3 contains the parameter estimates for the total and specific indirect effects of the mediators on the association between family support and survivor MVPA. The total indirect effect of the mediators and the specific indirect effect of self-efficacy were significant as evidenced by confidence intervals that did not contain zero. As illustrated in Figure 2, family support was positively associated with all three mediators, but only self-efficacy was significantly related to survivor MVPA.
Table 3.
Indirect Effects of Family and Peer Support on MVPA through Proposed Mediators (ab Paths)a
| Model | Data | Boot | Bias | SE | 95% CIb |
|---|---|---|---|---|---|
| Family Support | |||||
| Total | 0.1007 | 0.1019 | 0.0012 | 0.0488 | 0.0232 to 0.2223 |
| Perceived Benefits | 0.0205 | 0.0181 | −0.0025 | 0.0322 | −0.0420 to 0.0903 |
| Perceived Barriers | 0.0083 | 0.0087 | 0.0004 | 0.0221 | −0.0294 to 0.0629 |
| Self-efficacy | 0.0719 | 0.0752 | 0.0033 | 0.0460 | 0.0060 to 0.1893 |
| Peer Support | |||||
| Total | 0.0685 | 0.0678 | −0.0008 | 0.0420 | −0.0052 to 0.1610 |
| Perceived Benefits | 0.0160 | 0.0152 | 0.0007 | 0.0209 | −0.0085 to 0.0838 |
| Perceived Barriers | 0.0049 | 0.0033 | −0.0017 | 0.0148 | −0.0123 to 0.0541 |
| Self-efficacy | 0.0477 | 0.0493 | 0.0016 | 0.0320 | 0.0038 to 0.1337 |
Note. MVPA: Moderate-Vigorous Physical Activity. Data: Indirect effect calculated in original sample. Boot: Mean of the indirect effect estimates calculated across all bootstrap samples. Bias: Difference between indirect effect of original sample and mean of the indirect effect estimates calculated across all bootstrap samples. SE: Standard deviation of the bootstrap estimates of the indirect effect. CI: Confidence Interval.
5,000 resamples
bias-corrected confidence intervals
Taken as a whole, these results indicate that the positive association between family support and survivor MVPA is partially mediated through increases in self-efficacy. While perceived benefits and barriers contribute to the significant total effect of the mediation model, they do not act as independent mediators. Given that these variables did not emerge as significant, no contrasts were performed amongst the mediators.
Peer Support
Figure 3 presents the direct effects of peer support on survivor MVPA and the indirect effects of peer support on MVPA via the proposed mediators of perceived benefits, perceived barriers, and self-efficacy. After controlling for survivor gender, family income, and total treatment-related effects, the total effect of peer support on survivor MVPA was significant (c = 0.46, p< .001; overall R2= 0.43). After adjusting for the indirect effects of the mediators, the direct effect of peer support remained significant (c′= .38, p< .001) suggesting a partial mediation scenario similar to the findings for family support. After controlling for all other variables in the model, a partial effect of family income (p = .02) on survivor MVPA was shown, indicating that survivors with higher family income evidence greater levels of MVPA.
Figure 3.
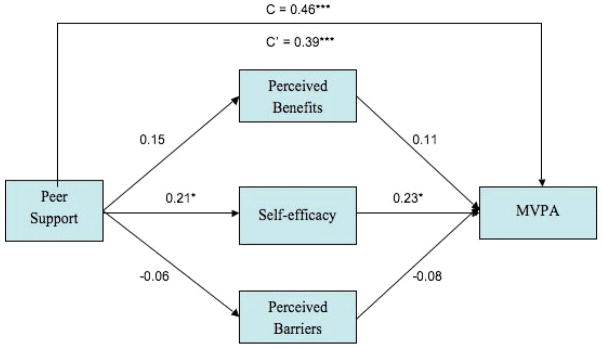
A multiple mediation model of the associations between friend support and survivor moderate- vigorous physical activity via survivor’s self-efficacy for PA. Standardized regression coefficients from a bootstrap procedure are provided alone the paths. * p < 0.05, ** p < 0.01, ***p < 0.001.
To further examine the indirect effects of peer support on survivor MVPA, mediators were analyzed both together and separately. Table 3 contains the parameter estimates for the total and specific indirect effects of the mediators on the association between peer support and survivor MVPA. Only the specific indirect effect of self-efficacy was significant in this model. As illustrated in Figure 3, peer support was positively associated with self-efficacy that in turn was positively associated with survivor MVPA, indicating that increases in family support are associated with increases in survivor MVPA via increases in survivor self-efficacy for PA. Again, as perceived benefits and barriers were not significant, no contrasts were performed amongst the mediators.
Discussion
The present study evaluates mediational relations between social and cognitive influences on child and adolescent survivor PA. Results indicated that self-efficacy partially mediated the relations between social support and child and adolescent cancer survivors’ PA. Survivors who reported greater family and peer support endorsed greater self-efficacy for PA. In turn, greater self-efficacy was associated with higher levels of survivor PA. It is possible that survivors who receive greater support for PA from family and peers are more likely to feel confident in their ability to engage in PA despite limiting physical or psychological effects of cancer. This confidence may allow them to more readily re-engage in PA following treatment. Also, engagement in PA may create a positive feedback loop whereby PA elicits continued support from family and peers thus reinforcing survivors’ perceptions of self-efficacy and continued PA engagement.
In addition to partial mediation, the results also support previous findings [19, 24–29] for the direct influence of social support on child and adolescent survivors’ PA. After controlling for self-efficacy, family and peer support maintained significant positive associations with survivor PA. This finding is consistent with conceptual models that propose both direct and indirect relations between social influences and PA among pediatric cancer survivors [20]. The finding of both direct and indirect influences of social support on survivor PA may reflect differences in the types of social support underlying these relations. We might tentatively hypothesize, for example, that direct influences of social support may reflect the physical facilitation of PA behaviors such as transportation and family and peer engagement with the survivor whereas indirect influences may reflect cognitive and emotional support including encouragement and problem-solving assistance.
Direct influences were also noted for control variables in each model. Gender was associated with survivor PA in the family support model and family income was associated with survivor PA in the peer support model. These may reflect parenting style and access to resources, but given the lack of consistent associations across models, future studies should include these variables in consideration of both family and peer support simultaneously to determine their influence on survivor PA.
A few findings were unexpected. Despite positive associations with survivor PA and contrary to the proposed hypotheses, perceived benefits and barriers of PA were not found to mediate the relations between social support and survivor PA. One possible explanation is that perceived benefits and barriers are subcomponents of a broader construct reflecting behavioral expectancies, so do not serve as independent mediators. Future research should explore this and other possibilities. Also unexpected was the fact that age, when analyzed as a continuous variable, was not associated with PA. We anticipated there might be a developmental component to PA engagement, but an association did not emerge despite the finding that older survivors were less likely to meet ACS recommendations compared to younger survivors.
Taken as a whole, results suggest the processes underlying childhood cancer survivors PA parallel those in healthy youth [17,21]. This discovery has implications for intervention development, as the majority of interventions with child and adolescent survivors utilize physical therapy models to improve PA [7,9] without addressing concurrent cognitive and environmental influences that are known to impact healthy youth [17–19,21] and apparently impact pediatric cancer survivors as well.
As an example of how this information might impact intervention development, work with healthy children and adolescents has successfully increased PA through social support [17,23]. Interventions with child and adolescent survivors might similarly target family and peer support, and survivor self-efficacy, to increase PA levels among pediatric cancer survivors. Such an intervention could be implemented immediately upon completion of medical treatment, could include peer support from fellow survivors, and could have immense impact on cognitive and social factors that subsequently improve survivor PA levels. Intervening immediately following treatment completion and prior to the development of long-term physical performance limitations found in adult survivors of childhood cancer [50, 51] may provide survivors with the skills necessary to maintain physical activity levels despite the onset of physical late effects and may serve as a protective factor against the development of some of these late effects.
Several limitations of the present study should be considered. First, while the directions of our models are based on past theoretical and empirical work, cross-sectional mediation models limit our ability to infer causality. Second, survivor PA was based on self-report telephone survey data. While the GLTEQ has demonstrated concurrent validity against objective measures of PA in healthy children and adults, there is potential for over-estimation of actual activity. Use of a telephone survey may also have introduced over-estimation of actual activity due to socially desirable answering [52]. Third, while family support for physical activity assessed the amount that family members engaged in PA with the survivor, it did not assess the amount that others engaged in PA not in the presence of the survivor and thus may not have captured the full effect of behavioral modeling. Fourth, the study was conducted during the fall, winter, and early spring, and as such may not capture the influence of warmer months that may alter PA levels. Finally, despite the inclusion of a heterogeneous sample of child and adolescent survivors, subsets of some individual diagnostic or treatment categories (i.e., osteosarcoma, transplant recipients) were small and thus we were unable to evaluate models based on these factors. Of course, the lack of significance of treatment-related effects in both models suggests diagnostic and treatment-related variables may not influence survivor PA.
Overall, our findings indicate self-efficacy mediates relations between social support and child and adolescent survivors’ PA. In addition, after controlling for self-efficacy, social support maintains direct influences on survivor PA. Future interventions should target family and peer support as well as survivor self-efficacy to increase PA behaviors in pediatric cancer survivors.
Acknowledgments
This work has been supported in part by the National Cancer Institute sponsored Cancer Prevention and Control Training Program, Grant No. P30 CA13148-39 and R25 CA047888.
References
- 1.Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidem Biomar. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Weiner SL, Simone JV. Childhood cancer survivorship: Improving care and quality of life. National Academics Press; Washington, D.C: 2003. [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. New Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, Yasui Y, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The childhood cancer survivor study. J Natl Cancer I. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCI. National Cancer Institute FactSheet: Physical activity and cancer. 2009 Retrieved from http://www.cancer.gov/cancertopics/factsheet/prevention/physicalactivity.
- 6.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA-Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 7.Wolin KY, Ruiz JR, Tuchman H, Lucia A. Exercise in adult and pediatric hematological cancer survivors: An intervention review. Leukemia. 2010;24:1113–1120. doi: 10.1038/leu.2010.54. [DOI] [PubMed] [Google Scholar]
- 8.San Juan AF, Wolin K, Lucia A. Physical activity and pediatric cancer Survivorship. Recent Res Cancer. 2011;186:319–347. doi: 10.1007/978-3-642-04231-7_14. [DOI] [PubMed] [Google Scholar]
- 9.Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: A review of the literature. Ann Behav Med. 2010;39:232–249. doi: 10.1007/s12160-010-9192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter C, Muller C, Hoffmann C, Boos J, Rosenbaum D. Physical activity and childhood cancer. Pediatr Blood Cancer. 2010;54:501–510. doi: 10.1002/pbc.22271. [DOI] [PubMed] [Google Scholar]
- 11.San Juan AF, Fleck SJ, Chamorro-Vina C, Mate-Munoz JL, Moral S, Perez M, et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med Sci Sport Exer. 2007;39:13–21. doi: 10.1249/01.mss.0000240326.54147.fc. [DOI] [PubMed] [Google Scholar]
- 12.Castellino SM, Casillas J, Hudson MM, Mertens AC, Whitton J, Brooks SL, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 13.Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: Targets for intervention. Cancer. 2009;115:642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnegan L, Wilkie DJ, Wilbur J, Campbell RT, Zong S, Katula S. Correlates of physical activity in young adult survivors of childhood cancers. Oncol Nurs Forum. 2007;34:E60–E69. doi: 10.1188/07.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- 15.Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidem Biomar. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 16.Keats MR, Culos-Reed N, Courneya KS, McBride M. Understanding physical activity in adolescent cancer survivors: An application of the theory of planned behavior. Psycho-oncology. 2007;16:448–457. doi: 10.1002/pon.1075. [DOI] [PubMed] [Google Scholar]
- 17.Lubans DR, Foster C, Biddle SJH. A review of mediators of behavior in interventions to promote physical activity among children and adolescents. Prev Med. 2008;47:463–470. doi: 10.1016/j.ypmed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sport Exer. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Heitzler CD, Lytle LA, Erickson DJ, Barr-Anderson D, Sirard JR, Story M. Evaluating a model of youth physical activity. Am J Health Behav. 2010;34:593–606. doi: 10.5993/ajhb.34.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilliam MB, Schwebel DC. Physical activity in child and adolescent cancer survivors: A review. Health Psychology Review. 2011:iFirst article:1–19. doi: 10.1080/17437199.2011.603641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields CA, Spink KS, Chad K, Muhajarine N, Humbert L, Odnokon P. Youth and adolescent physical activity lapsers: Examining self-efficacy as a mediator of the relationship between family social influence and physical activity. J Health Psychol. 2008;13:121–130. doi: 10.1177/1359105307084317. [DOI] [PubMed] [Google Scholar]
- 22.USDHHS. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 23.Dishman RK, Motl RW, Saunders RP, Felton G, Ward DS, Dowda M, Pate RR. Self-efficacy partially mediates the effect of a school-based physical-activity intervention among adolescent girls. Prev Med. 2004;38:628–636. doi: 10.1016/j.ypmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefriend W, Schwebel DC. Social, demographic, and medical influences on physical activity in child and adolescent cancer survivors. J Pediatr Psychol. 2011 doi: 10.1093/jpepsy/jsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustafson SL, Rhodes RE. Parental correlates of physical activity in children and early adolescents. Sports Med. 2006;36:79–97. doi: 10.2165/00007256-200636010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Heitzler CD, Martin SL, Duke J, Huhman M. Correlates of physical activity in a national sample of children aged 9–13 years. Prev Med. 2006;42:254–260. doi: 10.1016/j.ypmed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Whitt-Glover MC, Taylor WC, Floyd MF, Yore MM, Yancey AK, Matthews CE. Disparities in physical activity and sedentary behaviors among U.S. children and adolescents: Prevalence, correlates, and intervention implications. J Public Health Pol. 2009;30:S309–334. doi: 10.1057/jphp.2008.46. [DOI] [PubMed] [Google Scholar]
- 28.Strauss RS, Rodzilsky D, Burack G, Colin M. Psychosocial correlates of physical activity in healthy children. Arch Pediat Adol Med. 2001;155:897–902. doi: 10.1001/archpedi.155.8.897. [DOI] [PubMed] [Google Scholar]
- 29.van der Horst K, Chin A, Paw MJ, Twisk JWR, van Mechelen W. A brief review on correlates of physical activity and sedentariness in youth. Med Sci Sport Exer. 2007;39:1241–1250. doi: 10.1249/mss.0b013e318059bf35. [DOI] [PubMed] [Google Scholar]
- 30.Fein AJ, Plotnikoff R, Wild CT, Spence JC. Perceived environment and physical activity in youth. Int J Behav Med. 2004;11:135–142. doi: 10.1207/s15327558ijbm1103_2. [DOI] [PubMed] [Google Scholar]
- 31.Keats MR, Culos-Reed N. A theory driven approach to encourage physical activity in pediatric cancer survivors: A pilot study. J Sport Exercise Psy. 2009;31:267–283. doi: 10.1123/jsep.31.2.267. [DOI] [PubMed] [Google Scholar]
- 32.Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Demark-Wahnefried, W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum. 2008;35:121–130. doi: 10.1188/08.ONF.121-130. [DOI] [PubMed] [Google Scholar]
- 33.Zakarian JM, Hovell MF, Hofstetter CR, Sallis JF, Keating KJ. Correlates of vigorous exercise in a predominantly low SES and minority high school population. Prev Med. 1994;23:314–321. doi: 10.1006/pmed.1994.1044. [DOI] [PubMed] [Google Scholar]
- 34.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0. National Cancer Institute; Bethesda, MD: 2003. [Google Scholar]
- 35.Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sport Exer. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sport Exer. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using Caltrac accelerometer and five questionnaires. Med Sci Sport Exer. 1994;26:376–382. [PubMed] [Google Scholar]
- 39.Norris JM, Moules NJ, Pelletier G, Culos-Reed SN. Families of young pediatric cancer survivors: A cross-sectional survey examining physical activity level and health-related quality of life. J Pediatr Oncol Nurs. 2010;27:196–208. doi: 10.1177/1043454209358411. [DOI] [PubMed] [Google Scholar]
- 40.Taylor WC, Sallis JF, Dowda M, Freedson PS, Eason K, Pate RR. Activity patterns and correlates among youth: Differences by weight status. Pediatr Exerc Sci. 2002;14:418–431. [Google Scholar]
- 41.Sallis JF, Taylor WC, Dowda M, Freedson PS, Pate RR. Correlates of vigorous physical activity for children in grades 1 through 12. Comparing parent-reported and objectively measured physical activity. Pediatr Exerc Sci. 2002;14:30–44. [Google Scholar]
- 42.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Meth. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 43.Preacher KJ, Hayes A. SPSS INDIRECT macro syntax reference. Retrieved June 5, 2011, from http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html.
- 44.Mallinckrodt B, Abraham W, Wei M, Russell D. Advances in testing the statistical significance of mediation effects. J Couns Psychol. 2006;53:372–378. [Google Scholar]
- 45.Williams J, MacKinnon D. Resampling and distribution of the product methods for testing indirect effects in complex models. Struct Equ Modeling. 2008;15:23–51. doi: 10.1080/10705510701758166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKinnon D. Contrasts in multiple mediator models. In: Rose J, Chassin L, Presson C, Sherman S, editors. Multivariate applications in substance use research. Mahwah; Erlbaum: 2002. pp. 141–160. [Google Scholar]
- 47.Shrout PE, Bolger N. Mediation in experimental and non-experimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 48.Davis AM, Bennett KJ, Befort C, Nollen N. Obesity and related health behaviors among urban and rural children in the United States: Data from the National Health and Nutrition Examination Survey 2003–204 and 2005–2006. J Pediatr Psychol. 2011;36:669–676. doi: 10.1093/jpepsy/jsq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welk GJ, Wood K, Morss G. Parental influences on physical activity in children: An exploration of potential mechanisms. Pediatr Exerc Sci. 2003;15:19–33. [Google Scholar]
- 50.Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143:639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 51.Ness KK, Hudson MM, Ginsberg JP, Nagarajan R, Kaste SC, Marina N. Physical performance limitations in the childhood cancer survivor study cohort. J Clin Oncol. 2009;27:2382–2389. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27:281–291. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]