Abstract
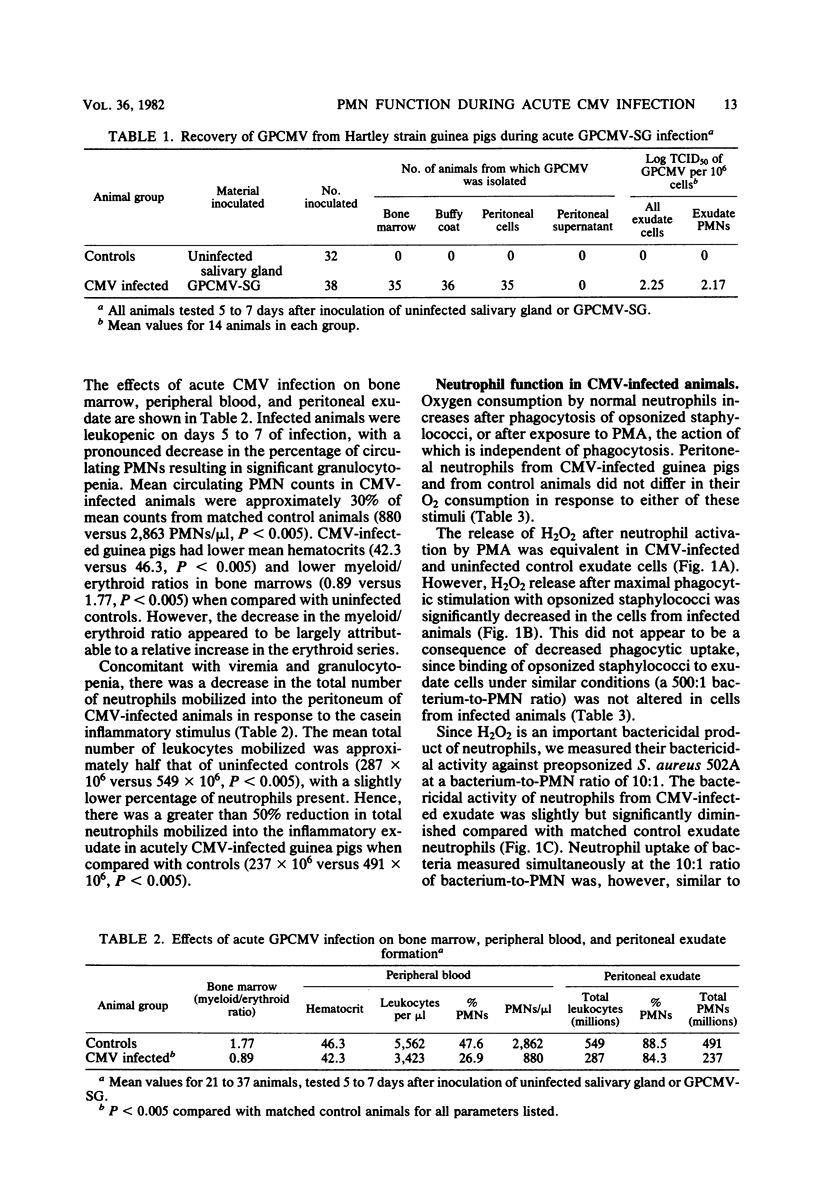
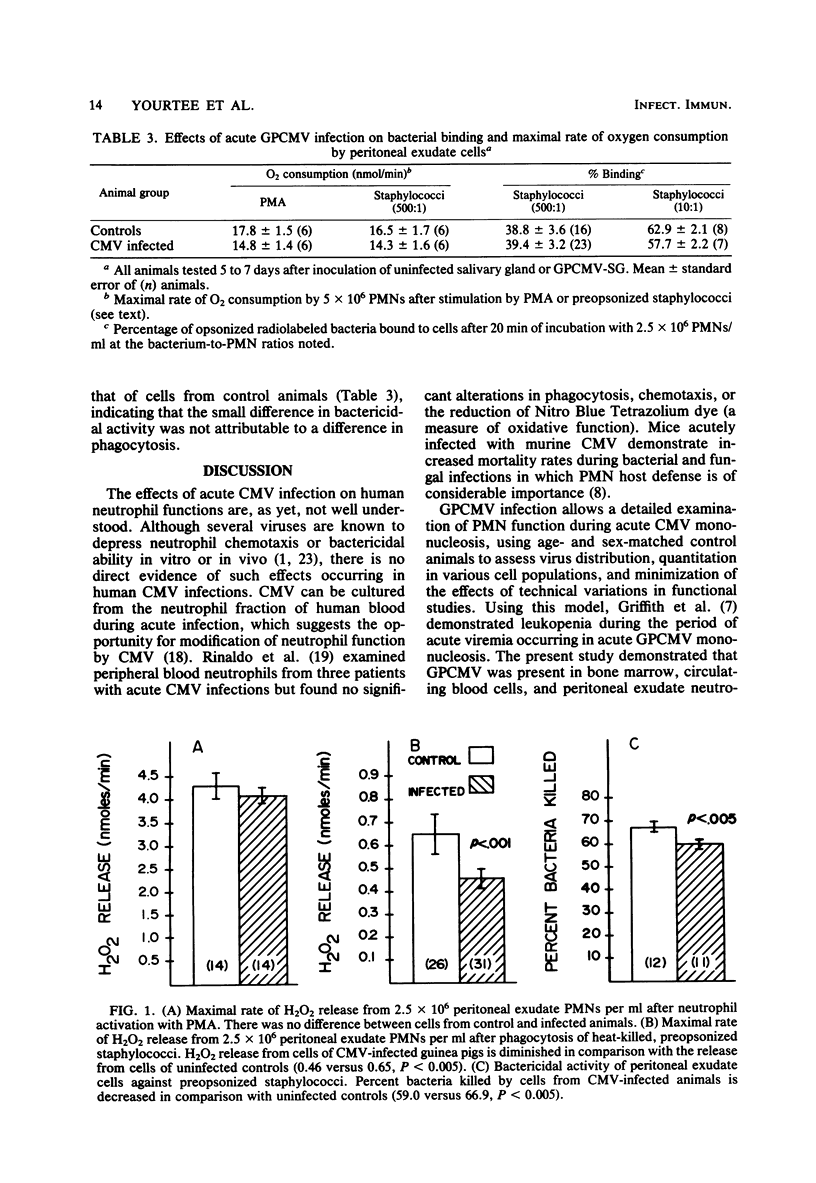
The mobilization and functional characteristics of polymorphonuclear leukocytes (PMNs) at the site of an inflammatory stimulus were studied during acute cytomegalovirus infection in guinea pigs. Weanling Hartley strain guinea pigs were inoculated subcutaneously with approximately 10(6) 50% tissue culture infective doses of virulent salivary gland-passaged guinea pig cytomegalovirus. The virus was uniformly present in bone marrow, buffy coat, and casein-elicited peritoneal exudate cells 5 to 7 days after the inoculation. The mean numbers of circulating PMNs in the animals were 2,862/microliters in uninfected controls and 880/microliters in infected animals. The total peritoneal PMNs recovered 14 h after casein injection were 491 X 10(6) and 237 X 10(6) in control and infected animals, respectively. The number of 50% tissue culture infective doses of guinea pig cytomegalovirus per 10(6) purified peritoneal PMNs was 10(2.17). Neutrophil O2 consumption was similar in infected and control animals in response to either stimulation by a neutrophil activator, phorbol myristate acetate, or phagocytosis of Staphylococcus aureus. However, the maximal rate of H2O2 release and the percent killing of S. aureus by peritoneal exudate cells from infected animals were significantly reduced compared with uninfected controls during acute infection. Granulocytopenia, a decreased mobilization of PMNs to the site of the inflammatory stimulus, a diminished cellular release of H2O2 in response to a bacterial stimulus, and a modest reduction in bacterial killing were demonstrated during experimental acute cytomegalovirus infection. Such reductions in granulocyte number and function at inflammatory sites during this type of infection could alter antimicrobial defenses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Rabson A. R., Sher R., Koornhof H. J. Defective neutrophil motility in children with measles. J Pediatr. 1976 Jul;89(1):27–32. doi: 10.1016/s0022-3476(76)80921-3. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Griffith B. P., Tarsio M., Hsiung G. D. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis. 1980 Nov;142(5):732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Hastings K., Hsiung G. D. Cytomegalovirus infection in guinea pigs. III. Persistent viruria, blood transmission, and viral interference. J Infect Dis. 1979 Dec;140(6):914–920. doi: 10.1093/infdis/140.6.914. [DOI] [PubMed] [Google Scholar]
- Choi Y. C., Hsiung G. D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978 Aug;138(2):197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Griffith B. P., Lucia H. L., Bia F. J., Hsiung G. D. Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun. 1981 May;32(2):857–863. doi: 10.1128/iai.32.2.857-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. B., Jr, ROWLANDS D. T., Jr, RIFKIND D. INFECTIOUS PULMONARY DISEASE IN PATIENTS RECEIVING IMMUNOSUPPRESSIVE THERAPY FOR ORGAN TRANSPLANTATION. N Engl J Med. 1964 Nov 12;271:1021–1027. doi: 10.1056/NEJM196411122712001. [DOI] [PubMed] [Google Scholar]
- Hamilton J. R., Overall J. C., Glasgow L. A. Synergistic effect on mortality in mice with murine cytomegalovirus and Pseudomonas aeruginosa, Staphylococcus aureus, or Candida albicans infections. Infect Immun. 1976 Oct;14(4):982–989. doi: 10.1128/iai.14.4.982-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. B., Djerassi I., Schwartz E., Root R. K. Polymorphonuclear leukocytes prepared by continuous-flow filtration leukapheresis: viability and function. Blood. 1974 Nov;44(5):707–713. [PubMed] [Google Scholar]
- Hsiung G. D., Choi Y. C., Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978 Aug;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey D. K., Olsen G. A., Overall J. C., Jr, Glasgow L. A. Alteration of host defense mechanisms by murine cytomegalovirus infection. Infect Immun. 1977 Dec;18(3):754–760. doi: 10.1128/iai.18.3.754-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E., Reeves W., Ray G., Flournoy N., Lerner K. G., Sale G. E., Thomas E. D. A prospective analysis interstitial pneumonia and opportunistic viral infection among recipients of allogeneic bone marrow grafts. J Infect Dis. 1977 Dec;136(6):754–767. doi: 10.1093/infdis/136.6.754. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Balfour H. H., Jr, Marker S. C., Fryd D. S., Howard R. J., Simmons R. L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 1980 Jul;59(4):283–300. [PubMed] [Google Scholar]
- Rand K. H., Pollard R. B., Merigan T. C. Increased pulmonary superinfections in cardiac-transplant patients undergoing primary cytomegalovirus infection. N Engl J Med. 1978 Apr 27;298(17):951–953. doi: 10.1056/NEJM197804272981705. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Black P. H., Hirsch M. S. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis. 1977 Nov;136(5):667–678. doi: 10.1093/infdis/136.5.667. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Stossel T. P., Black P. H., Hirsch M. S. Polymorphonuclear leukocyte function during cytomegalovirus mononucleosis. Clin Immunol Immunopathol. 1979 Mar;12(3):331–334. doi: 10.1016/0090-1229(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Tolkoff-Rubin N. E., Russell P. S., Hirsch M. S. Infectious disease syndromes attributable to cytomegalovirus and their significance among renal transplant recipients. Transplantation. 1977 Dec;24(6):458–464. doi: 10.1097/00007890-197712000-00010. [DOI] [PubMed] [Google Scholar]
- Ruutu P. Depression of rat neutrophil exudation and motility by influenza virus. Scand J Immunol. 1977;6(11):1113–1120. doi: 10.1111/j.1365-3083.1977.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Schleupner C. J., Glasgow L. A. Peritoneal macrophage activation indicated by enhanced chemiluminescence. Infect Immun. 1978 Sep;21(3):886–895. doi: 10.1128/iai.21.3.886-895.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M., McIntyre P. A. The requirement for membrane sialic acid in the stimulation of superoxide production during phagocytosis by human polymorphonuclear leukocytes. J Exp Med. 1976 Jun 1;143(6):1308–1316. doi: 10.1084/jem.143.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]


