Abstract
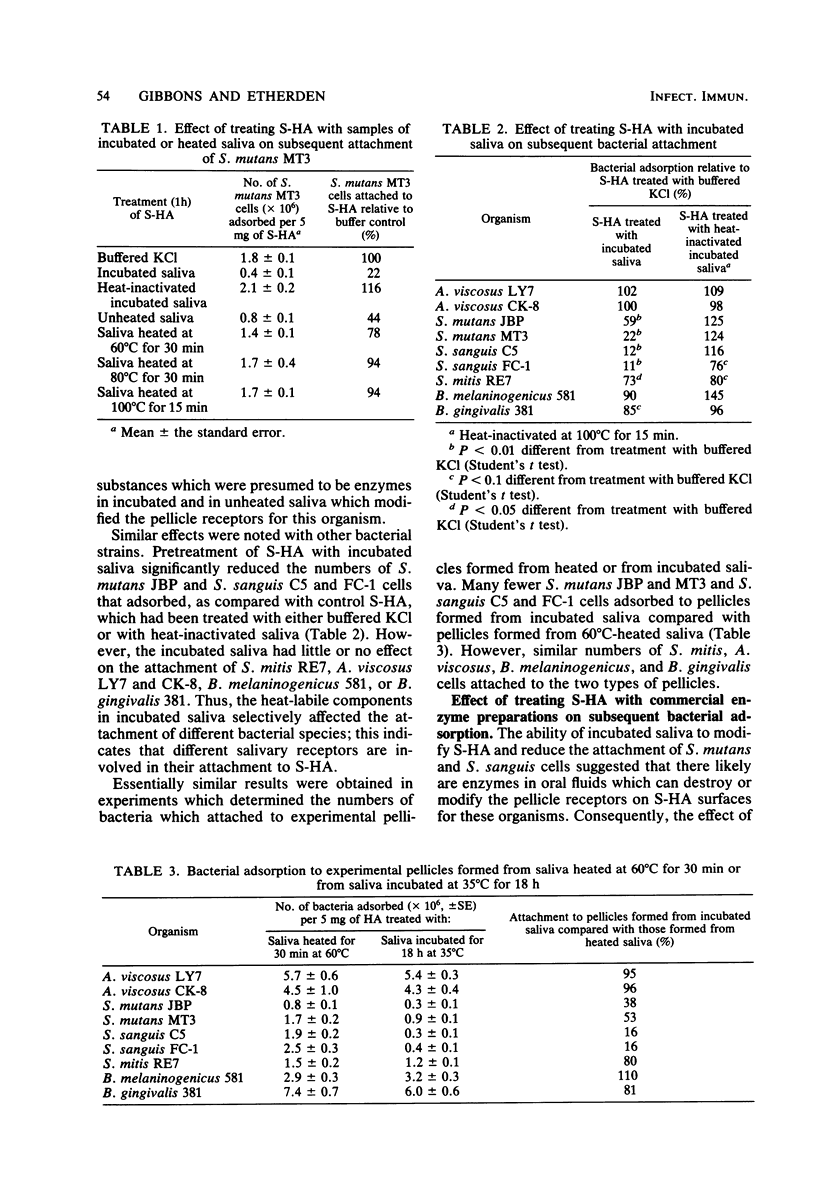
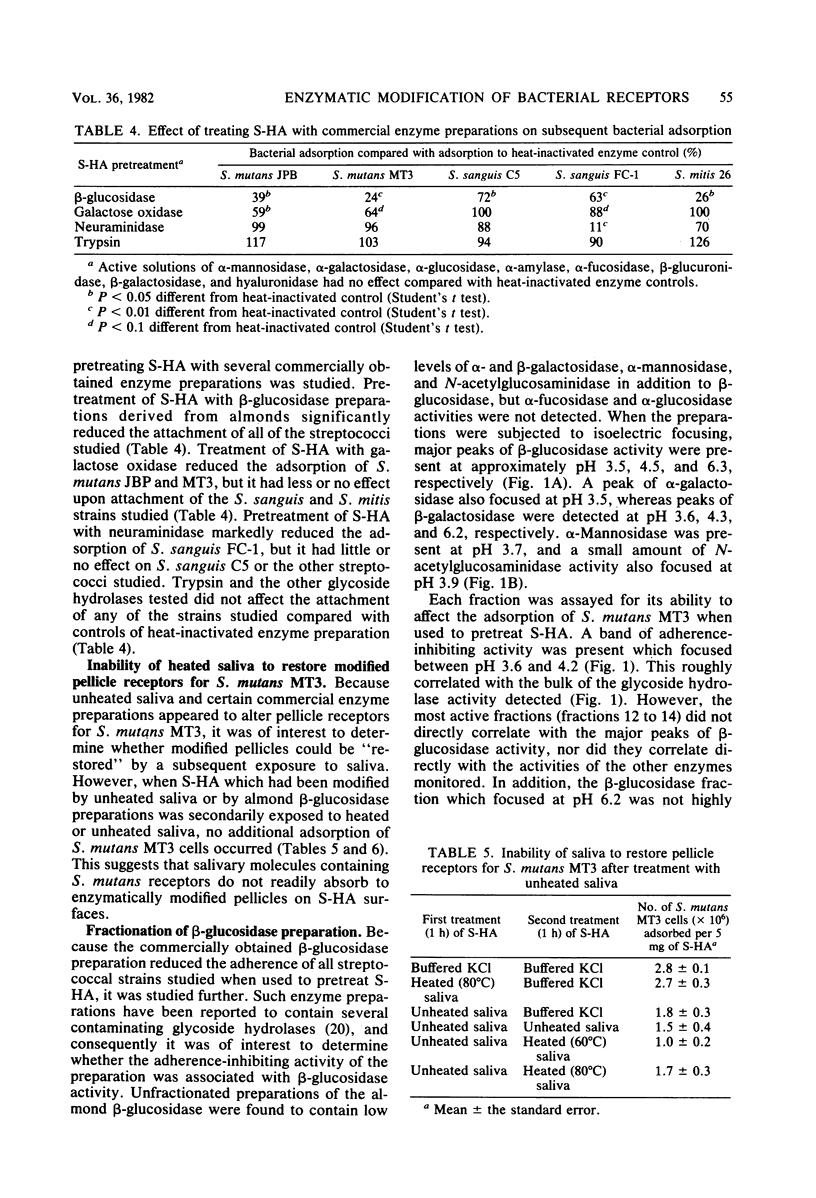
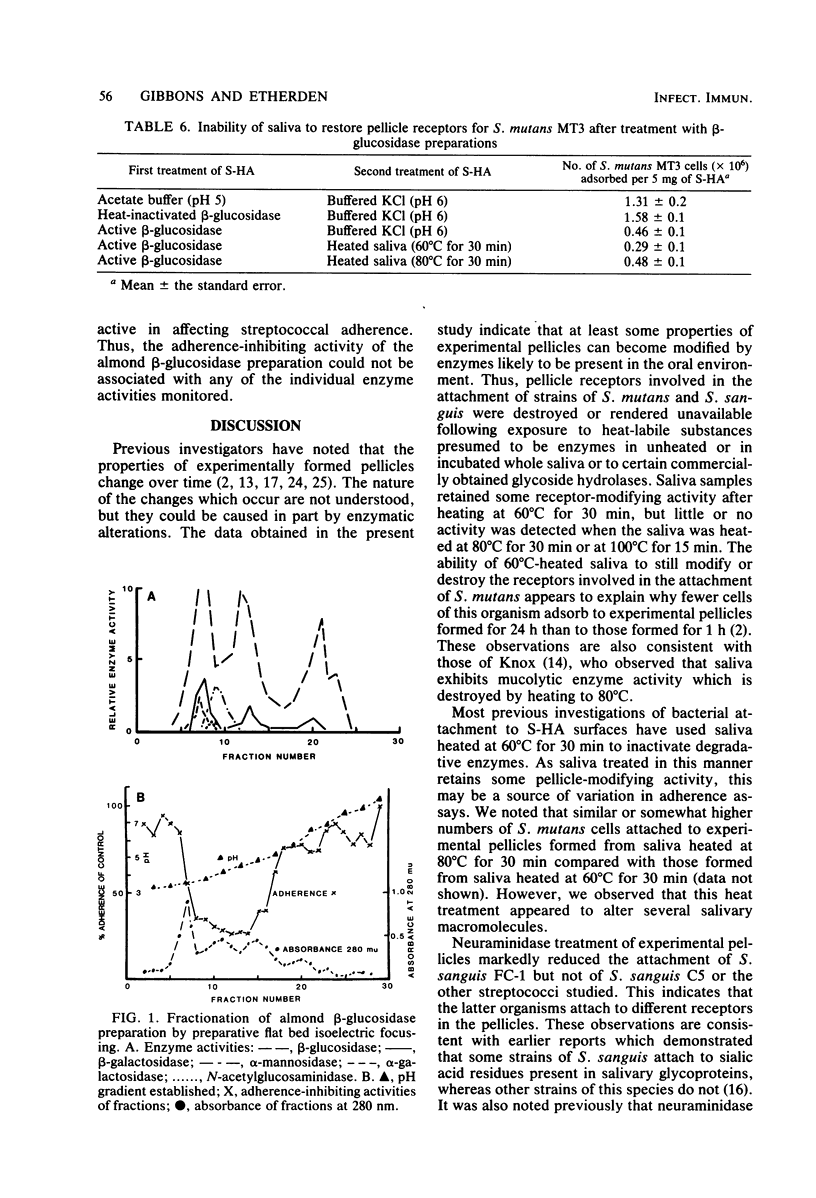
Certain properties of experimental pellicles formed by the adsorption of salivary components on hydroxyapatite surfaces change over time. To determine whether enzymes likely to be present in the oral environment could induce such changes, pellicles were treated with saliva which had been incubated for 18 h at 35°C to promote the elaboration of microbial enzymes. This treatment markedly reduced the numbers of Streptococcus mutans MT3 and JBP and S. sanguis FC-1 and C5 cells which attached, but it had little or no effect on the attachment of S. mitis RE7, Actinomyces viscosus LY7 and CK-8, Bacteroides gingivalis 381, or B. melaninogenicus subsp. intermedius 581. Heating the incubated saliva at 60°C for 30 min partially reduced its pellicle-modifying activity, whereas heating at 80°C for 30 min or 100°C for 15 min completely eliminated such activity. This indicated that the saliva contained heat-labile substances, presumably enzymes, which could affect the pellicle receptors involved in the attachment of S. mutans and S. sanguis. Treatment of saliva-treated hydroxyapatite with commercially obtained enzyme preparations also affected bacterial attachment. Thus, treatment with galactose oxidase reduced the numbers of the S. mutans strains which attached, whereas treatment with neuraminidase reduced the adsorption of S. sanguis FC-1 but not that of S. sanguis C5. Treatment with β-glucosidase preparations derived from almonds significantly reduced the attachment of all of the streptococcal strains studied, but, when subjected to isoelectric fractionation, the adherence-inhibiting activity did not correlate directly with β-glucosidase activity. Treatment of the pellicles with trypsin or eight other glycosidases did not affect streptococcal attachment. Exposure of the enzymatically modified pellicles to fresh saliva did not restore the streptococcal receptors. Collectively, the data suggest that some bacterial receptors in the pellicle coating of teeth can be modified by enzymes likely to be present in the oral environment, and these interactions may affect oral bacterial ecology.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman R. A., Schaefer S. J., Fitzgerald R. J., Rosner D., Shklair I. L., Walter R. G. Differential utilization of proteins in saliva from caries-active and caries-free subjects as growth substrates by plaque-forming streptococci. J Dent Res. 1979 Oct;58(10):2019–2027. doi: 10.1177/00220345790580101501. [DOI] [PubMed] [Google Scholar]
- EICHEL B., LISANTI V. F. LEUCOCYTE METABOLISM IN HUMAN SALIVA. Arch Oral Biol. 1964 May-Jun;9:299–314. doi: 10.1016/0003-9969(64)90062-7. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M., Johnson G. L. Proteolytic activity of Candida albicans: action on human salivary proteins. Infect Immun. 1978 Dec;22(3):861–866. doi: 10.1128/iai.22.3.861-866.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Dankers I. Lectin-like constituents of foods which react with components of serum, saliva, and Streptococcus mutans. Appl Environ Microbiol. 1981 Apr;41(4):880–888. doi: 10.1128/aem.41.4.880-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- KNOX K. W. Observations on the action of mucolytic enzymes on salivary mucoid. J Dent Res. 1953 Jun;32(3):374–378. doi: 10.1177/00220345530320031101. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey C., Stowell E. C. The adsorption of human salivary proteins and porcine submaxillary mucin by hydroxyapatite. Arch Oral Biol. 1967 Jul;12(7):815–828. doi: 10.1016/0003-9969(67)90104-5. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Linder L., Wadström T., Lindberg A. A. Formation of glycoside-hydrolases by oral streptococci. Arch Oral Biol. 1973 Mar;18(3):391–402. doi: 10.1016/0003-9969(73)90163-5. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Bahn A. N. Carbohydrate hydrolases of oral streptococci. Arch Oral Biol. 1969 Jul;14(7):735–744. doi: 10.1016/0003-9969(69)90165-4. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J., DALE A. C., BORTNICK L., ROSENTHAL E., MACDONALD J. B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol. 1963 May-Jun;8:275–280. doi: 10.1016/0003-9969(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Sloan J., Lee Y. C. Mannosidase, glucosidase, and galactosidase in sweet almond emulsin. Arch Biochem Biophys. 1970 Mar;137(1):122–127. doi: 10.1016/0003-9861(70)90418-2. [DOI] [PubMed] [Google Scholar]
- Shizukuishi S., Taniguchi T., Shibata S., Nakamura R., Tsunemitsu A., Uesugi Y. Changes of serological activity by alpha-L-fucosidase isolated from Streptococcus sanguis ATCC 10557. J Dent Res. 1979 Feb;58(2):656–659. doi: 10.1177/00220345790580022101. [DOI] [PubMed] [Google Scholar]
- WRIGHT D. E. THE SOURCE AND RATE OF ENTRY OF LEUCOCYTES IN THE HUMAN MOUTH. Arch Oral Biol. 1964 May-Jun;9:321–329. doi: 10.1016/0003-9969(64)90064-0. [DOI] [PubMed] [Google Scholar]
- Zahradnik R. T., Moreno E. C., Burke E. J. Effect of salivary pellicle on enamel subsurface demineralization in vitro. J Dent Res. 1976 Jul-Aug;55(4):664–670. doi: 10.1177/00220345760550042101. [DOI] [PubMed] [Google Scholar]
- Zahradnik R. T., Propas D., Moreno E. C. Effect of salivary pellicle formation time on in vitro attachment and demineralization by Streptococcus mutans. J Dent Res. 1978 Nov-Dec;57(11-12):1036–1042. doi: 10.1177/00220345780570110601. [DOI] [PubMed] [Google Scholar]


