Abstract
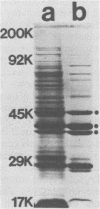
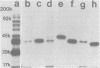
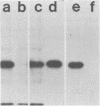
Monoclonal antibodies directed against several different Haemophilus influenzae type b outer membrane proteins with apparent molecular weights of 45,000, 39,000, and 37,000 were identified by a radioimmunoprecipitation method. Five monoclonal antibodies, including both immunoglobulin G and M isotypes, were specific for the same H. influenzae type b major outer membrane protein (39,000 molecular weight). One of these immunoglobulin G monoclonal antibodies (6A2) was shown to be directed against a cell surface-exposed antigenic determinant of the 39,000-molecular-weight protein, whereas the other monoclonal antibodies directed against this same protein were apparently specific for antigenic determinants not exposed on the H. Influenzae type b cell surface. The cell surface-exposed protein antigenic determinant recognized by monoclonal antibody 6A2 was not unique to the H. influenzae type b strain used as the source of outer membrane vesicles for generating immune spleen cells, but was found in a majority of independently isolated strains of H. influenzae type b. These data indicate that there is antigenic cross-reactivity among H. influenzae type b strains with regard to cell surface-exposed proteins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. The use of monoclonal antibodies in the study of lactose-sensitive adherence of Actinomyces viscosus T14V. J Reticuloendothel Soc. 1980 Dec;28(Suppl):73s–79s. [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoff S. P. On the surface of Haemophilus influenzae. J Infect Dis. 1981 May;143(5):747–748. doi: 10.1093/infdis/143.5.747. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Rockwell R. Experimental Haemophilus influenzae type b meningitis: immunological investigation of the infant rat model. Infect Immun. 1978 Jun;20(3):705–713. doi: 10.1128/iai.20.3.705-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripenberg M., Nissinen A., Väisänen E., Linder E. Demonstration of antibodies against Yersinia enterocolitica lipopolysaccharide in human sera by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Sep;10(3):279–284. doi: 10.1128/jcm.10.3.279-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H. Antigenic diversity of the serotype antigen complex of Neisseria gonorrhoeae: analysis by an indirect enzyme-linked immunoassay. Infect Immun. 1980 Apr;28(1):101–110. doi: 10.1128/iai.28.1.101-110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Norden C. W. Further studies on the immunology of the infant rat experimental model of Haemophilus influenzae type B meningitis. Br J Exp Pathol. 1978 Oct;59(5):544–550. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Cannon J. G., Mittler R. S. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun. 1981 May;32(2):641–648. doi: 10.1128/iai.32.2.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin R. A., Kennett R. Use of monoclonal antibodies in an enzyme immunoassay for rapid identification of group B Streptococcus types II and III. J Clin Microbiol. 1980 Apr;11(4):332–336. doi: 10.1128/jcm.11.4.332-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Wilkins J., Williams F. F., Wehrle P. F., Portnoy B. Agglutinin response to pertussis vaccine. I. Effect of dosage and interval. J Pediatr. 1971 Aug;79(2):197–202. doi: 10.1016/s0022-3476(71)80101-4. [DOI] [PubMed] [Google Scholar]