Abstract
Background
Upregulated by atheroprotective flow, the transcription factor Krüppel-like factor 2 (KLF2) is crucial for maintaining endothelial function. MicroRNAs (miRNAs) are non-coding small RNAs that regulate gene expression at the post-transcriptional level. We examined the role of miRNAs, particularly miR-92a, in the atheroprotective flow-regulated KLF2.
Methods and Results
Dicer knockdown increased the level of KLF2 mRNA in human umbilical vein endothelial cells (HUVECs), suggesting that KLF2 is regulated by miRNA. In silico analysis predicted that miR-92a could bind to the 3’ untranslated region (3’UTR) of KLF2 mRNA. Overexpression of miR-92a precursor (pre-92a) decreased the expression of KLF2 and the KLF2-regulated endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM) at mRNA and protein levels. A complementary finding is that miR-92a inhibitor (anti-92a) increased the mRNA and protein expression of KLF2, eNOS, and TM. Subsequent studies revealed that atheroprotective laminar flow downregulated the level of miR-92a to induce KLF2 and the level of this flow-induced KLF2 was reduced by pre-92a. Furthermore, miR-92a level was lower in HUVECs exposed to the atheroprotective pulsatile shear flow (PS) than under atheroprone oscillatory shear flow. Anti-Ago1/2 immunopreciptation coupled with RT-PCR revealed that PS decreased the functional targeting of miR-92a/KLF2 mRNA in HUVECs. Consistent with these findings, mouse carotid arteries receiving pre-92a exhibited impaired vasodilatory response to flow.
Conclusions
Atheroprotective flow patterns decrease the level of miR-92a, which in turn increases KLF2 expression to maintain endothelial homeostasis.
Keywords: shear stress, microRNAs, KLF2, vasodilation, endothelial cells
Introduction
The vascular endothelium, located at the interface between the circulating blood and the vessel wall, is exposed to shear stress resulting from blood flow. The endothelium in straight parts of the artery tree is subjected to pulsatile shear stress with a significant forward direction, which is an important physiological stimulus enhancing vessel compliance and conferring anti-thrombotic, -adhesive, and -inflammatory effects. In contrast, disturbed flow patterns at the arterial bifurcations and curvatures can cause endothelial dysfunction, which initiates atherosclerosis1–4.
The transcription factor Krüppel-like factor 2 (KLF2) is a critical integrator for endothelial lineage development and vascular functions (reviewed in references 5–7). KLF2 can be highly induced by shear stress with a significant forward direction (whether steady laminar flow or pulsatile flow), as well as HMG-CoA reductase inhibitors (i.e., statins). Previous studies have shown that KLF2 can be induced by shear stress at both transcriptional and posttranscriptional levels. The induction of KLF2 mRNA by laminar shear stress has been suggested to be mediated through the MEK5/ERK5/MEF2 pathway, which is AMPK dependent8–13. Shear stress increases the stability of KLF2 mRNA, with attendant elevation of KLF2 protein14. The molecular basis of such an increased stability of KLF2 mRNA has not been established.
MicroRNAs (miRNAs) are non-coding small RNAs that regulate gene expression at the post-transcriptional level15–17. Ranging from 18 to 24 nt (22 nt in general), miRNAs bind to the 3’ untranslated region (3’UTR) of their target mRNAs, with ensuing suppression of protein translation or enhancement of mRNA degradation. Analysis of miRNAs expressed in arterial walls and cultured vascular endothelial cells (ECs) has shown that approximately 40 miRNAs are highly expressed in ECs18–20. These miRNAs play important roles in regulating blood vessel 4 development, wound healing, redox signaling, inflammatory responses, and angiogenesis (reviewed by reference 21). Using miRNA microarrays, we and others have shown that laminar shear stress upregulates a set of miRNAs in ECs in vitro and in vivo22–25. Functionally, miR-21 increases NO bioavailability and reduces EC apoptosis, miR-19a and miR-23b regulate EC proliferation22–24, and miR-10a is anti-proinflammatory25. Several recent reports demonstrated that the miR-17~92 cluster regulates cardiac development, EC proliferation, and angiogenesis (reviewed by reference 26). In this cluster, miR-92a was the first miRNA identified to regulate angiogenesis. Loss- and gain-of-function experiments showed that miR-92a inhibited angiogenesis in vitro and in vivo27. Importantly, miR-92a overexpression in human umbilical vein ECs (HUVECs) suppressed the expression of several KLF2-regulated genes such as endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM)27. The lack of miR-92a binding sites in the 3’UTR of these genes suggests that a mechanism other than direct targeting of miR-92a is involved.
Here, we report that atheroprotective flow causes downregulation of miR-92a in ECs, which in turn elevates the KLF2 mRNA. The functional consequences of the flow-regulated miR-92a/KLF2, including the augmentation of eNOS and TM levels and increase of NO bioavailability, can be mimicked by inhibition of miR-92a. These findings suggest a new paradigm of mechanotransduction that involves the shear modulation of miRNAs, KLF2 expression, and vascular homeostasis.
Materials and Methods
miRNA and mRNA RT-PCR
Quantitative Real-time PCR (qRT-PCR) was performed to measure the level of miR-92a with the TaqMan miRNA assay kit according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). For the quantification of KLF2, eNOS, and TM mRNA, qRT-PCR was performed with the iQ SYBR Green supermix (Bio-Rad, Hercules, CA).
Knockdown and overexpression of miR-92a and KLF2
miR-92a was knocked down by using anti-92a (Ambion Inc, Austin, TX). T h e overexpression of miR-92a was achieved by transfecting cells with miR-92a precursor (pre-92a) (Ambion). Dicer1 was knocked down by using small interfering RNA (siRNA) obtained from Qiagen (Valencia, CA). The transfections were carried out by the use of lipofectamine 2000 (Invitrogen, Carlsbad, CA). For KLF2 overexpression, Ad-KLF2 was used to infect HUVECs.
Shear stress experiments
A parallel-plate flow system was used to impose shear stress on ECs cultured in flow channels by established methods28. Laminar flow, pulsatile shear flow (PS), and oscillatory shear flow (OS) were applied to ECs with shear stress of 12 dyn/cm2, 12±4 dyn/cm2, and 0±4 dyn/cm2, respectively.
Western blot analysis
Western blot analysis was performed with the use of antibodies against eNOS (Cell Signaling, Beverly, MA), TM (ABcam, Cambridge, MA), KLF2, histone H1 (Santa Cruz Biotechnology, Santa Cruz, CA), and α-tubulin (Sigma, St Louis, MO). The anti-KLF2 antibody was obtained by immunizing rabbits with 2 separate synthetic peptides (CALSEPILPSFST-amide and Ac-ALSEPILPSFST-Ahx-C-amide) corresponding to human KLF2 (21st Century Biochemicals, Marlboro, MA). The antiserum was purified by affinity column and tested by ELISA.
Statistical analysis
Data are expressed as either mean±SD or mean±SEM from at least three independent experiments. Two groups were compared by Student t test. Differences among multiple groups were evaluated by ANOVA followed by the Bonferroni posthoc test or Dunnett’s test with Prism 5 for Windows (GraphPad Software Inc, San Diego, CA). p < 0.05 were considered statistically significant.
The detailed methods for plasmid construction and luciferase assay, immunoprecipitation (IP) of miR-induced silencing complex (miRISC), NO bioavailability assay, flow-induced vasodilation, and computational analysis for KLF2-regulated miRNAs are described in the Supplemental Methods.
Results
miRNAs are involved in the regulation of KLF2 expression
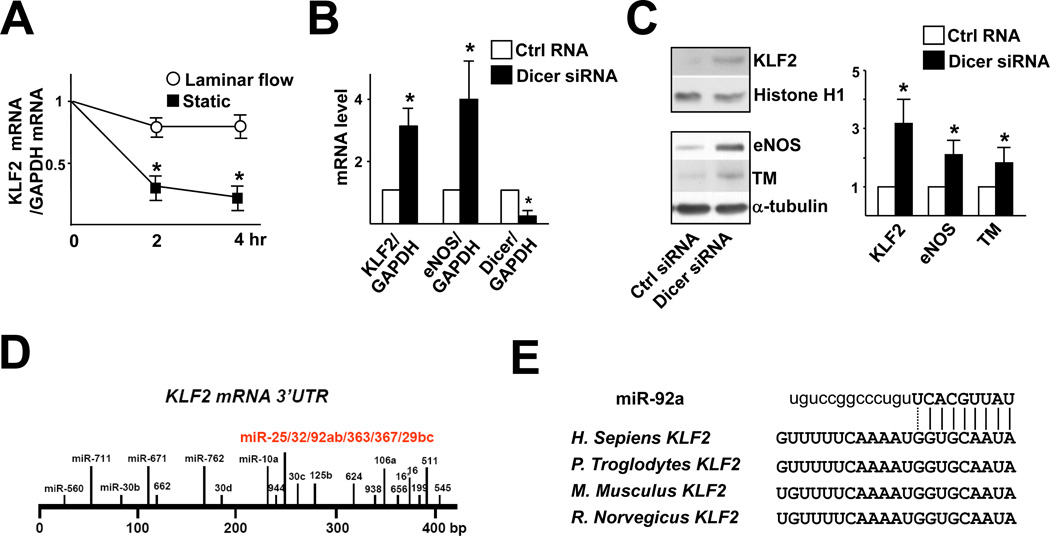
To investigate whether the shear stress-induction of KLF2 can be mediated at the posttranscriptional level, HUVECs were pre-sheared with laminar flow for 6 hr and then treated with 5,6-dichloro-1-β-D-ribobenzimidazole (DRB) to terminate transcription so that KLF2 mRNA stability could be monitored. As shown in Fig. 1A, the degradation rate of KLF2 mRNA in HUVECs exposed to laminar flow was much slower than that under static controls. To explore whether miRNAs are involved in the regulation of KLF2, we knocked down Dicer to block the miRNA biogenesis and found that the levels of KLF2 mRNA and protein increased (Fig. 1B, C). The levels of eNOS and TM, which are downstream targets of KLF2, also increased in ECs with Dicer knockdown (Fig. 1C).
Figure 1. miRNAs are involved in the regulation of KLF2 mRNA.
(A) HUVECs were subjected to laminar flow (12 dyn/cm2) for 6 hr. After the addition of DRB (2 µg/ml), then the cells were continuously exposed to laminar flow or static conditions for additional 2 and 4 hr. The levels of KLF2 and GAPDH mRNAs were measured by qRT-PCR, and the KLF2/GAPDH mRNA ratio is plotted as a percentage of that in the untreated static cells. (B,C) HUVECs were transfected with 20 nM Dicer siRNA or control RNA for 48 hr. qRT-PCR (B) and Western blot analysis (C) were performed to detect the mRNA levels of KLF2 and eNOS and protein levels of KLF2, eNOS, and TM, respectively. Histone H1 served as the internal control of nuclear extracts. The bar graphs are mean±SD from 3 independent experiments. * p<0.05 between Dicer siRNA and control RNA, analyzed by ANOVA followed by Dunnett’s test (A) or Student t test (B,C). (D) The miRNA binding sites in the KLF2 3’UTR are predicted by bioinformatics algorithms. (E) The seed region of miR-92a and its target sequences at the KLF2 3’UTR of several mammalian species: Homo sapiens, Pan troglodytes, Mus Musculus, and Rattus Norvegicus
By using miRanda, microCosm, and TargetScan, we explored the putative miRNA binding sequences at the 3’UTR of KLF2 mRNA. Twenty miRNA binding sites with 45 miRNAs were predicted (Fig. 1D). miRanda, microCosm, and TargetScan predicted, in common, that the segment between 228 to 249 nt contains binding sequences for 8 miRNAs (i.e., miR-25, -32, -92a, -92b, -363, -367, -29a, and -29b). Analysis of the secondary structure of this segment by RNAfold (http://www.tbi.univie.ac.at) revealed that the miRNA binding locus was located in “unstable” regions, characteristic of a miRNA target site. Among these putative 8 miRNAs, miR-29 and miR-92a are highly expressed in ECs18–20. Interestingly, a nucleotide sequence, GGUGCAAUA, complementary to the seed region of miR-92a, is highly conserved among the human, chimpanzee, mouse, and rat KLF2 3’UTR (Fig. 1E).
miR-92a targets KLF2 mRNA
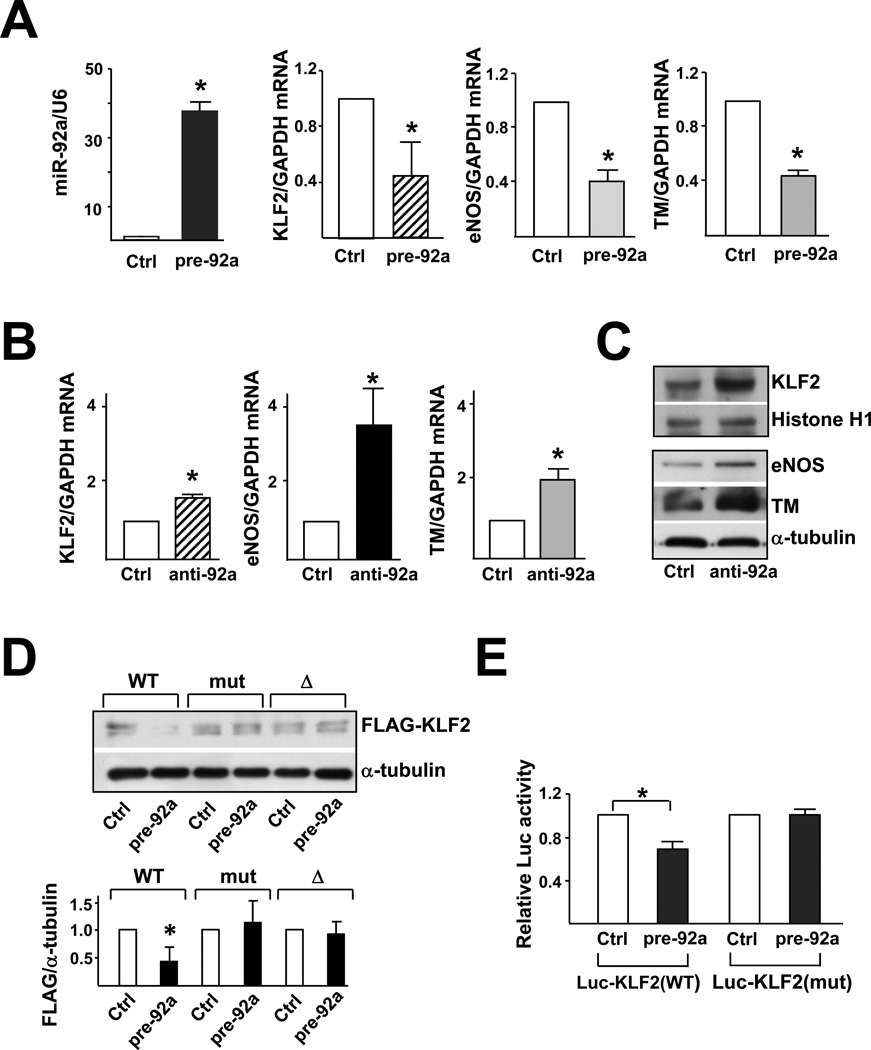
To explore whether miR-92a targets KLF2 mRNA, HUVECs were transfected with pre-92a. qRT-PCR was performed and confirmed the increased expression of miR-92a and decreased level of KLF2 mRNA in pre-92a-transfected cells, as compared with the control RNA-transfected HUVECs (Fig. 2A). Furthermore, the mRNA level of eNOS and TM decreased by ~50%. In complementary experiments, HUVECs transfected with anti-92a exhibited higher levels of KLF2, eNOS, and TM mRNAs (Fig. 2B); and protein (Fig. 2C).
Figure 2. miR-92a targets KLF2 mRNA and decreases KLF2 translation.
HUVECs were transfected with 20 nM miR-92a precursor (pre-92a) or control RNA. At 48 hr, the levels of miR-92a relative to U6 RNA and KLF2, eNOS, and TM mRNA as ratios to GAPDH were assessed by qRT-PCR. In (B) and (C), HUVECs were transfected with 20 nM miR-92a inhibitor (anti-92a) or control RNA. The mRNA levels of KLF2, eNOS, and TM as ratios to GAPDH were assessed by qRT-PCR. T h e protein levels of KLF2, Histone H1 and eNOS were determined by Western blotting. (D) HEK293 cells were transfected with the wild-type FLAGKLF2 (WT), FLAG-KLF2 (mut) (miR-92a binding site mutation), or Flag-KLF2 (Δ) (miR-92a 24 binding site deletion) together with 20 nM pre-92a or control RNA for 48 hr. The cells were then lysed, and the level of exogenously expressed FLAG-KLF2 fusion proteins was detected by Western blot analysis with anti-FLAG. Shown in the bottom panel is the densitometry analysis of the protein amount normalized to that in the control RNA-transfected cells. (E) HEK293 cells were transfected with the wild-type Luc-KLF2-3’UTR(WT) or Luc-KLF2-3’UTR(mut) together with 20 nM pre-92a or control RNA and CMV-β-gal. The luciferase activity was normalized to that of β-gal. The data represent mean±SD from 3 independent experiments. * p<0.05 between cells transfected with pre-92a and control RNA.
To demonstrate the direct targeting of KLF2 3’UTR by miR-92a, we created a CMV-driven expression plasmid encoding the wild-type KLF2–3’UTR fused with a FLAG tag [FLAGKLF2(WT)]. We also fused FLAG to a mutated KLF2–3’UTR in which the miR-92a binding site was altered [FLAG-KLF2(mut)] or deleted [FLAG-KLF2(Δ)]. Together with pre-92a or control RNA, these KLF2 expression plasmids were transfected into HEK293 cells, which have a high transfection efficiency and a low level of endogenous KLF2. As shown in Fig. 2D, pre-92a, but not control RNA, decreased the level of FLAG-KLF2(WT) fusion protein. In parallel experiments, the expression of FLAG-KLF2(mut) or FLAG-KLF2(Δ) was unaffected by the cotransfected pre-92a.
We also created reporter constructs containing luciferase fused to the wild-type KLF2 3’UTR [Luc-KLF2(WT)] or the mutated KLF2 3’UTR [Luc-KLF2(mut)]. HEK293 cells were co-transfected with Luc-KLF2(WT) or Luc-KLF2(mut) together with pre-92a or control RNA. As shown in Fig. 2E, the transfected pre-92a decreased the luciferase activity of Luc-KLF2(WT), as compared with cells co-transfected with control RNA but was unable to decrease the luciferase activity of Luc-KLF2(mut). Together, the data from Fig. 2 suggest that the 3’UTR of KLF2 mRNA contains a functional miR-92a target site. The interaction of miR-92a with KLF2 mRNA through this site causes the degradation of KLF2 mRNA and/or decreased translation of KLF2.
Shear stress-regulation of KLF2 is mediated by miR-92a
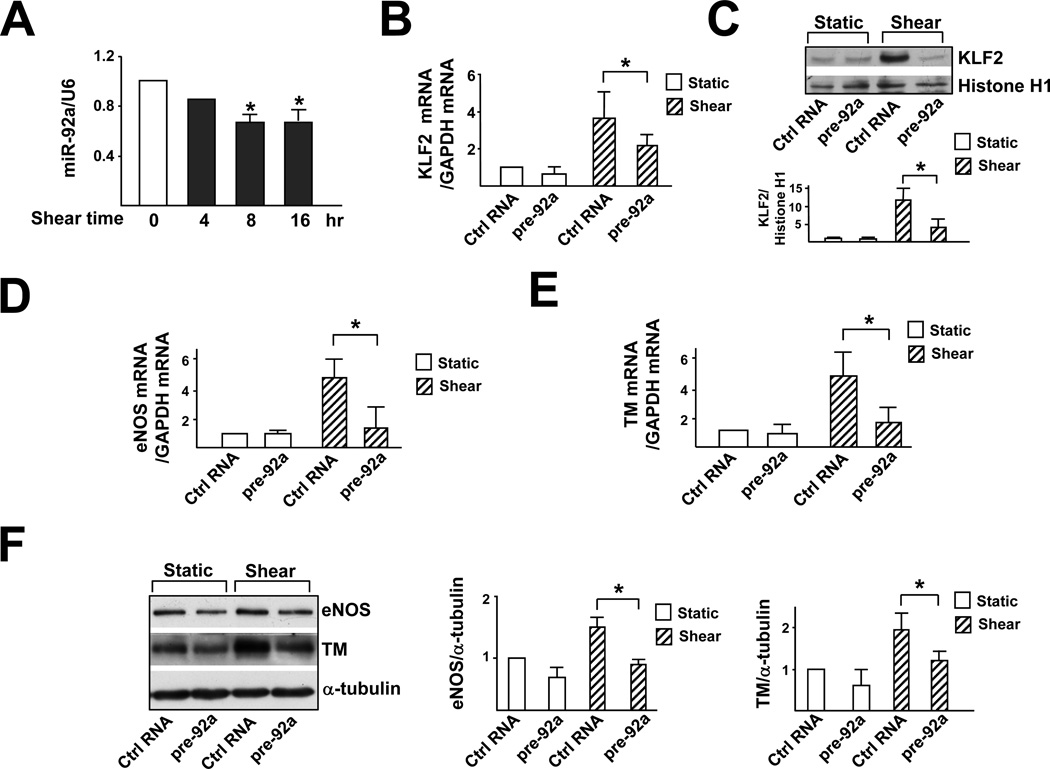
Because shear stress upregulates, while miR-92a downregulates, KLF2 mRNA in ECs, we examined the effect of laminar flow on miR-92a. As shown in Fig. 3A, the level of miR-92a in HUVECs decreased after exposure to laminar flow for 8 hr, and this decrease lasted for at least 16 hr. To explore whether miR-92a is involved in the shear stress-regulated KLF2, HUVECs were transfected with pre-92a and then exposed to laminar flow. As shown in Fig. 3B, C, pre-92a transfection attenuated shear stress induction of KLF2 at both mRNA and protein levels. Furthermore, the shear stress-induction of eNOS and TM was downregulated by pre-92a in a similar manner (Fig. 3D–F).
Figure 3. Shear stress-induction of KLF2 is mediated through miR-92a.
(A) HUVECs were exposed to laminar flow for 4, 8 or 16 hr. qRT-PCR was performed to detect the level of miR-92a, which was normalized to that of U6 RNA. * p<0.05 compared with static controls (time 0), analyzed by one-way ANOVA followed by Dunnett’s test. (B–F) HUVECs were transfected with 20 nM control RNA or pre-92a for 48 hr and then exposed to laminar flow for 8 hr. (B) KLF2 mRNA level was detected by qRT-PCR and (C) protein level was assessed by Western blot analysis. (D,E) eNOS and TM mRNA levels were detected by qRT-PCR and (F) protein level was assessed by Western blot analysis, and the results of statistical analyses are shown in the right. The data represent mean±SD from 3 independent experiments. * p<0.05 between the indicated groups, analyzed by two-way ANOVA followed by the Bonferroni posthoc test.
Differential regulation of miR-92a by PS versus OS
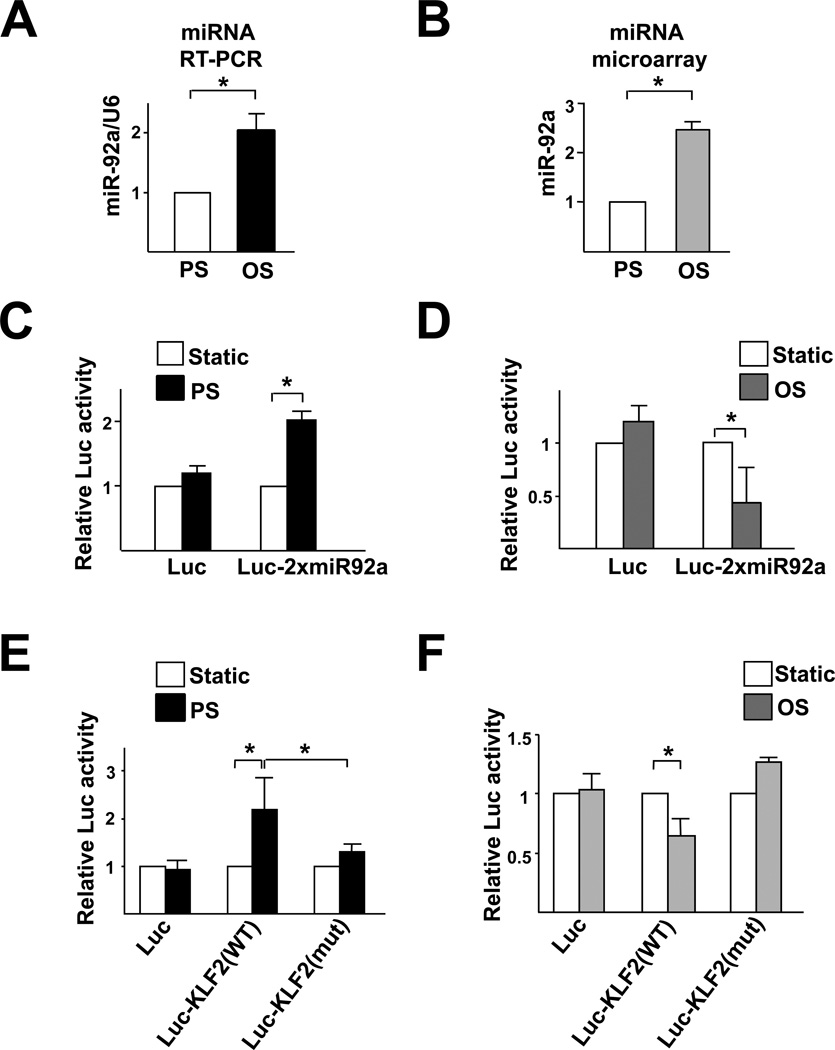
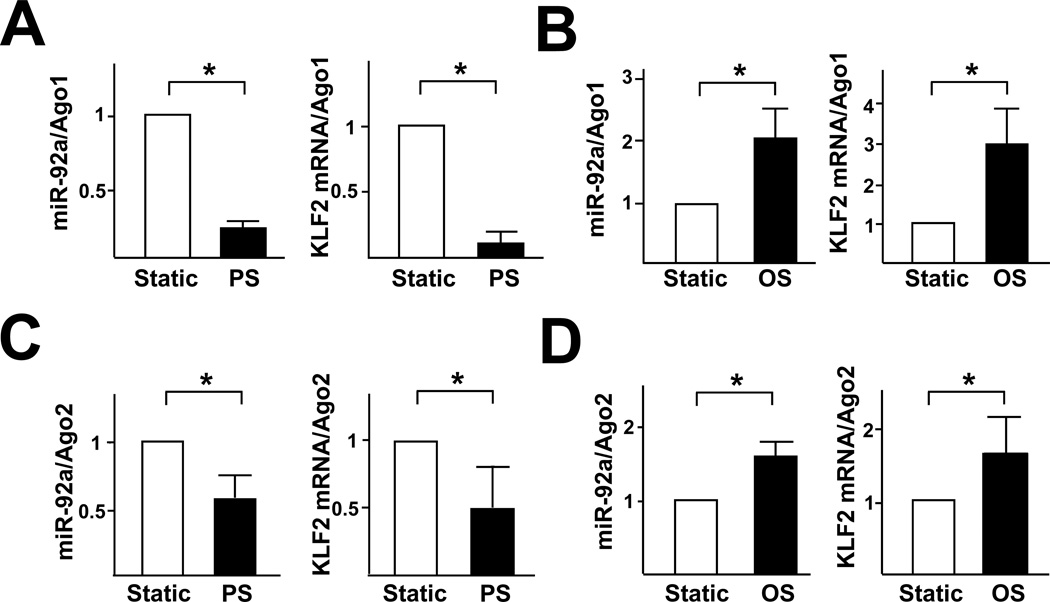
Because of the atheroprotective versus atheroprone natures associated with PS and OS, we compared the miR-92a levels in HUVECs subjected to PS and OS. Both qRT-PCR and miRNA microarray showed that the expression of miR-92a was attenuated in ECs exposed to PS as compared with OS (Fig. 4A, B). To further test the flow regulation of miR-92a/KLF2, we created a reporter construct in which luciferase was fused to 2 copies of the miR-92a binding site found in the KLF2 3’UTR (Luc-2xmiR92a). As shown in Fig. 4C, PS caused a 2-fold increase in luciferase activity in bovine aortic endothelial cells (BAECs), as compared with static controls. In contrast, OS significantly reduced the luciferase activity, as compared with static controls (Fig. 4D). Furthermore, PS increased whereas OS slightly decreased the luciferase activity of Luc-KLF2(WT) (Fig. 4E, F).
Figure 4. PS down-regulates, but OS up-regulates, miR-92a expression in ECs.
(A) HUVECs were exposed to PS (12±4 dyn/cm2) or OS (0±4 dyn/cm2) for 8 hr. qRT-PCR was performed to detect the level of miR-92a, which was normalized to that of U6 RNA. (B) The expression of miR-92a in ECs exposed to PS or OS flow assessed by miRNA microarray. (C,D) BAECs were transfected with Luc-2×miR92 reporter or control plasmid for 24 hr and then exposed to PS or OS flow for 12 hr. The luciferase activity was measured and normalized to β-gal activity. (E, F) BAECs were transfected with Luc-KLF2(WT) or Luc-KLF2(mut) for 24 hr and then exposed to PS or OS flow for 12 hr. The luciferase activity was measured and normalized to β-gal activity. The data represent mean±SD from 3 independent experiments. * p<0.05 between the 2 groups being compared by Student t test (A,B) or two-way ANOVA (C–F) followed by the Bonferroni posthoc test.
Because miRNA targeting mRNAs depends on the association of the miRNA/mRNA complex with Ago proteins to form miRISC, we investigated the association of miR-92a and KLF2 mRNA with Ago1 and Ago2 in HUVECs under PS or OS. As shown in Fig. 5A and C, the levels of miR-92a and KLF2 mRNA associated with Ago1 or Ago2 immunoprecipitated from HUVECs subjected to PS were lower than that in static controls. In contrast, OS increased the miRISC-associated miR-92a and KLF2 mRNA (Fig. 5B,D). The expression of neither Ago1 nor Ago2 was affected by the applied PS and OS (Suppl. Fig. 1). In the isotype controls, miR-92a was not detected in the immunoprecipitates (data not shown).
Figure 5. miRISC regulates miR-92a.
HUVECs were exposed to PS (A,C) or OS (B,D) for 8 hr. The Ago1- or Ago2-associated miRNAs and mRNAs were enriched by IP with the use of anti-Ago1 (A,B) or anti-Ago2 (C,D). mRNA levels of miR-92a and KLF2 were detected by qRT-PCR and normalized to those of Ago1 or Ago2 protein. The data represent mean±SD from 3 independent experiments. * p<0.05 for PS or OS vs. static control, as analyzed by Student t test.
miR-92a regulates NO bioavailability
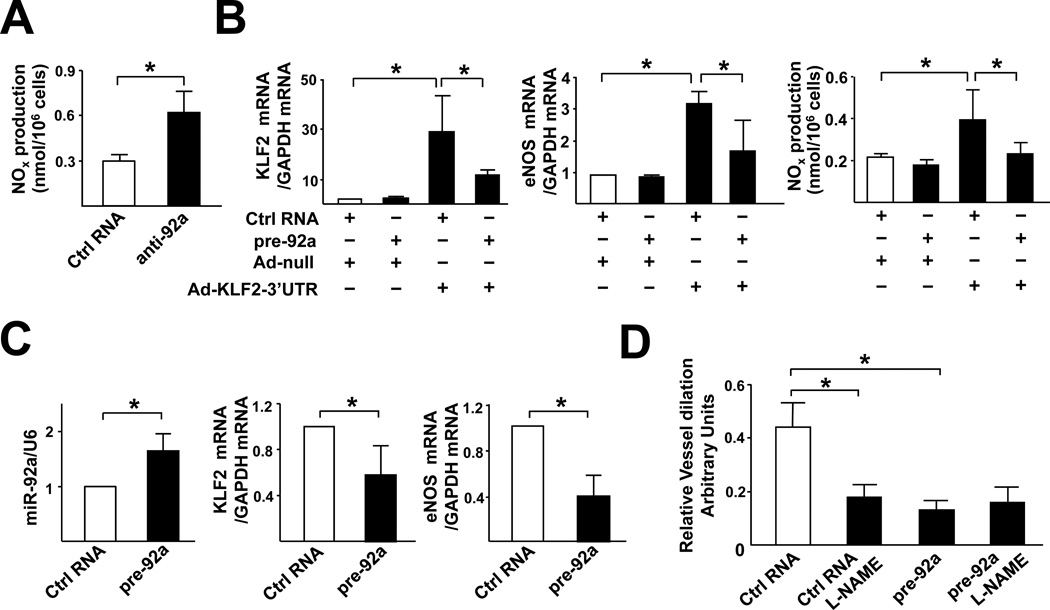
A functional consequence of the KLF2 induction of eNOS is the increase in NO bioavailability in ECs. Hence, we examined whether NO production is affected by miR-92a. As shown in Fig. 6A, miR-92a knockdown by anti-92a enhanced NO production in HUVECs. To demonstrate that the effects of pre-92a on eNOS and NO is mediated by it's targeting of KLF2, HUVECs were infected with Ad-KLF2 containing its 3’UTR (Ad-KLF2–3’UTR). As shown in Fig.6B, the level of eNOS mRNA and NO increased in HUVECs infected with Ad-KLF2–3’UTR together with control RNA. When HUVECs were infected with Ad-KLF2–3’UTR together with pre-92a, the induction of eNOS mRNA and the associated NO was much reduced. However, such an induction was not affected if pre-92a was co-transfected with Ad-KLF2 lacking 3’UTR (Suppl. Fig. 2).
Figure 6. miR-92a regulates endothelial function in vitro and ex vivo.
(A) HUVECs were transfected with control RNA or anti-92a. After 48 hr, the NO bioavailability was detected by fluorometric assay and expressed as nmol/106 cells. In (B), HUVECs were transfected with pre-92a and infected with Ad-KLF2-3’UTR or Ad-null for 48 hr. The level of KLF2 and eNOS mRNA was assessed by qRT-PCR and NO bioavailability was measured. (C) pre-92a or control RNA was administered to the carotid arteries of C57BL6 mice by pluronic gel F-127. Five days later, the arteries were isolated. The expression levels of miR-92a, KLF2 and eNOS in the isolated vessels (n=6) were assessed by qRT-PCR. miR-92a level was normalized to that of U6 RNA, whereas KLF2 and eNOS levels were normalized to that of GAPDH. The data represent mean±SD. (D) The flow-induced vasodilation ex vivo was measured by use of the SoftEdge Acquisiton Subsystem in the presence or absence of L-NAME (1 µM). The dilation ability is defined as the percentage of the diameter change of the flow-induced dilation compared to the diameter change of the PE-induced constriction. The bars represent mean±SEM. p<0.05 between the 2 groups being compared by Student t test (A,C) or two-way ANOVA followed by the Bonferroni posthoc test (B,D).
To determine the role of miR-92a in regulating vascular functions in vivo, we delivered pre-92a into the mouse carotid artery by a local delivery system. Carotid arteries were used because the associated flow conditions are well defined29 and gene expression in these vessels can be manipulated by pluronic gel-based delivery. As shown in Fig. 6C, the expressions of KLF2 and eNOS were significantly lower in vessels receiving pre-92a. The flow-mediated vasodilation was then investigated. As shown in Fig. 6D and suppl. Table 1, the flow-induced vasodilation was suppressed in vessels transfected with pre-92a compared to those of control RNA. L-NAME was used to examine whether the inhibitory effect of miR-92a was due to the eNOS suppression. Introduction of L-NAME into the system decreased the flow-induced vasodilation in carotid arteries treated with control RNA, but had little effect on vessels receiving pre-92a. In addition, acetylcholine induced the dilation of vessels administered with control RNA, but not those with pre-92a. These results indicate that miR-92a is critical in regulating the eNOS-dependent vasodilation responding to flow.
Discussion
Shear stress with a forward direction has been shown to upregulate KLF2 in ECs5–7, which in turn modulates the expression of ~70% of the genes that are responsive to shear stress30. Early studies demonstrated that shear stress enhances KLF2 transcription via the binding of several transcription factors (TFs) to the promoter of the KLF2 gene (see supplemental Table 2). Post-translational modifications (e.g., phosphorylation and acetylation/deacetylation) of these TFs play important roles in changing the transcriptional activity of KLF213, 31. Results from the present study show that shear stress also regulates KLF2 expression at the post-transcriptional level, which is mediated by the decreased level of miR-92a. Thus, the expression of KLF2 can be modulated at multiple levels, including transcription, post-transcriptional via miR-92a, and post-translational modifications.
miR-92a belongs to the miR-17~92 cluster. Also known as oncomiR-1, the miR-17~92 cluster is located in the third intron of the C13orf25 locus at 13q31-q3232. Overexpression of miR-92a in ECs blocks angiogenesis in vitro and in vivo27, which suggests that miR-92a is a negative regulator of some endothelial functions. It has since been found that other miRNAs of the miR-17~92 cluster, such as miR-17, -18a, -19a, and -20a, are also anti-angiogenic33. Sharing the same promoter, various miRNAs within the miR-17~92 cluster would be suppressed by PS and/or induced by OS in a similar manner. Indeed, the expression of miR-17, -18a, -19b, and -20a in HUVECs was downregulated by PS but upregulated by OS (Suppl. Fig. 3). However, other miRNAs within the miR-17~92 cluster may not target KLF2 mRNA, since only the seed sequence of miR-92a is complementary to the KLF2 3'UTR.
The expression of the miR-17~92 cluster can be modulated by several transcription factors or signaling molecules. Overexpression of c-Myc, cyclin D1, and E2F increased the expression of the miR-17~92 cluster in cancer cells34–36. ChIP assay has shown the binding of c-Myc, E2F, and cyclin D1 to the upstream promoter region of the miR-17~92 cluster. In addition, a stat3 binding site is present in this promoter37. Given the positive effect of c-Myc, cyclin D1, E2F, and stat3 on the induction of the miR-17~92 cluster, one would assume that OS upregulates and that PS downregulates these proteins. Prolonged laminar flow has been shown to suppress the expression of these proteins38–40. Since miR-92a targets the KLF2 3’UTR, the PS-downregulation of miR-92a should lead to an elevation of KLF2 expression at the posttranscriptional level. Consequently, the “de-suppressed” KLF2 transactivates its target genes (e.g., eNOS, and TM), which are otherwise suppressed under static or OS conditions. In addition to the regulation of NO and its consequent vasodilation, this mechanism involving miR-92a targeting KLF2 may also regulate other KLF2-dependent genes such as von Willebrand factor, FLK1, and Tie-2, which are critical for EC lineage development and vascular functions27, 41, 42.
The results presented in Figs. 2 and 3 show that miR-92a regulates KLF2 at both mRNA and protein levels. When assembled into miRISC, the mature miRNAs can cause the degradation of their target mRNAs or interfere with the translational process15–17. Among the Ago family members, Ago1 mediates the miRNA-induced translational inhibition16, 43. Recent studies have shown that the Ago1-involved miRISC could also destabilize target mRNAs by deadenylation and 5'→3′ decay after decapping16, 43. Neither of the mechanisms requires a perfect match between miRNAs and the 3’UTR of the targeted mRNAs. However, mRNA degradation modulated by Ago2 requires a near-perfect complementary sequence in the 3′UTR of the target44, 45. The miR-92a/KLF2 mRNA targeting lacks a perfect match, since only the seed region of miR-92a (8 nt) is complementary to the KLF2 3’UTR. The RNA chaperone model46 suggests that Ago1 may facilitate the association of the guide strand of miR-92a with the KLF2 mRNA, and then Ago2 is recruited to the miRISC complex. This model explains the increased association of miR-92a and KLF2 mRNA with both Ago1 and Ago2 in ECs under OS (Fig. 5B,D).
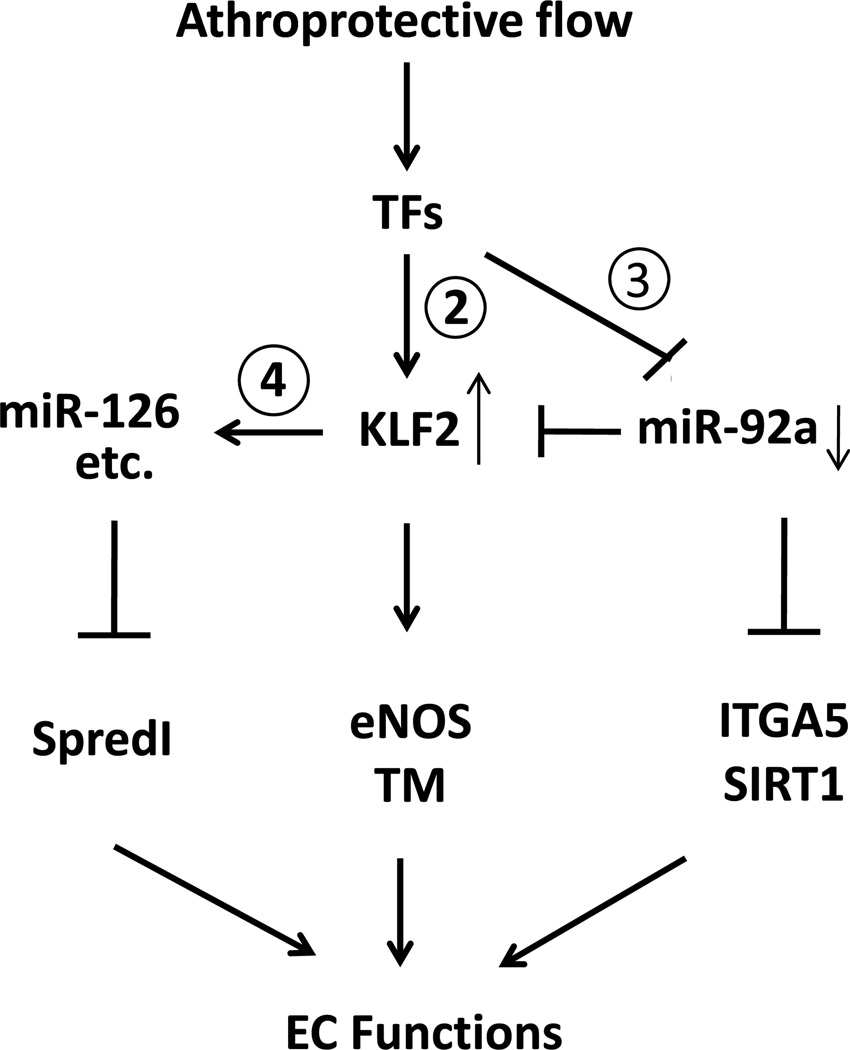
Based on the knowledge gained from the current study and those in the literature, Fig. 7 is drawn to summarize a network of molecular events leading to the induction of KLF2 in ECs by atheroprotective flow. Among the TFs modulated by the imposed flow, some are directly involved in the induction of the klf2 gene (Supplemental Table 2), whereas others may downregulate the miR-17~92 cluster (Supplemental Table 3). In this model, the upregulated KLF2, functioning as a TF, transactivates a panel of genes related to EC lineage, as well as miRNAs47. Recently, Harris et al. showed that KLF2 regulates the transcription of miR-12648. In addition to miR-126, bioinformatics analysis revealed a set of shear stress-upregulated miRNAs that have the KLF2-binding site in their promoter region (Supplemental Table 4). Moreover, the downregulation of miR-92a leads to augmentation of IGTA5 (beneficial)27, and upregulation of miR-126 results in attenuation of SpredI (detrimental)47. Collectively, these miRNA-regulated events and KLF2 targets (e.g., eNOS, TM) maintain endothelial homeostasis and some aspects of the EC lineage.
Figure 7. Shear stress regulation of KLF2.
The diagram shows the regulatory circuitry of the responses of transcription factors and miRNAs to atheroprotective shear flow. The circled numerals refer to the Table numbers. Shear stress with a forward direction regulates the expressions of KLF2 and miR-92a through several TFs (in Supplemental Tables 2 and 3, respectively). Serving as a transcription factor, KLF2 transactivates the expression of downstream genes such as eNOS and TM. In addition, KLF2 may bind to the promoter region of some miRNAs, including miR-126, to upregulate their transcription directly (Supplemental Table 4). In turn, the network of KLF2 and miRNAs regulates the expression of factors that control anti-inflammatory, anti-thrombotic, anti-proliferative, anti-angiogenic, anti-oxidant, and anti-fibrotic effects to maintain EC functions. The methods for computational analysis are described in the supplements.
Supplementary Material
Clinical summary.
Upregulated by atheroprotective flow, the transcription factor Kruppel-like factor 2 (KLF2) is a crucial integrator for maintaining endothelial functions. MicroRNAs (miRNAs) are non-coding small RNAs that regulate gene expressio n at the post-transcriptional level. In the present study, we examined the role of miR-92a in the atheroprotective flow-regulated KLF2. Overexpression of miR-92a precursor (pre-92a) decreased the expression of KLF2 and the KLF2-regulated endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM) in vascular endothelial cells. In contrast, miR-92a inhibitor (anti-92a) increased the expression of KLF2, eNOS, and TM. The clinical implication is that atheroprotective flow downregulated the level of miR-92a which in turn induced KLF2. Furthermore, overexpression of KLF2 rescued the pre-92a-suppressed KLF2 and eNOS mRNA and NO production, demonstrating that miR-92a directly targets KLF2 and hence affects the KLF2-mediated endothelial functions. Consistent with these findings, mouse carotid arteries receiving pre-92a exhibited impaired vasodilatory response to flow. This newly defined miR-92a-KLF2 pathway suggests that miR-92a may be a therapeutic target to improve vascular functions.
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health Grants HL89940 and HL106579 (S.C., J.S.), HL076686 (G.G.-C.), an Ibercaja Research Fellowship, the Red Heracles de Investigación Cardiovascular, and the Research Foundation of the Hospital Clinico Universitario de Valencia (Spain to A.L.-F.); the Howard Hughes Medical Institute Research Training Fellowship for Medical Students and a Seed Training Grant from the American Medical Association (G.V.), Taiwan National Science Council I-RiCE Program Grant NSC-99-2911-I-009-101 (W.W., H.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 2.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 3.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 4.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 5.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 6.Nayak L, Lin Z, Jain MK. ‘Go with the flow’ - How Krüppel-like factor 2 regulates the vasoprotective effects of shear stress. Antioxid Redox Signal. 2010 Oct 4; doi: 10.1089/ars.2010.3647. Online ahead of editing: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–43. [PubMed] [Google Scholar]
- 8.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 9.Kato Y, Zhao M, Morikawa A, Sugiyama T, Chakravortty D, Koide N, Yoshida T, Tapping RI, Yang Y, Yokochi T, Lee JD. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem. 2000;275:18534–18540. doi: 10.1074/jbc.M001573200. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Lin Z, SenBanerjee S, Jain MK. Tumor necrosis factor alpha-mediated reduction of KLF2 is due to inhibition of MEF2 by NF-kappaB and histone deacetylases. Mol Cell Biol. 2005;25:5893–5903. doi: 10.1128/MCB.25.14.5893-5903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CC, Ornatsky OI, McDermott JC, Cruz TF, Prody CA. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 1998;26:4771–4777. doi: 10.1093/nar/26.20.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn SJ, Li D, Lee LK, Winoto A. Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase. Mol Cell Biol. 2005;25:8553–8566. doi: 10.1128/MCB.25.19.8553-8566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Thienen JV, Fledderus JO, Dekker RJ, Rohlena J, van Ijzendoorn GA, Kootstra NA, Pannekoek H, Horrevoets AJ. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72:231–240. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–460. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 17.Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–249. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 19.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 21.Sen CK, Gordillo GM, Khanna S, Roy S. Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res. 2009;46:527–540. doi: 10.1159/000226221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A. 2010;107:3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonauer A, Dimmeler S. The microRNA-17–92 cluster: still a miRacle? Cell Cycle. 2009;8:3866–3873. doi: 10.4161/cc.8.23.9994. [DOI] [PubMed] [Google Scholar]
- 27.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 28.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 29.Quan A, Ward ME, Kulandavelu S, Adamson SL, Langille BL. Endothelium-independent flow-induced dilation in the mouse carotid artery. J Vasc Res. 2006;43:383–391. doi: 10.1159/000094414. [DOI] [PubMed] [Google Scholar]
- 30.Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, Dimmeler S. Members of the microRNA-17–92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T, McCue P, Pestell RG. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 37.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 38.Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J Biol Chem. 2003;278:32994–32999. doi: 10.1074/jbc.M304799200. [DOI] [PubMed] [Google Scholar]
- 39.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) Circ Res. 2000;86:185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- 40.Ni CW, Hsieh HJ, Chao YJ, Wang DL. Shear flow attenuates serum-induced STAT3 activation in endothelial cells. J Biol Chem. 2003;278:19702–19708. doi: 10.1074/jbc.M300893200. [DOI] [PubMed] [Google Scholar]
- 41.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 42.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 45.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Li S, Qi HH, Chowdhury D, Shi Y, Novina CD. Distinct passenger strand and mRNA cleavage activities of human Argonaute proteins. Nat Struct Mol Biol. 2009;16:1259–1266. doi: 10.1038/nsmb.1712. [DOI] [PubMed] [Google Scholar]
- 47.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1990–1997. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.