ABSTRACT
Objective:
We aimed to study various measures of blood pressure (BP) in the subacute phase of ischemic stroke to determine whether any of them predicted clinical outcome.
Methods:
In this retrospective observational study, a consecutive series of patients hospitalized for ischemic stroke within 48 hours of onset were enrolled. The subacute stage of stroke was defined as the time period from 72 hours of symptom onset to discharge or transfer. During this period, mean, maximum, maximum − minimum, SD, and coefficient of variation of systolic BP (SBP) and diastolic BP (DBP) were determined. A baseline severity-adjusted analysis was performed using each patient’s 3-month modified Rankin Scale score as the primary outcome.
Results:
Among a total of 2,271 patients, the median number of BP measurements was 34 per person and the median interval from onset to discharge was 8.7 days. Measures of variability of BP were associated with poor outcome. One SD increase of maximum − minimum (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.12–1.42), SD (OR, 1.20; 95% CI, 1.07–1.34), or coefficient of variation (OR, 1.21; 95% CI, 1.09–1.35) for SBP, but not mean level of SBP (OR, 0.92; 95% CI, 0.79–1.07), was independently associated with poor outcome. Results were similar for DBP.
Conclusion:
This study shows that variability of BP, but not average BP in the subacute stage of ischemic stroke, is associated with functional outcome at 3 months after stroke onset.
Approximately 80% of patients with acute ischemic stroke present with elevated blood pressure (BP).1,2 Increased BP in the acute stage of ischemic stroke may be associated with poor functional outcome because there is augmentation of cerebral edema, hemorrhagic transformation, or stroke recurrence.3-6 Consequently, current guidelines recommend modest BP reduction in the acute stage, although there is uncertainty related to benefits of lowering of elevated BP.7-10 Low BP levels were actually harmful in some studies.11 In the chronic phase of stroke, however, the efficacy of antihypertensive medications for recurrent stroke prevention has been well established, and thus, aggressive BP lowering is recommended.12,13
There is a paucity of information about the clinical impact of BP during the transitional period between the acute and chronic stages of ischemic stroke (i.e., the subacute stage). Elevated BP in the acute stage of ischemic stroke usually decreases spontaneously in the ensuing 7 to 10 days, and approximately 40% of patients were discharged to home without further BP management.14,15
BP fluctuation or variability may be an important predictor of stroke risk and outcome.6,16 In this research, we studied multiple BP parameters. The aim of the study was to investigate whether BP in the subacute phase of ischemic stroke was associated with 3-month function outcome and, if so, which BP parameter was most highly associated or predictive of outcome.
METHODS
Standard protocol approvals, registrations, and patient consents
The study protocol was approved by the local institutional review board. The board permitted the study to be conducted without patient consent because this was a retrospective, registry-based study.
Patients
In this observational study based on a prospective stroke registry,17 we identified a consecutive series of patients who were hospitalized at Seoul National University Bundang Hospital between January 1, 2004, and January 31, 2009. All study subjects had ischemic stroke within 48 hours of symptom onset and relevant ischemic lesions on cranial CT or brain MRI. Potential study subjects were excluded if they had died during hospitalization, were discharged just before death because the patient’s family wanted them to die at home according to Korean tradition, were transferred to departments other than rehabilitation units because of medical or neurologic complications of index stroke, had missing daily BP data, had hospitalization stay of <72 hours or >21 days, or were not assessed for functional status at 3 months after stroke onset.
Supine BP was measured in the nonparalyzed arm using a standard mercury sphygmomanometer and entered manually into the electronic medical record (EMR) as a part of the clinical nursing routine for patients cared for on general hospital units. In the emergency room, stroke unit, or intensive care unit, BP was measured using a noninvasive BP monitoring device (IntelliVue MP20; Philips Medizin Systeme, Böblingen, Germany) and recorded automatically into the EMR. BP data from hospitalization were downloaded from the clinical data warehouse of our institution’s EMR. For BP and other acute stroke management, we follow the current guidelines proposed by the American Stroke Association17 and the Korean Stroke Society.18
Clinical characteristics, vascular risk factors, acute management strategies, and other laboratory findings were gathered directly from the stroke registry or by reviewing medical records in some cases. The subacute stage of ischemic stroke was defined as the time period during which patients were stabilized neurologically and medically. Operationally, it was defined as the time period after 72 hours from ischemic stroke symptom onset and until the day of discharge or transfer to rehabilitation units in the given period of 4 to 21 days from symptom onset.
To characterize the BP status of each individual, we obtained the following parameters for systolic BP (SBP) and diastolic BP (DBP), respectively: mean (an average of values), maximum, maximum − minimum, SD, and coefficient of variation (CV) (SD × 100/mean). BP measurements representing the acute stage were restricted to the first 24 hours after admission and the mean of SBP and DBP was obtained for each patient.
Outcome assessment
Patients were prospectively followed up at 3 months from stroke onset by telephone interview as part of an institutional quality-of-care monitoring program for hospitalized stroke patients. A dedicated and trained stroke nurse (M.H. Yang) was responsible for performing the outcome assessment using the modified Rankin scale (mRS).19
The baseline severity-adjusted analysis (responder analysis) was applied for dichotomization of outcome status.20 That is, poor outcome was defined as a 3-month mRS score of 2 to 6 if the baseline National Institutes of Health Stroke Scale (NIHSS) score was ≤7 points, mRS score of 3 to 6 if the NIHSS score was 8 to 14 points, and mRS score of 4 to 6 if the NIHSS score was ≥15 points.
Statistical analysis
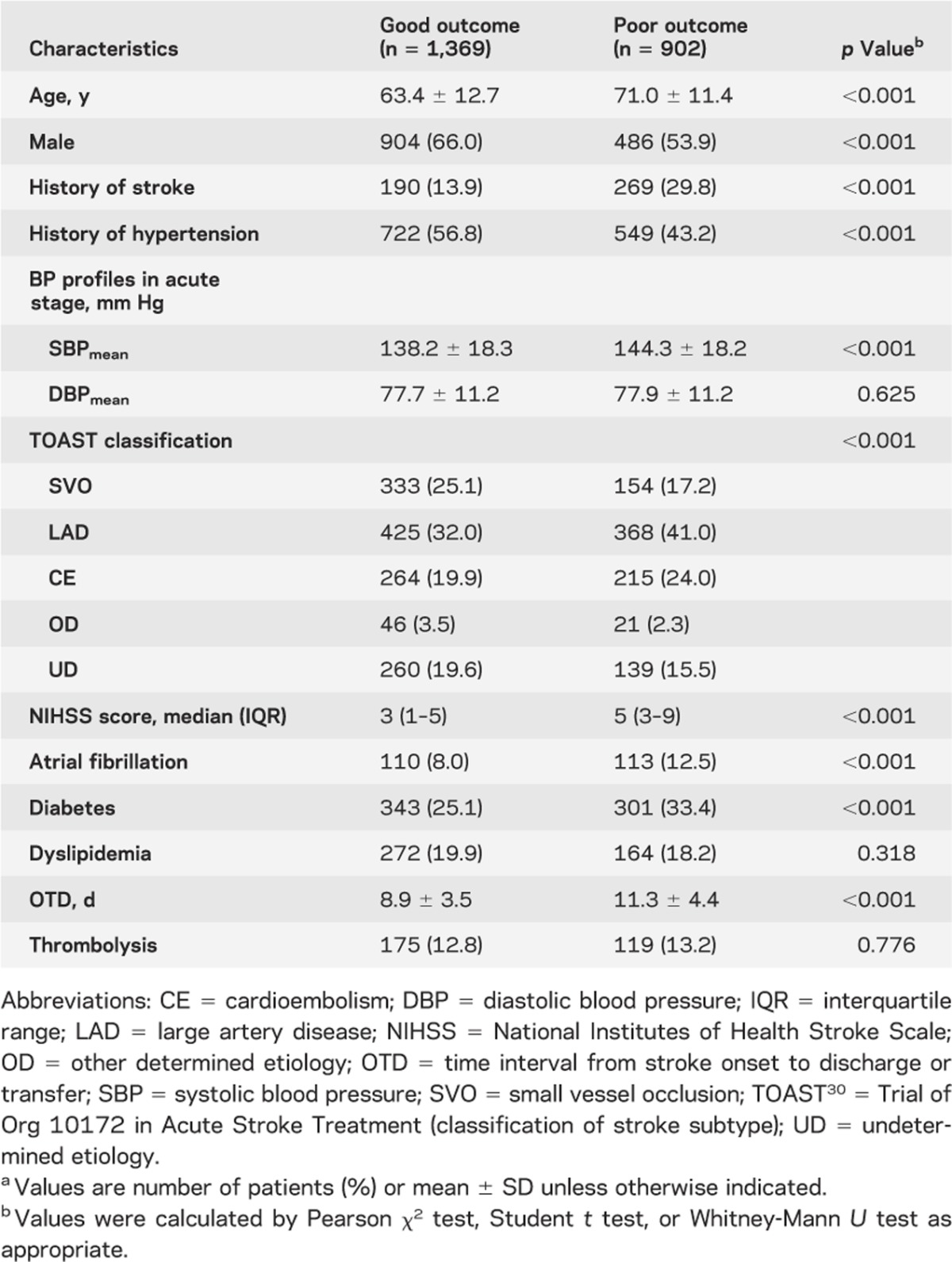
Values are presented as mean ± SD, median (interquartile range [IQR]) for continuous variables, or as the number (%) of subjects for categorical variables.
Comparisons of baseline characteristics between those with good and poor outcomes were made using the Pearson χ2 test, Mann-Whitney test, or Student t test according to the type of variable (table 1). In multivariable analysis, variables with p values <0.2 from comparisons of baseline characteristics, and associations with outcome that were biologically plausible, were chosen as potential confounders for statistical adjustment. Selected variables were age, sex, history of stroke, history of hypertension, baseline NIHSS score, stroke subtype, diabetes mellitus, atrial fibrillation, SBPmean in the acute stage, and time interval from onset to discharge or transfer.
Table 1.
Comparisons of baseline characteristics according to 3-month functional outcomea
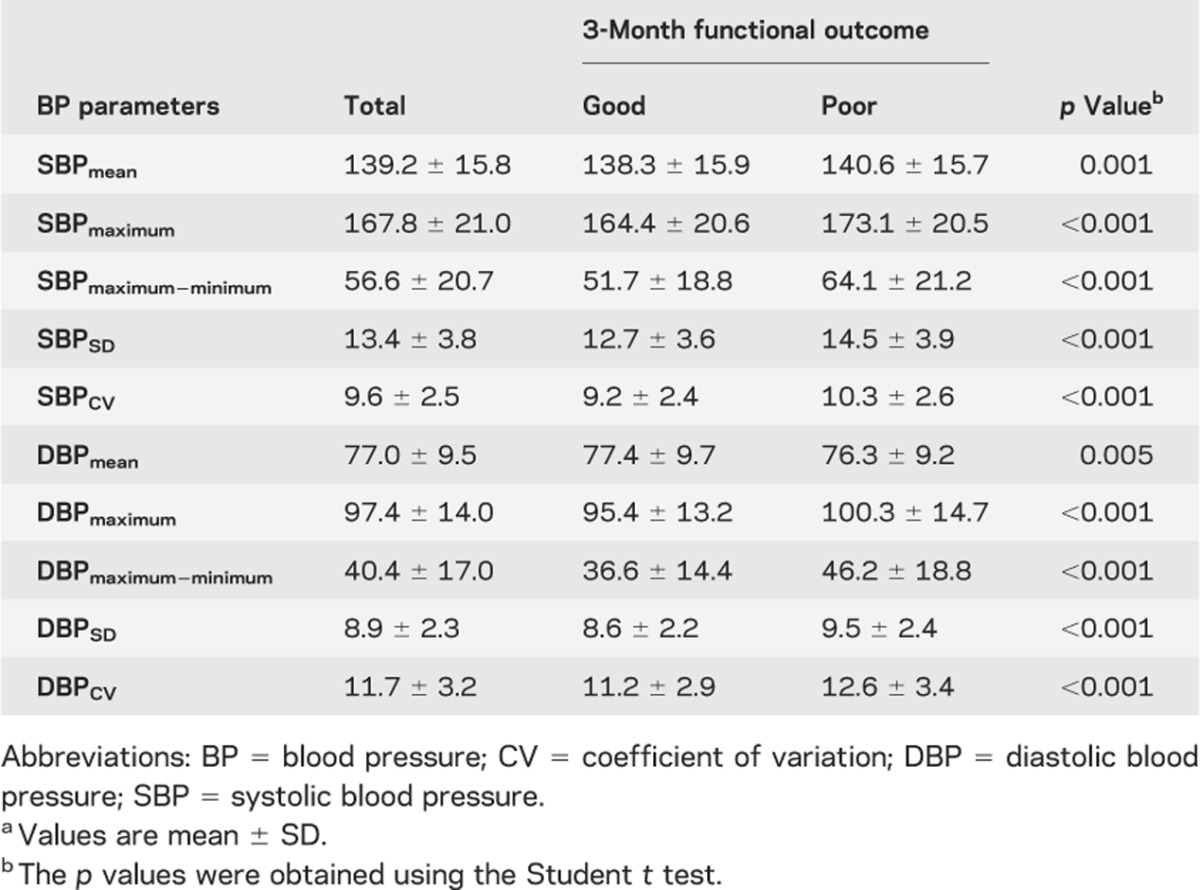
To analyze the relationship between subacute BP status and clinical outcome, each mean value of the BP parameters was compared between those with good and poor outcomes using the Student t test (table 2).
Table 2.
Comparisons of BP parameters according to 3-month functional outcomea
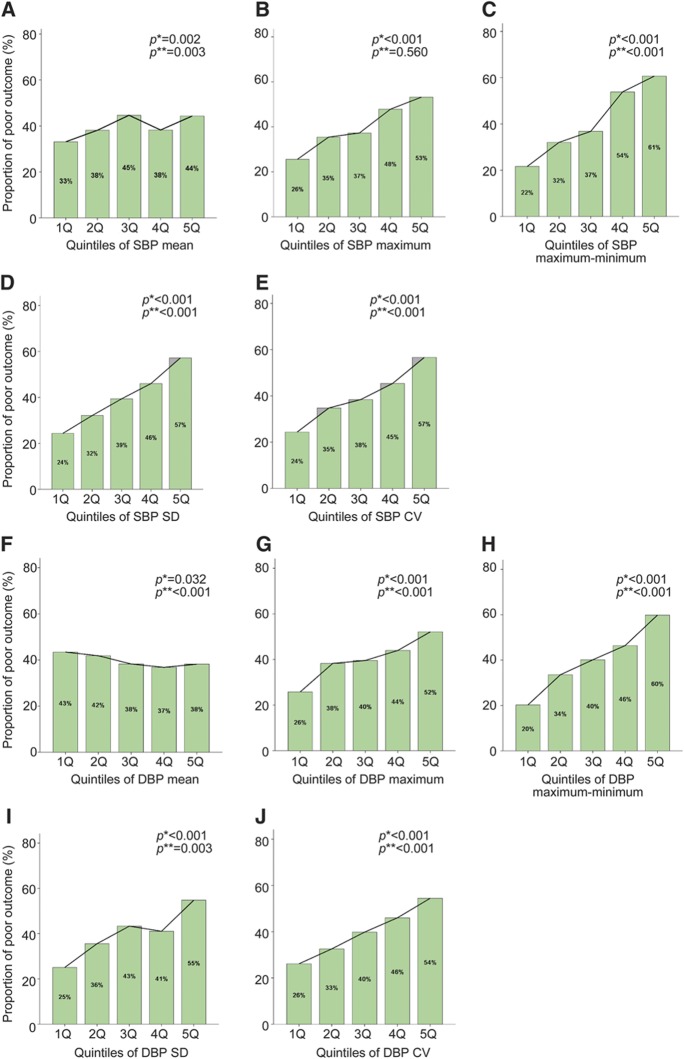
To examine the linearity of the relationship between subacute BP status and outcome, BP parameters were categorized into quintiles and analyzed by the χ2 test for linear trend (figure). Then, odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using multiple logistic regression analysis (tables e-1 and e-2 on the Neurology® Web site at www.neurology.org). The likelihood ratio test for trends was used to elucidate a dose-response relationship between the quintiles of each BP parameter and risk of poor outcome.
Figure. Proportion of 3-month poor outcome (dark bar) according to the quintiles of blood pressure parameters.
(A) Systolic blood pressure (SBP)mean, (B) SBPmaximum, (C) SBPmaximum−minimum, (D) SBPSD, (E) SBPCV, (F) diastolic blood pressure (DBP)mean, (G) DBPmaximum, (H) DBPminimum, (I) DBPmaximum−minimum, (J) DBPSD, and (K) DBPCV. p Values are for χ2 *linear trend test or for †likelihood ratio test for trend adjusted by age, sex, history of stroke, history of hypertension, baseline National Institutes of Health Stroke Scale score, stroke subtype, diabetes mellitus, atrial fibrillation, SBPmean at acute stage, and time from onset to discharge. CV = coefficient of variation.
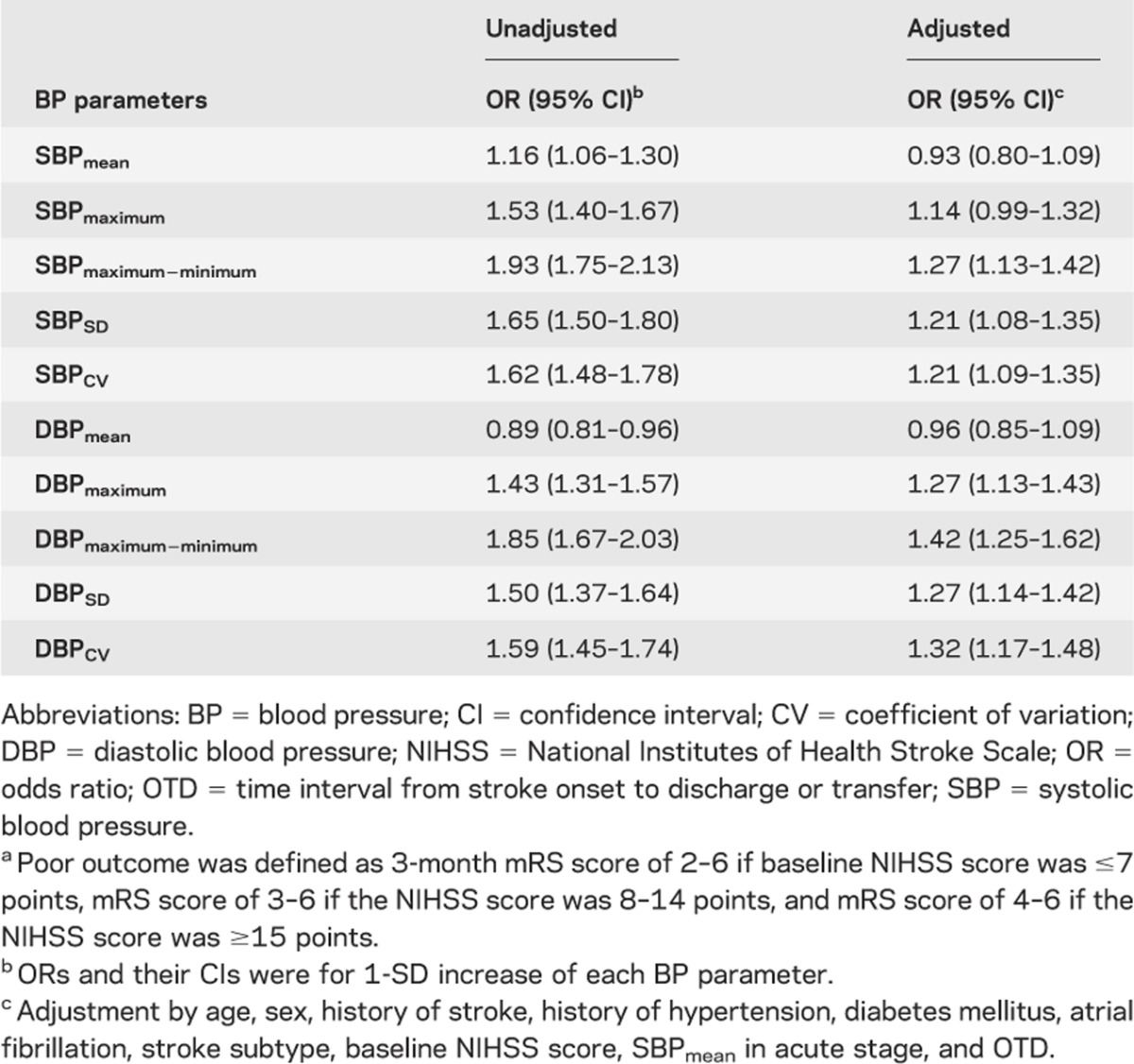
We also computed an adjusted OR of each BP parameter, using multiple logistic regression models, for change of 1 SD, after examining a collinearity of the model, and compared the impact of each BP parameter on poor functional outcome (table 3). As a sensitivity analysis, multiple logistic regression analyses for the BP parameters were repeated 1) using simple dichotomization of mRS score into 0–2 vs 3–6, instead of the baseline severity-adjusted dichotomization (table e-3); and 2) including subjects who were initially excluded because of death during hospitalization, discharge to die at home, and hospitalization for >21 days (table e-4).
Table 3.
Odds ratios for poor outcome according to 1-SD increase of each BP parametera
All statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL). A 2-sided p value of <0.05 was generally considered a minimum level of statistical significance.
RESULTS
Among 2,811 patients who initially met the study eligibility criteria, 540 were eventually excluded for the following reasons: 91 died during hospitalization, were discharged to die at home by request, or were transferred to other departments because of complications; 39 had missing daily BP data; 338 were discharged <4 days or after 21 days from stroke onset; and 72 were not assessed for functional outcome at 3 months. As a result, a total of 2,271 patients were included in the analysis. Their mean age was 66.4 years (SD, 12.8 years), and 61.2% were male. The median NIHSS score was 3 (IQR, 2–7) and 294 patients (12.9%) received thrombolysis. The patients excluded were older, had more severe neurologic deficits, and had longer duration of hospitalization compared with those included.
In an analysis of baseline severity-adjusted dichotomization of functional outcome, 902 patients (39.7%) were classified as having a poor outcome at 3 months, whereas 647 patients (28.5%) were categorized as such according to a dichotomization of the mRS at a cut point of ≥3 or <3. Comparisons of baseline characteristics between those with good and poor outcome groups are presented in table 1. Consistent with previous research, age, history of stroke, SBPmean in the acute stage, baseline NIHSS score, stroke subtype, diabetes mellitus, and atrial fibrillation were associated with the 3-month functional outcome.21-25
In the predefined subacute stage, the median number of BP measurements was 34 (IQR, 20–60). The median time from symptom onset to discharge or transfer was 8.7 days (IQR, 6.8–11.9 days). The profiles of BP parameters in the subacute stage are summarized in table 2. Compared with the average BP level in the acute stage, significant but minimal BP reduction (mean difference, 1.5 mm Hg; 95% CI, 0.94–1.98 mm Hg) was observed in the subacute stage (p < 0.001 by the paired t test). The proportion of patients with SBPmean ≥140 mm Hg was 46.5% (n = 1,056) and for DBPmean ≥90 mm Hg was 9.4% (n = 213), whereas the proportion of patients with SBPmean ≥170 mm Hg was 3.1% (n = 71) and for DBPmean ≥110 mm Hg was 0.2% (n = 4).
In bivariate analysis, the average values of all the BP parameters of interest differed significantly by 3-month outcome status. Patients with poor outcome had higher BP levels, except for mean DBP, and a wider range of BP variability than those with good outcome (table 2).
The analysis by quintile categorization of BP parameters showed a dose-response relationship between most of the BP parameters and poor outcome (figure). The odds of a poor outcome were linearly associated with increasing quintile of BP parameters except for maximum of SBP (tables e-1 and e-2).
Because the linearity assumption was satisfied, we calculated the adjusted ORs by 1-SD change of individual BP parameters and compared the magnitude of their impact on functional outcome. Maximum − minimum, SD, and CV of SBP and maximum, maximum − minimum, SD, and CV of DBP were independently associated with poor outcome, and among them maximum − minimum parameters of both SBP and DBP were most highly associated with poor outcome (table 3). None of the multivariable models with different BP parameters were found to suffer from a collinearity (a variance inflation factor ranges from 1.018 to 2.346 among models for SBP parameters, and from 1.018 to 1.958 among those for DBP parameters).
When substituting the simply dichotomized outcome for the baseline severity–adjusted outcome, or including the patients who were initially excluded, the association between BP variability and poor outcome remained significant (tables e-3 and e-4).
DISCUSSION
The objective of this study was to evaluate the effect of various BP parameters during the subacute stage of ischemic stroke on outcome. A main finding was that BP variability during the subacute stage was independently associated with 3-month functional outcome.
Another key finding was that the level of BP remained elevated in the subacute stage of ischemic stroke. Specifically, the mean BP in approximately half the patients met accepted criteria for hypertension (140/90 mm Hg cut point) although such patients had been medically treated and stabilized and were soon to be discharged or transferred to another unit. These findings are consistent with previous studies such as ACCESS (Acute Candesartan Cilexetil Therapy in Stroke Survivors),10 COSSACS (effects of antihypertensive treatment after acute stroke in the Continue or Stop post-Stroke Antihypertensives Collaborative Study),8 and SCAST (the angiotensin-receptor blocker candesartan for treatment of acute stroke),9 in which SBPmean was in the 135 to 150 mm Hg range during a similar time period.
A simple comparison by functional outcome status showed a statistically significant difference for all the BP parameters although the difference was modest in most cases, and the average level of BP, not BP variability, lost its statistical significance after adjustments. Findings from the COSSACS support our observations at least in part because continuation of BP medication lowered the BP level successfully but did not improve clinical outcome.8 Regarding the influence of BP on clinical outcome, this study and the COSSACS differ from previous ones in which BP level as well as variability in the acute stage were associated with clinical outcome.11,26 The discriminative effect of BP between the acute and subacute stages will need to be confirmed in subsequent well-designed studies.
In the subacute stage, BP variability is associated with worse functional outcome at 3 months. As observed in the figure, the proportion with poor outcome among patients with the highest quintile of maximum − minimum, SD, and CV of both SBP and DBP is >2 times that of those in the lowest quintile. Furthermore, there was a statistically significant dose-response relationship. Our findings have practical ramifications and suggest that we may need to focus more attention on BP variability rather than usual BP level. Furthermore, additional research is needed to confirm or refute the modest relationship of BP variability during the subacute stage and to determine whether control of BP variability has any clinically significant benefit in patient management.
How then, might BP variability influence functional outcome? First, we speculate that BP variability during this time period may increase the risk of stroke recurrence and other vascular events, including clinically silent ischemic strokes, which might worsen functional outcome. It has been reported that BP variability is a risk factor for stroke and other end-organ damage.16,27,28 Second, greater BP variability can exacerbate hypoperfusion of the brain. During the acute stage of ischemic stroke, high BP variability may be associated with lesion growth.4,29 Autoregulation may also be impaired in the subacute stage,30,31 and a hypoperfused brain is more likely to be affected by systemic BP changes. However, it should be noted that these explanations are speculative. Three to five days from stroke onset (the starting point of the subacute stage by our definition) is a large enough time interval during which the ischemic penumbra may be dead or salvaged. To clarify the causal relationship of BP variability and outcome, neuroimaging findings, such as lesion growth, recurrence, and hemorrhagic transformation, should be analyzed; however, this information was not available in the current study.
We adopted baseline severity-adjusted dichotomization to define functional outcome. Recently it was suggested that this method is useful for detecting a true signal and improving study power.30,31 The simple dichotomization of function outcome (mRS scores 0–2 vs 3–5), which was performed as a sensitivity analysis in this study, provided similar results.
It should be noted that there was no contribution of thrombolysis to functional outcome in this study (table 1). This finding might be explained by an imbalance of baseline characteristics between patients who received thrombolysis and those who did not. For example, the median baseline NIHSS score in patients with thrombolysis was 11 (IQR, 5–17), which is much higher than that in those without thrombolysis (median, 3; IQR, 1–5).
We observed a modest inverse relationship between mean SBP and DBP and poor outcome. This may have occurred as a chance finding or as a function of overall medical care and clinical stabilization leading to a better functional outcome in patients with lower BP.
Limitations of our research include the retrospective nature of the study in a single-center, community-based hospital. Furthermore, we did not consider the possible influence of antihypertensive medications or class of antihypertensive medications, or the perfusion status of the ischemic brain, which may influence BP variability or outcome.16,32 However, one would expect the influence of antihypertensive medications to be reflected in BP status. Finally, we cannot fully exclude the possibility that BP variability may have been the result of stroke severity, rather than the cause of poor functional outcome, because a deteriorating or fluctuating clinical course may lead to a variable BP profile. However, the adjustment of outcomes for baseline severity suggests that BP variability was causal.
Our study shows that BP status in the subacute stage of stroke influences 3-month outcome, and BP variability during this period may be a key independent predictor of clinical outcome.
Supplementary Material
Glossary
- BP
blood pressure
- CI
confidence interval
- COSSACS
Continue or Stop post-Stroke Antihypertensives Collaborative Study
- CV
coefficient of variation
- DBP
diastolic blood pressure
- EMR
electronic medical record
- IQR
interquartile range
- mRS
modified Rankin scale
- NIHSS
National Institutes of Health Stroke Scale
- OR
odds ratio
- SBP
systolic blood pressure
Footnotes
Supplemental data at www.neurology.org
Editorial, page 2014
AUTHOR CONTRIBUTIONS
J. Kang: drafting/revising the manuscript and contribution of vital reagents/tools/patients. Y. Ko: study concept or design and acquisition of data. J.H. Park: analysis or interpretation of data and acquisition of data. W.-J. Kim: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, and study supervision. M.S. Jang: drafting/revising the manuscript and acquisition of data. M.H. Yang: drafting/revising the manuscript and acquisition of data. J.-S. Lee: analysis or interpretation of data and statistical analysis. J. Lee: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and statistical analysis. M.-K. Han: drafting/revising the manuscript and contribution of vital reagents/tools/patients. P. Gorelick: drafting/revising the manuscript, study concept or design, and analysis or interpretation of data. H.-J. Bae: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, statistical analysis, study supervision, and obtaining funding.
DISCLOSURE
J. Kang, Y. Ko, J.H. Park, W.-J. Kim, M.S. Jang, M.H. Yang, J.-S. Lee, J. Lee, and M.-K. Han report no disclosures. P. Gorelick reports serving on study steering committees for acute stroke treatment for Brainsgate (IMPACT-24) and D-Pharm (MASCI), serves as the Director of the US DIAS 4 in the acute ischemic stroke Clinical Coordinating Center for H. Lundbeck, and serves as a consultant to Genetech and Boehringer Ingelheim. H.-J. Bae received honoraria for lectures from MSD Korea, AstraZeneca Korea, BMS Korea, Sanofi Aventis Korea, Ostuka Korea, Pfizer Korea, Handok Pharmaceutical Company, and Chong Kun Dang Pharmaceutical; received funding for a trip from BMS Korea, Pfizer Korea, Dae Woong Pharmaceutical Company, and Ostuka Korea; received consultant fees from Yu Yu Pharmaceutical Company; and received compensation for board membership from Bayer Korea, Novartis Korea, MSD Korea, and Boehringer-Ingelheim Korea. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol 2009;8:938–948 [DOI] [PubMed] [Google Scholar]
- 2.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18–24 [DOI] [PubMed] [Google Scholar]
- 3.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–322 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez FJ, Segura T, Castellanos M, et al. Cerebral hemodynamic reserve and early neurologic deterioration in acute ischemic stroke. J Cereb Blood Flow Metab 2004;24:1267–1271 [DOI] [PubMed] [Google Scholar]
- 5.Aslanyan S, Fazekas F, Weir CJ, Horner S, Lees KR. Effect of blood pressure during the acute period of ischemic stroke on stroke outcome: a tertiary analysis of the GAIN International Trial. Stroke 2003;34:2420–2425 [DOI] [PubMed] [Google Scholar]
- 6.Stead LG, Gilmore RM, Vedula KC, Weaver AL, Decker WW, Brown RD., Jr Impact of acute blood pressure variability on ischemic stroke outcome. Neurology 2006;66:1878–1881 [DOI] [PubMed] [Google Scholar]
- 7.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276 [DOI] [PubMed] [Google Scholar]
- 8.Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol 2010;9:767–775 [DOI] [PubMed] [Google Scholar]
- 9.Sandset EC, Bath PM, Boysen G, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011;377:741–750 [DOI] [PubMed] [Google Scholar]
- 10.Schrader J, Luders S, Kulschewski A, et al. The ACCESS study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke 2003;34:1699–1703 [DOI] [PubMed] [Google Scholar]
- 11.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315–1320 [DOI] [PubMed] [Google Scholar]
- 12.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041 [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145–153 [DOI] [PubMed] [Google Scholar]
- 14.Christensen H, Meden P, Overgaard K, Boysen G. The course of blood pressure in acute stroke is related to the severity of the neurological deficits. Acta Neurol Scand 2002;106:142–147 [DOI] [PubMed] [Google Scholar]
- 15.Harper G, Castleden CM, Potter JF. Factors affecting changes in blood pressure after acute stroke. Stroke 1994;25:1726–1729 [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010;375:938–948 [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:e478–e534 [DOI] [PubMed] [Google Scholar]
- 18.Yoon B-W, Lee B-C, Heo J, et al. Clinical practice guidelines for stroke [online]. Available at: http://www.stroke-crc.or.kr/. Accessed May 15, 2012
- 19.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–1096 [DOI] [PubMed] [Google Scholar]
- 20.Saver JL, Yafeh B. Confirmation of tPA treatment effect by baseline severity-adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke 2007;38:414–416 [DOI] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131 [DOI] [PubMed] [Google Scholar]
- 22.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000;31:1062–1068 [DOI] [PubMed] [Google Scholar]
- 23.Sandercock P, Bamford J, Dennis M, et al. Atrial fibrillation and stroke: prevalence in different types of stroke and influence on early and long term prognosis (Oxfordshire community stroke project). BMJ 1992;305:1460–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megherbi SE, Milan C, Minier D, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project. Stroke 2003;34:688–694 [DOI] [PubMed] [Google Scholar]
- 25.Weimar C, Ziegler A, Konig IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol 2002;249:888–895 [DOI] [PubMed] [Google Scholar]
- 26.Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke 2000;31:463–468 [DOI] [PubMed] [Google Scholar]
- 27.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991;54:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010;375:906–915 [DOI] [PubMed] [Google Scholar]
- 29.Delgado-Mederos R, Ribo M, Rovira A, et al. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology 2008;71:552–558 [DOI] [PubMed] [Google Scholar]
- 30.Adams HP, Jr, Leclerc JR, Bluhmki E, Clarke W, Hansen MD, Hacke W. Measuring outcomes as a function of baseline severity of ischemic stroke. Cerebrovasc Dis 2004;18:124–129 [DOI] [PubMed] [Google Scholar]
- 31.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke 2007;38:3055–3062 [DOI] [PubMed] [Google Scholar]
- 32.Mattle HP, Kappeler L, Arnold M, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke 2005;36:264–268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.