Abstract
Background and Aim:
Intrathecal (IT) neostigmine has been used as an adjunct to spinal anesthesia. The purpose of this study was to determine whether a combination of low-dose neostigmine IT would enhance analgesia of a fixed dose of fentanyl IT, in patients undergoing unilateral total knee replacement (TKR) surgery with spinal anesthesia.
Settings and Design:
Forty-five patients scheduled for unilateral TKR were randomized to one of the three groups (n = 15) and prospectively studied using placebo-controlled, double-blinded design.
Materials and Methods:
A 19-G epidural catheter was introduced through the L3–L4 interspace with patient in the sitting position, followed by spinal anesthesia administration through the L3–L4 interspace. Fifteen milligrams of hyperbaric bupivacaine (3 ml) plus the test drug (0.5 ml) was administered IT. The test drug was normal saline (0.5 ml) in group I; fentanyl 20 mcg (0.4 ml) and normal saline (0.1 ml) in group II; and fentanyl 20 mcg (0.4 ml) and neostigmine 1 mcg (0.1 ml) in group III. Characteristics of sensory and motor block, heart rate, and blood pressure were recorded intraoperatively. Postoperatively, pain scores, postoperative nausea and vomiting (PONV) scores, and sedation scores, and postoperative analgesic dose were recorded.
Results:
Forty-five patients were enrolled in this study and 43 patients were subjected to statistical analysis. Overall 24-h visual analog score in group III was significantly less than in those who received fentanyl alone (P = 0.00). The durations of complete analgesia and effective analgesia were longer for all patients in group III compared with group II (P < 0.05) and group I (P < 0.005) patients. The total number of epidural top ups (rescue analgesia) required was less in group II (P < 0.05) and group III (P < 0.005) patients, compared with the control group. The incidence of nausea and vomiting was not increased in group III patients.
Conclusions:
The addition of 1 mcg neostigmine IT increased the duration of analgesia and decreased the analgesic consumption in 24 h in TKR. There was no increase in the incidence of adverse effects.
Keywords: Intrathecal fentanyl, intrathecal neostigmine, spinal neostigmine, total knee replacement surgery
Introduction
Intrathecal (IT) neostigmine has been used as an adjunct to spinal anesthesia (SA) for the prevention of acute perioperative pain. It has been shown to potentiate opioid analgesia[1–4] while reducing undesirable side effects such as somnolence and respiratory depression.[5,6] Though this multimodal pain therapy approach including spinal neostigmine and spinal opioids is efficacious, significant systemic side effects of IT neostigmine, especially nausea and vomiting, have been reported with doses higher than 6.25 mcg,[7] limiting it use in clinical practice. The benefits of adding lower neostigmine dose to potentiate fentanyl analgesia, however, have not been evaluated to date.
We planned to determine whether combination of low-dose (1 mcg) neostigmine IT would enhance analgesia from a fixed IT dose of fentanyl, in patients undergoing unilateral total knee replacement (TKR) surgery with SA.
Materials and Methods
After obtaining the institutional ethics committee clearance and written informed consent, 45 adult patients of American Society of Anaesthesiologists (ASA) 1 or 2, undergoing unilateral TKR surgery under regional anesthesia, were included in the study. Patients were randomly allocated into three groups of 15 patients each. The concept of visual analog scale (VAS), which consisted of a 10-cm line with 0 equaling “no pain at all” and 10 equaling “the worst possible pain,” was introduced before surgery.
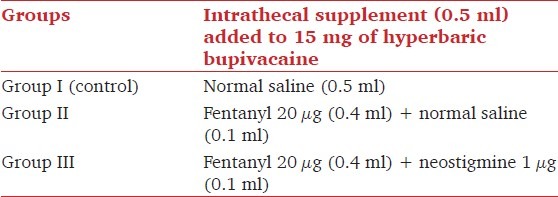
Patients were premedicated with 0.1–0.2 mg/kg oral diazepam on the morning of surgery. Intravenous (IV) preloading was done with Ringer's lactate as a bolus of 6–8 ml/kg given over 15 min before SA. A 19-G epidural catheter was introduced through the L3–L4 interspace with patient in the sitting position, followed by SA through the L3–L4 interspace. A total volume of 3.5 ml was injected IT, at a rate of 0.25 ml/sec, using a 26-G spinal needle. The IT drug injected was 15 mg hyperbaric bupivacaine (3 ml) plus the test drug (0.5 ml). The test drug was normal saline (0.5 ml) in Group I; fentanyl 20 mcg (0.4 ml) and normal saline (0.1 ml) in group II; and fentanyl 20 mcg (0.4 ml) and neostigmine (Neostigmine, Norris Medicines Ltd., Ankleshwar, India) 1 mcg (0.1 ml) in group III [Table 1]. Patients were placed in the supine position immediately after spinal injection. One anesthesiologist prepared the drug and administered the IT drug, while another anesthesiologist, who was blinded to the drug randomization, monitored the intraoperative and postoperative period.
Table 1.
Study groups
Intraoperative sensory loss assessment was done using pin-prick test at 5, 10, 15, 20 min after IT injection of the study drug and every 30 min thereafter, until the end of surgery. Motor blockade of lower extremities was measured using 4-point modified Bromage scale at 5 min intervals for the first 20 min after injection of the IT drug. Blood pressure was monitored noninvasively every 5 min throughout the surgery, and heart rate and oxyhemoglobin saturation were monitored continuously throughout the surgery. A decrease in mean arterial pressure of greater than 25% below the baseline preanesthetic value or less than 60 mmHg was treated by incremental doses of 6 mg mephenteramine IV. A decrease in heart rate of more than 15% below the baseline or 50 beats per min, whichever was lesser, was defined as bradycardia and was treated by atropine 0.5 mg IV.
Postoperative assessment included pain scores, postoperative nausea and vomiting (PONV) scores (5-point scale), and sedation scores (4-point scale), recorded at 30-min interval for first 4 h and then 4 hourly thereafter, for 24 h postoperatively. Duration of complete analgesia was taken as the time until VAS pain scores remained “0” following IT injection of the study drug. Duration of effective analgesia was the time until VAS pain scores were ≥3 cm or when the patient's first requested for supplemental analgesia, whichever appeared first. Subsequently, an epidural top up of 8 ml of 0.125% bupivacaine was administered as the rescue analgesic. If the pain score remained ≥3 even 30 min after epidural top up, additional analgesia with intramuscular diclofenac 1.5 mg/ kg was administered. The total number of rescue analgesics administered in 24 h was noted. “Overall 24-h VAS score” was obtained as a measurement of total pain experienced by the patient.
Nausea was scored by the patient using a 5-point scale. One or more emetic episodes were treated using ondansetron 4 mg IV. For patients experiencing more than one episode of nausea, the scores were averaged.
Sample size was selected to detect a projected difference of 75% between the neostigmine–fentanyl–bupivacaine and bupivacaine groups and 29% between the neostigmine–fentanyl–bupivacaine and fentanyl–bupivacaine groups, with respect to the primary variable, duration of effective analgesia, a type I error of 0.01, and a power of 0.85, and was based on a pilot study with 12 patients. Data from the pilot study for neostigmine–fentanyl–bupivacaine (mean 1 = 252 min), fentanyl-bupivacaine (mean 2 = 196 min), and bupivacaine (mean 3 = 144 min) groups with an estimated SD of 35 showed that 12 patients were required in each group. However, to compensate for possible dropouts, we included 15 patients in each group. The data were analyzed statistically. Demographic data (age, weight, height, gender) and duration of surgery were compared among the groups by one-way analysis of variance. Blood pressure, heart rate, maximum level and time to attain peak level of sensory block, time to complete motor blockade, and VAS scores were compared among the groups by two-way analysis of variance followed by Mann–Whitney test. P <0.05 was considered significant. The duration of complete analgesia, duration of effective analgesia, and the number of rescue analgesics in 24 h was compared using Kruskal–Wallis test, applied along with Mann–Whitney test. P <0.05 was considered significant.
Results
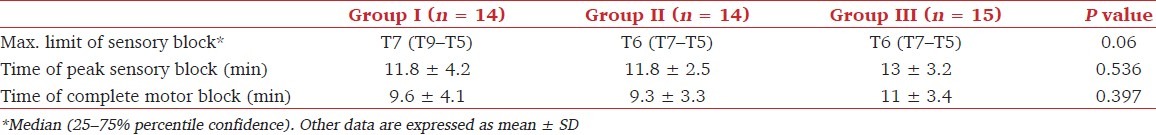
A total of 45 patients were recruited for the study. Two patients, one each from groups I and II, were excluded from the study as general anesthesia had to be administered due to the failure of SA and accidental dislodgement of epidural catheter, respectively. The three groups showed no differences with regard to age, weight, height, gender, and duration of surgery (P > 0.05) [Table 2]. The maximum level of sensory block, time to peak sensory block, and time to complete motor block were comparable among the groups [Table 3]. The intraoperative hemodynamic characteristics were also comparable and intraoperative mephenteramine consumption was similar among the groups.
Table 2.
Demographic characteristics and surgical duration
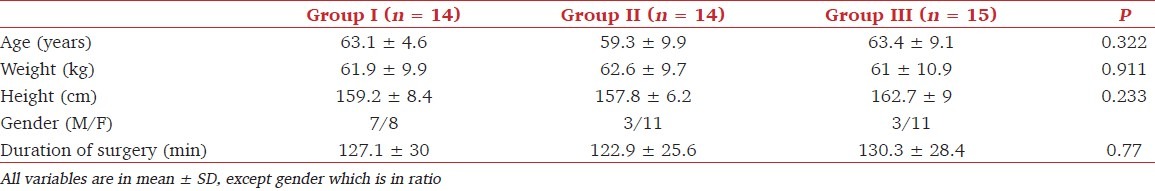
Table 3.
Characteristics of subarachnoid block
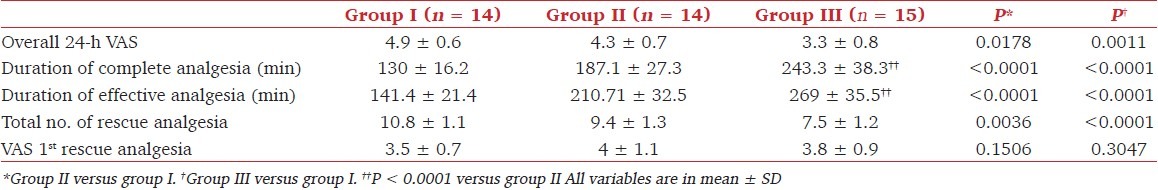
Analgesia characteristics are shown in Table 4. The baseline postoperative VAS scores were comparable in the three groups. However, overall 24-h VAS score in patients who received the combination of fentanyl with neostigmine was significantly less than in those who received fentanyl alone (P = 0.00). Overall 24-h VAS scores in the control group were significantly greater than in the fentanyl group (P = 0.00). The durations of complete analgesia and effective analgesia were longer for all patients in group III compared with group II (P < 0.05) and group I (P < 0.005). The total number of epidural top ups (rescue analgesia) required was less in the fentanyl group (P < 0.05) and fentanyl–neostigmine group (P < 0.005) compared with the control group.
Table 4.
Analgesia characteristics
Six patients in the control group required antiemetic once and only two patients twice. However, six patients in the fentanyl group and two patients in the fentanyl–neostigmine group received ondansetron once and no patient in either group needed antiemetic twice or more. This difference among the groups was not statistically significant (P > 0.05) [Figure 1]. No patient in any group had sedation score >2 at any time postoperatively.
Figure 1.
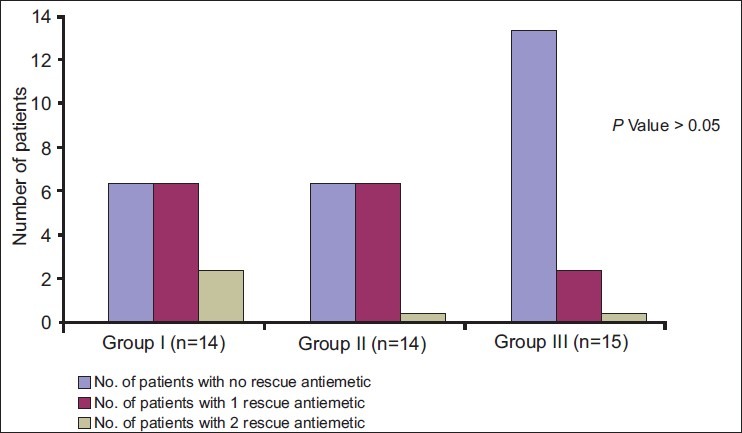
Antiemetic requirement in the three groups
Discussion
A dose-independent reduction of postoperative analgesia requirement and dose-dependent increase in the incidence of PONV has been demonstrated using various doses of IT neostigmine with bupivacaine.[8] Also, the fact that IT neostigmine enhances the analgesic action of opioid has been recognized.[4] These properties of IT neostigmine were best demonstrated in a study by Almeida et al.[9] The authors showed that the addition of 1–5 mcg neostigmine IT to 100 mcg morphine doubled the duration to first rescue analgesic in the patients undergoing major gynecological surgeries without increasing the incidence of adverse effects. However, the study was underpowered. Moreover, the risk of delayed respiratory depression with the use of neuraxial morphine is a great concern. We therefore hypothesized that 1 mcg IT neostigmine would augment the analgesic efficacy of IT fentanyl and bupivacaine, without increasing the incidence of untoward side effects. As TKR surgery involves severe pain in the postoperative period, we performed our study in this subset of patients.
We report an enhancement of the analgesic action of 20 mcg fentanyl IT by 1 mcg neostigmine IT in patients undergoing TKR surgery. The addition of a low dose of neostigmine increased the duration of complete analgesia and effective analgesia by 75% and 78%, respectively. All patients who received IT neostigmine in combination with IT bupivacaine and fentanyl required less epidural top ups in 24 h. “Overall 24-h VAS scores” were also better in these patients. However, no increase in the incidence of nausea and vomiting was noted with addition of 1 mcg neostigmine IT to fentanyl–bupivacaine IT combination.
IT injection of neostigmine produces analgesic effects. The inhibition of spinal cholinesterase by neostigmine results in an increase of endogenous acetylcholine, which is most likely released from intrinsic cholinergic neurons within the dorsal horn of the spinal cord.[10] These cholinergic neurons terminate in the vicinity of primary afferent express muscarinic receptors.[11,12] The endogenous acetylcholine produces analgesic effect through muscarinic presynaptic inhibition of glutamatergic afferents, similar to how it has been described in the neostriatum.[13] Muscarinic receptor antagonists have been shown to reverse the analgesic effects of IT neostigmine.[14] A tonic cholinergic activity is an important prerequisite for the effectiveness of neostigmine.[15,16] The enhanced analgesic efficacy of IT neostigmine results from greater release of spinal acetylcholine from the more intense and prolonged discomfort of postoperative pain,[17,18] and consequent action at muscarinic M1 and M3[14,19] and presynaptic nicotinic receptors[20] present in the cholinergic interneurons at the lamina III and V of the dorsal horn.[21] An action at nicotinic receptors at the dorsal horn ganglion[22] and at the spinal meninges[23] has also been suggested.
A possible explanation for the effect of small doses of IT neostigmine in enhancing the duration of analgesia produced by opioid relates to the mechanism of action of opioids in producing analgesia. Opioid increases the concentration of norepinephrine in lumbar cerebrospinal fluid which in turn produces analgesia in part by activating spinal cholinergic neurons to release acetylcholine.[24] This effect gets further accentuated in patients under noxious stimuli such as surgery.[16]
In our study, the time to reach maximum level of sensory block and the peak level attained was not influenced by the use of IT neostigmine. Similar effect was demonstrated by Lauretti et al,[25] in patients undergoing vaginal hysterectomy. This is possibly due to a difference in the onset of action of IT neostigmine[26] and IT bupivacaine.[27] The time to development of complete motor block was also not altered. No patient in the IT neostigmine–fentanyl–bupivacaine group developed intraoperative hypotension or bradycardia requiring treatment. Although similar effects on hemodynamics during SA using low-dose IT neostigmine were observed by few researchers,[7,27] this could not be explained on the basis of cardiostimulatory effect of spinal neostigmine as this requires large doses in humans.[26]
Use of low-dose IT neostigmine in an attempt to reduce the incidence of untoward side effects, particularly PONV, while retaining its analgesic efficacy has been tried by many investigators. In patients undergoing below knee surgery, Lauretti et al[8] showed a dose-independent reduction of postoperative analgesia requirement, but a dose-dependent increase in the incidence of PONV following addition of various doses of IT neostigmine (ranging from 25 to 100 mcg) to 15 mg of hyperbaric bupivacaine 0.5%. Even the dose as low as 6.25 mcg has been associated with high incidence of PONV.[7] Almeida et al, demonstrated a trend toward more nausea with doses higher than 1 mcg in patients undergoing major gynecological surgeries.[9] These observations are further supported by the present study. We observed a lower incidence of emesis and lesser need for antiemetic (though not significant) in patients receiving 1 mcg IT neostigmine as an adjunct to IT bupivacaine and IT fentanyl. This could be due to lower VAS scores in this group. Neostigmine 1 mcg IT can be considered a safe dose for retaining the analgesic efficacy while avoiding PONV. All patients were either awake or arousable to command in the postoperative period.
The commercially available preparation of neostigmine containing methylparaben as an antioxidant was used in the present study. Although solutions containing preservatives should not be injected IT, methyl- and propyl-paraben have not been demonstrated to be toxic. Phase I tolerability and safety study of the commercially available neostigmine formulations in human volunteers found no evidence of toxicity.[28] Moreover, preservative-free preparation of neostigmine is no longer available and all studies on IT neostigmine utilized neostigmine solutions containing preservatives.[7]
In conclusion, the addition of 1 mcg neostigmine IT increased the duration of postoperative analgesia and decreased the analgesic consumption in TKR surgery. There was no increase in the incidence of adverse effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Flodmark S, Wrammer T. The analgesia action of morphine, serine, and prostigmine studied by a modified Handy-Wolff-Goodel method. Acta Physiol Scand. 1945;9:88–96. [Google Scholar]
- 2.Pleuvry BJ, Tobias MA. Comparison of the antinociceptive activity of physostigmine, oxotremorine and morphine in the mouse. Br J Pharmacol. 1971;43:706–14. doi: 10.1111/j.1476-5381.1971.tb07205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauretti GR, Mattos AL, Reis MP, Pereira NL. Combined intrathecal fentanyl and neostigmine: therapy for postoperative abdominal hysterectomy pain relief. J Clin Anesth. 1998;10:291–6. doi: 10.1016/s0952-8180(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 4.Lauretti GR, Reis MP, Prado WA, Klamt JG. Dose response study of intrathecal morphine versus intrathecal neostigmine, their combination, or placebo for postoperative analgesia in patients undergoing anterior and posterior vaginoplasty. Anesth Analg. 1996;82:1182–7. doi: 10.1097/00000539-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock M, Davidson JT, Rosin AJ, Schnieden H. Effect of physostigmine on morphine-induced postoperative pain and somnolence. Br J Anaesth. 1982;54:429–34. doi: 10.1093/bja/54.4.429. [DOI] [PubMed] [Google Scholar]
- 6.Willette RN, Doorley BM, Sapru HN. Activation of cholinergic mechanisms in the medulla oblongata reverse intravenous opioid-induced respiratory depression. J Pharmacol Exp Ther. 1987;240:352–8. [PubMed] [Google Scholar]
- 7.Liu SS, Hodgson PS, Moore JM, Trautman WJ, Burkhead DL. Dose-response effects of spinal neostigmine added to bupivacaine spinal anesthesia in volunteers. Anesthesiology. 1999;90:710–7. doi: 10.1097/00000542-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lauretti GR, Mattos AL, Reis MP, Prado WA. Intrathecal neostigmine for post operative analgesia after orthopaedic surgery. J Clin Anesth. 1997;9:473–7. doi: 10.1016/s0952-8180(97)00103-7. [DOI] [PubMed] [Google Scholar]
- 9.Almeida RA, Lauretti GR, Mattos AL. Antinociceptive effect of low-dose intrathecal neostigmine combined with intrathecal morphine following gynecological surgery. Anesthesiology. 2003;98:495–8. doi: 10.1097/00000542-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro-da-Silva A, Cuello AC. Choline acetyltransferase-immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. J Comp Neurol. 1990;295:370–84. doi: 10.1002/cne.902950303. [DOI] [PubMed] [Google Scholar]
- 11.Day NS, Berti-Mattera LN, Eichberg J. Muscarinic cholinergic receptor-mediated phosphoinositide metabolism in peripheral nerve. J Neurochem. 1991;56:1905–13. doi: 10.1111/j.1471-4159.1991.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 12.Wanke E, Bianchi L, Mantegazza M, Guatteo E, Mancinelli E, Ferroni A. Muscarinic regulation of Ca2+ currents in rat sensory neurons: Channel and receptor types, dose-response relationships and cross-talk pathways. Eur J Neurosci. 1994;6:381–91. doi: 10.1111/j.1460-9568.1994.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 13.Barral J, Galarraga E, Bargas J. Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain Res Bull. 1999;49:285–9. doi: 10.1016/s0361-9230(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 14.Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg. 1997;85:847–53. doi: 10.1097/00000539-199710000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo M, Gebhart GF. Tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1991;46:211–22. doi: 10.1016/0304-3959(91)90078-C. [DOI] [PubMed] [Google Scholar]
- 16.Bouaziz H, Tong C, Eisenach JC. Postoperative analgesia from intrathecal neostigmine in sheep. Anesth Analg. 1995;80:1140–4. doi: 10.1097/00000539-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Krukowski JA, Hood DD, Eisenach JC, Mallak KA, Parker RL. Intrathecal neostigmine for post-cesarean section analgesia: dose response. Anesth Analg. 1997;84:1269–75. doi: 10.1097/00000539-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Eisenach JC, Detweiler DJ, Tong C, D’Angelo R, Hood DD. Cerebrospinal fluid norepinephrine and acetylcholine concentrations during acute pain. Anesth Analg. 1996;82:621–6. doi: 10.1097/00000539-199603000-00034. [DOI] [PubMed] [Google Scholar]
- 19.Honda K, Harada A, Takano Y, Kamiya H. Involvement of M3 muscarinic receptors of the spinal cord in formalin-induced nociception in mice. Brain Res. 2000;859:38–44. doi: 10.1016/s0006-8993(99)02456-7. [DOI] [PubMed] [Google Scholar]
- 20.Wamsley JK, Lewis MS, Yong WS, 3rd, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. 1981;1:176–91. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyosawa A, Katsurabayashi S, Akaike N, Pang ZP, Akaike N. Nicotinie facilitates glycine release in the rat spinal dorsal horn. J Physiol. 2001;536:101–10. doi: 10.1111/j.1469-7793.2001.t01-1-00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–82. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 23.Ummenhofer WC, Brown SM, Bernards CM. Acetylcholinesterase and butyrylcholinesterase are expressed in the spinal meninges of monkeys and pigs. Anesthesiology. 1998;88:1259–65. doi: 10.1097/00000542-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Bouaziz H, Tong C, Yoon Y, Hood DD, Eisenach JC. Intravenous opioids stimulate norepinephrine and acetylcholine release in spinal cord dorsal horn. Systematic studies in sheep and an observation in a human. Anesthesiology. 1996;84:143–54. doi: 10.1097/00000542-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Lauretti GR, Hood DD, Eisenach JC, Pfeifer BL. A multi-center study of intrathecal neostigmine for analgesia following vaginal hysterectomy. Anesthesiology. 1998;89:913–8. doi: 10.1097/00000542-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Malinovsky JM, Renaud G, Le Corre P, Charles F, Lepage JY, Malinge M, et al. Intrathecal bupivacaine in humans: influence of volume and baricity of solutions. Anesthesiology. 1999;91:1260–6. doi: 10.1097/00000542-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Eisenach JC, Hood DD, Curry R. Phase I human safety assessment of intrathecal neostigmine containing methyl- and propylparabens. Anesth Analg. 1997;85:842–6. doi: 10.1097/00000539-199710000-00024. [DOI] [PubMed] [Google Scholar]


