Abstract
Thyroid hormone (TH) plays critical roles during vertebrate postembryonic development. TH production in the thyroid involves incorporating inorganic iodide into thyroglobulin. The expression of iodotyrosine deiodinase (IYD; also known as iodotyrosine dehalogenase 1) in the thyroid gland ensures efficient recycling of iodine from the byproducts of TH biosynthesis: 3′-monoiodotyrosine and 3′, 5′-diiodotyrosine. Interestingly, IYD is known to be expressed in other organs in adult mammals, suggesting iodine recycling outside the thyroid. On the other hand, the developmental role of iodine recycling has yet to be investigated. Here, using intestinal metamorphosis as a model, we discovered that the Xenopus tropicalis IYD gene is strongly up-regulated by TH during metamorphosis in the intestine but not the tail. We further demonstrated that this induction was one of the earliest events during intestinal metamorphosis, with IYD being activated directly through the binding of liganded TH receptors to a TH response element in the IYD promoter region. Because iodide is mainly taken up from the diet in the intestine and the tadpole stops feeding during metamorphosis when the intestine is being remodeled, our findings suggest that IYD transcription is activated by liganded TH receptors early during intestinal remodeling to ensure efficient iodine recycling at the climax of metamorphosis when highest levels of TH are needed for the proper transformations of different organs.
Thyroid hormone (TH) plays critical roles in adult organs and during development in vertebrates (1). TH is synthesized in the thyroid gland as either T3 or the less active precursor form, T4. Iodine is an indispensable component of TH and is mainly taken up from the diet through the digestive tract, mainly the small intestine. Insufficient iodine uptake causes the so-called iodine deficiency disorders, such as goiter formation and hypothyroidism (2).
The thyroid gland has two systems to ensure sufficient iodine for TH synthesis. One is iodine uptake at the basolateral membrane by the sodium-iodine symporter (3, 4). The other one is the regeneration of iodide through the deiodination of 3′-monoiodotyrosine (MIT) and 3′, 5′-diiodotyrosine (DIT), the main iodinated byproducts of TH synthesis. Iodotyrosine deiodinase (IYD; also known as iodotyrosine dehalogenase 1) facilitates iodide salvage by catalyzing deiodination of MIT and DIT in the thyroid (5–7). IYD is distinct from other iodotyronine deiodinases that are known to catalyze the deiodination of TH, i.e. iodothyronine deiodinase 1 (D1), deiodinase 2 (D2), deiodinase 3 (D3) (8, 9) because IYD acts on only MIT and DIT but not T4 and T3. Failure of IYD function causes iodotyrosine deiodinase deficiency, which was clinically identified in the 1950s and shows goitrous hypothyroidism with elevated levels of iodotyrosine in serum and urine. The enzyme, however, was not purified and characterized until 1979 (10, 11). More recently the IYD gene was identified (12), and more importantly in 2008, mutations in the human IYD gene were reported to be associated with iodotyrosine deiodinase deficiency (13), demonstrating the importance of IYD in maintaining physiological levels of TH.
Interestingly, IYD expression is not restricted to the thyroid gland. IYD mRNA has been detected in adult human liver and kidney, and analysis of human expressed sequence tag database indicated that IYD mRNA is also expressed in the digestive tract (small intestine, colon, rectum) (14). The expression of IYD in nonthyroidal organs suggests that IYD promotes efficient iodide recycling to protect the body against low dietary iodine by acting in broad organs.
The most important period of TH action during vertebrate development is the so-called postembryonic development, which corresponds to the period from a few months before to several months after birth in human (15). TH deficiency during this period leads to developmental defects, including cretinism, which is characterized by short stature and severe mental retardation (2). The difficulty to manipulate the uterus-enclosed mammalian postembryonic embryos has so far hindered mechanistic investigations on how TH regulates this developmental period.
Amphibian metamorphosis is a complex morphogenic event that is regulated by TH and resembles postembryonic mammalian development in many aspects, including having peak levels of plasma TH (2, 15, 16). TH receptors (TR) have dual functions during development (17–29). In premetamorphic tadpoles, unliganded TR heterodimerized with 9-cis retinoic acid receptors (RXR) bind to at least some of the target genes and recruit corepressor complexes to repress their expression to ensure proper premetamorphic growth (22, 30–32). During metamorphosis, TH binds to TR and recruit coactivator complexes, leading to gene activation and metamorphic transformation of different organs (24, 25, 33–37). Despite the enormous progress in our understanding of the role of TR during metamorphosis, most of the TR target genes during development remain to be identified and characterized.
We have been studying the remodeling of the intestine during Xenopus metamorphosis as a model to understand the gene regulation cascade induced by TH that is responsible for the transformation of the larval organ to the adult one. This process involves the degeneration of the larval epithelium through apoptosis and concurrent de novo development of the adult epithelial stem cell through dedifferentiation of some larval cells (38–41). Toward understanding how liganded TR induces intestinal remodeling, we initiated a chromatin immunoprecipitation (ChIP)-on-chip analysis of the intestine by using a set of microarray chips covering an 8-kb region flanking each putative promoter of 17,000 Xenopus tropicalis genes to look for genes bound by TR (Das, B., K. Matsuura, L. Fu, and Y.-B. Shi, unpublished observation). Although the ChIP-on-chip data are still being analyzed and validated, we observed that one of the putative TR target genes identified from the ChIP-on-chip assay is homologous to the mammalian IYD gene. This suggested an interesting possibility that the IYD gene might be up-regulated by TH to facilitate iodine recycling to ensure sufficient TH during metamorphosis since the animal stops feeding. Thus, we carried out detailed analysis of the Xenopus tropicalis IYD gene. Our results indicate that IYD is strongly up-regulated by TH specifically in the intestine but not the tail during Xenopus metamorphosis, supporting a role of IYD in organ-specific deiodination of MIT and DIT for iodine recycling when the intestine is being remodeled at the climax of metamorphosis. Furthermore, we show that this up-regulation is directly at the transcription level through the binding of TR to a TH response element (TRE) located in the promoter region of the IYD gene in the tadpole intestine during metamorphosis.
Materials and Methods
Experimental animals
Xenopus tropicalis tadpoles and Xenopus laevis frogs were purchased from Nasco (Fort Atkinson, MI). Tadpoles were staged according to Nieuwkoop and Faber (42). When indicated, the stage 54 tadpoles were treated with 10 nm T3 for 2 d at 22 C. All animal procedures were done as approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Use and Care Committee.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from tadpole small intestine or tail at indicated stages. cDNA was synthesized with 2.5 μg of total RNA in 50 μl by using the high-capacity cDNA archive kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions. qRT-PCR was carried out by using SYBR Green PCR master mix (Applied Biosystems). Because the expression of elongation factor-1α (EF1α) is stable during natural and T3-induced metamorphosis (43, 44), the level of IYD mRNA was normalized to that of EF1α as described previously (45). The primers for IYD were forward 5′-TGAGGACGGTGAAGATAAGGA-3′ and reverse 5′-ACTGAGTATCGGGCAGAGGA-3′.
Bioinformatics identification of TRE
The computational analysis program NHR-Scan (46) was used to search for putative TRE with the default setting.
Gel mobility shift assay
This was performed as described (45). Briefly, X. laevis TR and RXR proteins were made by using TNT SP6 quick coupled transcription/translation system (Promega, Madison, WI). Infrared (IR) dye IR700 (LI-COR, Lincoln, NE)-labeled TRE of the X. laevis TRβA gene was used as a probe. The sequences of the sense strands of the IYD TRE oligonucleotides used for competition are as follows: 5′-AATTCCATGACCGCATTTGACTTCTCCGTC-3′ for IYD TRE, and 5′-AATTCCATGATTGCATTTGACTTCTCCGTC-3′ for mutant IYD TRE (bold letters are the TRE half-sites, and the mutated nucleotides are underlined.). One hundred femtomoles of IR-labeled probe was used in each reaction, and wild-type and mutant IYD TRE oligonucleotides were diluted to 400 fmol/μl, 2 pmol/μl, and 10 pmol/μl to obtain 4×, 20×, and 100× unlabeled oligonucleotides as the competitors. The assays were done by incubating 1 μl of each TR and RXR protein from the in vitro translation reaction with 1 μl of the labeled probe (100 fmol/μl) and 1 μl of appropriately diluted unlabeled competitor solution at room temperature for 20 min. Samples were electrophoresed on 6% DNA retardation gels (Invitrogen, Grand Island, NY) and then scanned by using Odyssey infrared scanner (LI-COR).
Generation of promoter constructs
The IYD promoter region was PCR amplified from X. tropicalis genomic DNA with the primer pair 5′-CCCCGGTACCTATGCAATGGGCAAGCCGCAGGTCT-3′ (bearing KpnI site at its 5′-end) and 5′-CCCCCTCGAGGTATGATTGGTCGGCTATACGCCTGGA-3′ (bearing XhoI site at its 5′-end) by using high fidelity PrimeStar DNA polymerase (TaKaRa Bio Inc., Mountain View, CA). The region was chosen to include the putative TRE and basic core promoter. The PCR product was cloned into KpnI and XhoI sites of pGL4.10 firefly luciferase vector (Promega). The mutant promoter harboring the mutant TRE was generated from the wild-type clone by using QuickChange lightening site-direct mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA) according to the instructions of the manufacturer. The primer used for mutagenesis is: 5′-GTCCAAGATGAGAATTCCATGATTGCATTTGATTTCTCCGTCTGGGGTTTAATTG-3′ (the mutated nucleotides are underlined). The mutated promoter was confirmed by DNA sequencing.
Transcription assay in the X. laevis oocyte system
The plasmids containing X. tropicalis TRα and RXRβ were linearized and transcribed in vitro by using a T7 kit (Life Technologies/Ambion, Grand Island, NY) as previously described (29, 47). The cytoplasm of stage VI oocytes from X. laevis was injected with 230 pg per oocyte of the TR and RXR mRNA. Two hours later, the firefly luciferase reporter under the control of the wild-type or mutant promoter of X. tropicalis IYD gene (69 pg per oocyte) and the phRG-TK Renilla luciferase (Promega) (6.9 pg per oocyte) as an internal control were coinjected into the nucleus. After incubation at 18 C overnight in the presence or absence of 100 nm T3, the injected oocytes were prepared for luciferase assay using the dual-luciferase-reporter assay system according to the manufacture's protocol (Promega). Six oocytes per sample were lysed in 90 μl of 1× lysis buffer (Promega), and 10 μl of lysate was used for the luciferase assays. Three independent samples were done for each injection at the same time. The relative expression of firefly luciferase to Renilla luciferase was determined. Each data point represents the average of three groups. The data shown were representative of a few independent experiments with similar results.
ChIP assay
ChIP assays on tadpole intestine and tail were done as described previously (28) with anti-TR (new PB) antibody, which recognizes both TRα and TRβ (29). As a negative control, a polyclonal antibody against Id14, an extracellular protein (48), was also used. All the treatment and control groups had three replicas, and each replica consisted of a pool of six to eight tadpoles. All ChIP experiments were done twice with similar results.
For immunoprecipitation, the DNA concentration of the chromatin was diluted to 10 ng/μl with ChIP dilution buffer (Millipore, Billerica, MA). After precleaning with salmon sperm DNA/protein A-agarose beads, input samples were taken, and 500 μl of each chromatin sample was added to the tubes with the indicated antibodies and the salmon sperm DNA/protein A-agarose beads. The mixture was incubated overnight at 4 C. After incubation, the beads were washed sequentially with ChIP buffer I, ChIP buffer II, ChIP buffer III, and Tris/EDTA (Millipore). After the last wash, 200 μl of elution buffer was added to the samples as well as the input controls and incubated at 65 C overnight, and the DNA was purified by phenol/chloroform extraction.
The immunoprecipitated DNA was analyzed by quantitative PCR with the gene-specific primers for the TRE region of X. tropicalis IYD gene (5′-CCCCAGACGGAGAAGTCAAATGCGGT-3′ and 5′-GTGAGATTCACATCCATGTCTGAAGGTGCA-3′) and for exon 3 of X tropicalis IYD gene (5′-CCTGGACATTTGTTGTTGTTCA-3′ and 5′-GAGGACGTGCAATTTCTTCAAT-3′).
Statistical analysis
A Student t test was done, and where indicated, a star shows a pair of samples with significant difference (P < 0.05).
Results
Xenopus and mammalian iodotyrosine deiodinases are highly conserved
We obtained the coding sequences for X. tropicalis and X. laevis IYD from published cDNA and genomic sequences and deduced amino acid sequences. By using the Conserved Domain Database analysis from the National Center for Biotechnology Information (Bethesda, MD), we observed that X. laevis or X. tropicalis IYD is very similar to the mouse or human IYD (Fig. 1). It contains an iodotyrosine dehalogenase domain (Fig. 1) and belongs to the nicotinamide adenine dinucleotide hydroxide oxidase/flavin reductase superfamily (49).
Fig. 1.
The IYD gene is highly conserved in vertebrates. Amino acid sequence comparison among human, mouse, X. tropicalis, and X. laevis IYD gene. The amino acid residues identical to the human IYD sequence are shaded in gray. The conserved iodotyrosine dehalogenase domain of IYD is boxed.
Next, to determine the relationship between IYD and the well-known TH deiodinases (D1, D2, and D3) (50), we constructed a phylogenetic tree of human and Xenopus IYD and TH deiodinases. The result showed that the IYD are in a clade distinct from that for D1, D2, and D3 (not shown), suggesting that the IYD lineage diverged from the iodothyronine deiodinase lineage early during vertebrate evolution.
The expression of IYD is up-regulated in the intestine during natural and T3-induced metamorphosis
As indicated in the introductory text, a preliminary ChIP-on-chip assay of 17,000 putative promoter regions of X. tropicalis genome revealed that TR was bound to the IYD promoter region in the tadpole intestine, suggesting that IYD is a TR target gene during metamorphosis. To determine whether IYD is indeed regulated by T3 during metamorphosis, we treated premetamorphic tadpoles at stage 54 with T3 for 2 d. Total RNA was isolated from the intestine and tail and subjected to qRT-PCR analysis for IYD expression. The results showed that T3 strongly induced the expression of IYD mRNA in the intestine, whereas IYD expression remained very low, even in the presence of T3 in the tail (Fig. 2), suggesting tissue-specific induction of the gene by T3.
Fig. 2.
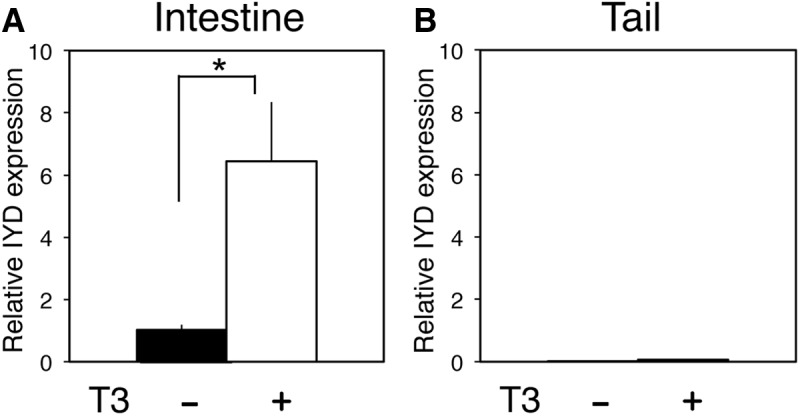
The expression of IYD is strongly up-regulated in the intestine but remains low in tail upon T3 treatment of premetamorphic X. tropicalis tadpoles. Premetamorphic tadpoles were treated with 10 nm T3 for 2 d, and total RNA was isolated from the intestine and tail. qRT-PCR was performed to examine IYD expression in the intestine (A) and tail (B). The IYD mRNA level was normalized to EF1α expression. Error bars indicate sem (n = 3). *, P < 0.05.
To investigate whether IYD plays a role during natural metamorphosis, we analyzed IYD mRNA by qRT-PCR in the intestine and tail at different stages from premetamorphic (stage 54), metamorphic climax (stages 58–64), to the end of metamorphosis (stage 66). As shown in Fig. 3, the expression of IYD was up-regulated in the intestine during metamorphosis with a peak level at stage 60 (Fig. 3A), whereas it remained very low throughout metamorphosis in the tail, although it was higher at climax (stage 62) (Fig. 3B). Similar up-regulation of the IYD gene was observed in the X. laevis intestine (data not shown). Thus, IYD is strongly regulated by T3 specifically in the intestine during both natural and T3-induced metamorphosis.
Fig. 3.
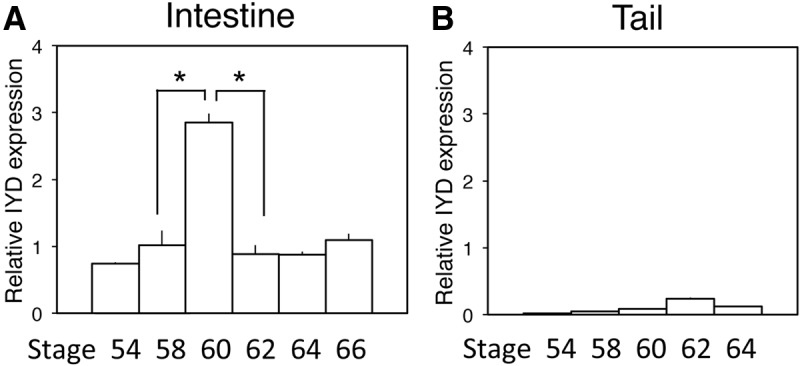
Developmental expression profiles of IYD in the intestine and tail during natural metamorphosis of X. tropicalis. Total RNA was isolated from the intestine and tail of X. tropicalis tadpoles at the indicated stages, and qRT-PCR was carried out to determine IYD expression. The IYD mRNA level was normalized to EF1α expression (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Note that in the intestine, the expression of IYD mRNA was dramatically up-regulated by stage 60 and then reduced by the end of metamorphosis (A). In contrast, in the tail, the IYD mRNA expression remained low throughout development (B). Error bars indicate sem (n = 3). *, P < 0.05.
TR/RXR heterodimer can bind to a putative TRE in the IYD gene promoter region in vitro
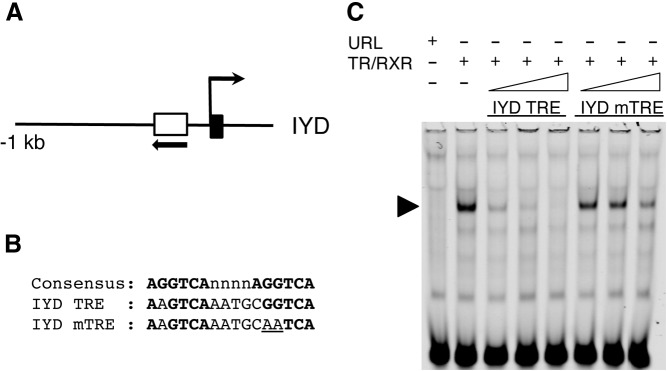
To determine how T3 regulates the IYD gene, we carried out a sequence analysis on the promoter region that was bound by TR in our preliminary ChIP-on-chip assay. We found a putative TRE near the predicted transcription start site (Fig. 4A). A gel mobility shift assay was carried out to determine whether TR could bind to the putative TRE. The IR-labeled TRE of X. laevis TRβA gene, a well-characterized TRE consisting of two nearly perfect direct repeats of AGGTCA separated by 4 bp (51), was mixed with in vitro-translated TR and RXR in the presence or absence of unlabeled competitors. The wild-type TRE from the IYD gene as well as the IYD TRE with one of the half-site mutated (Fig. 4A) were used as the competitors. As shown in Fig. 4B, a strong TRE-TR/RXR complex was formed between TR/RXR and the TRβA TRE, as expected. In the presence of the increasing levels of the IYD TRE, the complex formation was competed away dramatically, whereas the mutant IYD TRE was ineffective in the competition. Thus, the IYD TRE could bind to TR/RXR specifically in vitro.
Fig. 4.
TR binds to the TRE in the IYD gene in vitro. A, Schematic diagram of the promoter region of the X. tropicalis IYD gene. The IYD TRE is shown as a white box with arrow, indicating the orientation of TRE. Black box shows the first exon. B, Comparison of the sequences of the TRE of the IYD gene and the mutated TRE (mTRE) to the consensus TRE. The TRE half-sites are shown in bold letters. The mutated nucleotides in the mTRE used for the gel mobility shift assay are underlined. C, The IYD TRE binds to TR/RXR heterodimers in vitro. A gel mobility shift assay was done with TR/RXR heterodimers translated in reticulocyte lysate in vitro and labeled X. laevis TRβ promoter TRE, a well-characterized TRE (51), in the presence or absence of 4-, 20-, or 100-fold of unlabeled wild-type or mutant IYD TRE as the competitor. Unprogrammed reticulocyte lysate (URL) was used as a negative control. The arrowhead indicates the complex of TRβ TRE with TR/RXR. Note that the complex was competed away efficiently by an excess of unlabeled wild-type IYD TRE but not mutated IYD TRE, indicating specific binding of the IYD TRE to TR/RXR.
TR/RXR regulates the IYD promoter in vivo in a T3-dependent manner
We next investigated whether TR/RXR could activate the IYD promoter in the presence of T3 in vivo. For this purpose, we used the reconstituted X. laevis oocyte transcription system, which allows the analysis of the promoter in the context of chromatin in vivo (47). We microinjected mRNA encoding TR and RXR into the frog oocyte cytoplasm to allow the synthesis of the proteins. The reporter plasmid containing the IYD promoter driving the firefly luciferase was then injected together with an internal control plasmid phRG-tk expressing Renilla luciferase into oocyte nucleus. After culturing the oocytes in the presence or absence of T3 overnight, the oocytes were isolated for dual-luciferase assay with the ratio of firefly luciferase to Renilla luciferase activities as a measure of the IYD promoter activity. The results in Fig. 5 showed that the wild-type IYD promoter was expressed at a basal level in the oocyte in the absence of T3 and injected TR/RXR. Expression of TR/RXR in the absence of T3 had little effect on the promoter, whereas the addition of T3 in the presence of TR/RXR led to strong activation of the promoter, indicating that liganded TR/RXR activated the IYD promoter in vivo. When the TRE of the IYD promoter was mutated, the mutant promoter became unresponsive to TR/RXR and T3. Thus, TR/RXR can activate the IYD promoter via the TRE in the presence of T3 in vivo.
Fig. 5.
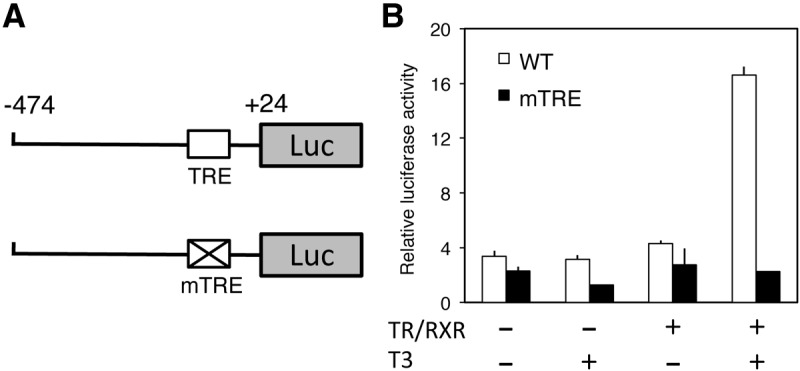
The TRE in X. tropicalis IYD promoter mediates activation by liganded TR. A, Schematic diagrams show the reporter constructs of the wild-type and mutant of X. tropicalis IYD promoter. B, Mutating the TRE eliminates the induction of the promoters by T3. Wild-type or mutant (mTRE) promoter construct was coinjected with the control Renilla luciferase construct phRG-tk into the nuclei of the oocytes with or without prior cytoplasmic injection of mRNA for X. tropicalis TRα and RXRβ. The oocytes were incubated at 18 C overnight in the presence or absence of 100 nm T3 and then used for dual-luciferase assays. The relative activities of the firefly luciferase to Renilla luciferase were plotted. Error bars indicate sem (n = 3).
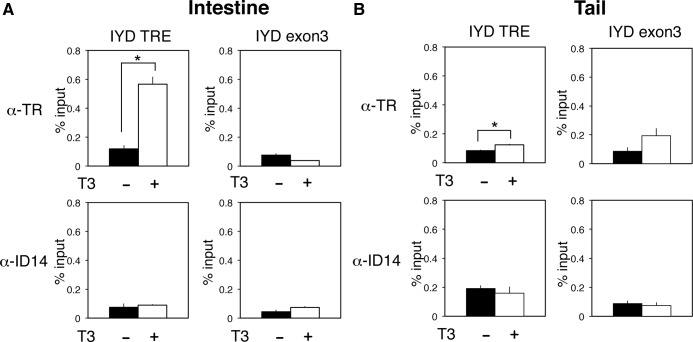
TR is strongly associated to the IYD TRE in the intestine but not tail of premetamorphic tadpoles treated with T3
As mentioned above, the IYD promoter region was found to be associated with TR in the intestine from our preliminary ChIP-on-chip assay of 17,000 putative promoter regions in X. tropicalis. To confirm the binding of TR to the TRE region of IYD gene in tadpoles, ChIP assays with a polyclonal antibody recognizing both TRα and TRβ were carried out on the intestine and tail of stage 54 premetamorphic tadpoles treated with or without T3 for 2 d. The results showed that in the absence of T3 treatment, there was little TR associated with the TRE region in either the intestine (Fig. 6A) or tail (Fig. 6B) because the ChIP signal for TR was comparable with that with a control polyclonal antibody against ID14, an extracellular matrix protein (48) in both the intestine or tail. After T3 treatment, TR binding to the IYD TRE was dramatically increased in the intestine (Fig. 6A) but not the tail. In addition, only background TR ChIP signal was observed in the exon 3 region of the IYD gene in both the intestine and tail, demonstrating the specificity of TR binding to the TRE region. Thus, TR was bound to the TRE of the IYD gene specifically in the intestine, consistent with the observed strong up-regulation of the IYD gene by T3 in the intestine but not tail during T3-induced and natural metamorphosis.
Fig. 6.
TR strongly associates specifically with the TRE in the IYD promoter in the intestine but not the tail in vivo. Stage 54 premetamorphic tadpoles were treated with or without T3 for 2 d, and the intestine (A) and tail (B) were isolated for ChIP assay with the anti-TR antibody (top panel) or anti-Id14 antibody (bottom panel), which served as a negative control for antibody specificity. The immunoprecipitated DNA was analyzed by qPCR for the presence of the TRE region of IYD promoter. A region of IYD exon 3 with no TRE was analyzed as a negative control for binding specificity. Note that little or no TR was bound to the TRE of the IYD gene in the absence of T3 in premetamorphic tadpoles. In the presence of T3, TR binding to the TRE in the intestine was strongly increased in the intestine, but not the tail, in agreement with the specific, strong regulation of IYD mRNA expression by T3 in the intestine but not the tail. There was no TR binding to IYD exon 3 in the presence or absence of T3. Only background signals were observed with the anti-ID14 antibody. Error bars indicate sem (n = 3). *, P < 0.05.
Discussion
It has been well established that TH levels peak during the postembryonic development: at the climax during amphibian metamorphosis and around birth in mammals. Iodine is essential for TH biosynthesis and is taken up via the digestive tract. Interestingly, during amphibian metamorphosis, tadpoles stop feeding while plasma TH reaches peak levels, suggesting that iodine is been efficiently recycled. Our studies here demonstrate that the X. tropicalis IYD gene is up-regulated by TH specifically in the intestine as one of the first events during intestinal remodeling, supporting a possible role of IYD in iodine recycling during metamorphosis.
The X. tropicalis IYD gene was initially identified as a putative TR target gene due to the binding of TR to its promoter region based on a preliminary ChIP-on-chip assay. Our gel mobility shift assay demonstrated that TR/RXR heterodimers could bind to a TRE located upstream of the transcription start site in vitro. More importantly, we showed that TR/RXR activated the promoter in the presence of T3 in the context chromatin in the frog oocyte transcription system in a TRE-dependent manner, indicating that TR activates the promoter directly at the transcription level.
TR/RXR has been shown to bind and repress target promoters in the absence of T3 by recruiting corepressor complexes, including in the frog oocyte system (52). Interestingly, there was little repression of the IYD promoter in the reconstituted frog oocyte system by unliganded TR/RXR. Although the underlying mechanism is unclear, a few possibilities exist. First, it is possible that tissue-specific factors uniquely important for the repression of the IYD gene might be missing in the oocyte. Alternatively, TR/RXR binding to the TRE is weak in the absence of TH in the context of this promoter in vivo because the TRE sequences and histone modifications can affect TR binding during metamorphosis (27, 53). Our in vivo ChIP assay supports the second possibility. By isolating the intestine and tail from premetamorphic tadpoles treated with or without T3 and subjecting them to a ChIP assay with an anti-TR antibody, we showed clearly that only background signals were present at the TRE of the IYD promoter in the absence of T3 in either the intestine or tail. T3 treatment led to strong increase in TR association with the TRE in the intestine but not the tail. These findings suggest that in the absence of T3, little or no TR was present at the TRE in tadpoles and the presence of T3 led to a strong increase in TR binding to the TRE specifically in the intestine. This agrees with a lack of repression of the promoter by TR in the frog oocyte in the absence of T3 as well as with the lack of a strong induction of the gene by T3 in the tail during metamorphosis.
The X. tropicalis IYD gene has little expression in the tadpole tail but is highly up-regulated in the intestine at the climax of metamorphosis. Its up-regulation in the intestine is directly at the transcriptional level, although the binding of liganded TR to the TRE in the promoter region. These results suggest that there is a predetermined genetic program to enable the intestine as an organ for iodine recycling during metamorphosis. This may make good sense physiologically because the tadpole intestine is the major organ for iodine uptake for TH biosynthesis before metamorphosis. Because the tadpole initiates metamorphosis and stops feeding, it becomes critical to ensure efficient utilization of the existing iodine in the animals. The normal physiological function of the intestine as the organ to process and absorb nutrients makes it an ideal organ to regenerate iodide from TH metabolites for TH biosynthesis in the thyroid gland. On the other hand, the tail likely lacks the efficient transport system to supply the regenerated iodide back to the thyroid gland even if it expressed IYD. Thus, it may not be surprising that IYD does not need to be up-regulated by TH in the tail. Clearly our studies raise a number of interesting questions for future studies. For example, why is TR strongly associated specifically with the IYD TRE in the intestine but not the tail, even though both organs express high levels of TR during metamorphosis? Also, does intestinal IYD indeed participate in iodide regeneration and is this required for tadpole metamorphosis? Future molecular and genetic studies should help to address these interesting questions.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Current address for K.F.: Division of Gene Structure and Function, Research Center for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi, Saitama, Japan 350-1241.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- D1
- deiodinase1
- D2
- deiodinase 2
- D3
- deiodinase 3
- DIT
- 3′, 5′-diiodotyrosine
- EF1α
- elongation factor 1α
- IR
- infrared
- IYD
- iodotyrosine deiodinase
- MIT
- 3′-monoiodotyrosine
- qRT-PCR
- quantitative RT-PCR
- RXR
- 9-cis retinoic acid receptor
- TH
- thyroid hormone
- TR
- thyroid hormone receptor
- TRE
- TH response element.
References
- 1. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 2. Hetzel BS. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford, UK: Oxford University Press [Google Scholar]
- 3. Jhiang SM. 2000. Regulation of sodium/iodide symporter. Rev Endocr Metab Disord 1:205–215 [DOI] [PubMed] [Google Scholar]
- 4. Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. 2003. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 [DOI] [PubMed] [Google Scholar]
- 5. Moreno JC, Visser TJ. 2010. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Mol Cell Endocrinol 322:91–98 [DOI] [PubMed] [Google Scholar]
- 6. Rokita SE, Adler JM, McTamney PM, Watson JA., Jr 2010. Efficient use and recycling of the micronutrient iodide in mammals. Biochimie (Paris) 92:1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kopp PA. 2008. Reduce, recycle, reuse—iodotyrosine deiodinase in thyroid iodide metabolism. N Engl J Med 358:1856–1859 [DOI] [PubMed] [Google Scholar]
- 8. St. Germain DL, Galton VA. 1997. The deiodinase family of selenoproteins. Thyroid 7:655–668 [DOI] [PubMed] [Google Scholar]
- 9. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg IN, Goswami A. 1979. Purification and characterization of a flavoprotein from bovine thyroid with iodotyrosine deiodinase activity. J Biol Chem 254:12318–12325 [PubMed] [Google Scholar]
- 11. Goswami A, Rosenberg IN. 1979. Characterization of a flavoprotein iodotyrosine deiodinase from bovine thyroid. Flavin nucleotide binding and oxidation-reduction properties. J Biol Chem 254:12326–12330 [PubMed] [Google Scholar]
- 12. Moreno JC. 2003. Identification of novel genes involved in congenital hypothyroidism using serial analysis of gene expression. Horm Res 60(Suppl 3):96–102 [DOI] [PubMed] [Google Scholar]
- 13. Moreno JC, Klootwijk W, van Toor H, Pinto G, D'Alessandro M, Lèger A, Goudie D, Polak M, Grüters A, Visser TJ. 2008. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N Engl J Med 358:1811–1818 [DOI] [PubMed] [Google Scholar]
- 14. Gnidehou S, Lacroix L, Sezan A, Ohayon R, Noël-Hudson MS, Morand S, Francon J, Courtin F, Virion A, Dupuy C. 2006. Cloning and characterization of a novel isoform of iodotyrosine dehalogenase 1 (DEHAL1) DEHAL1C from human thyroid: comparisons with DEHAL1 and DEHAL1B. Thyroid 16:715–724 [DOI] [PubMed] [Google Scholar]
- 15. Tata JR. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 [DOI] [PubMed] [Google Scholar]
- 16. Shi YB. 1999. Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons, Inc [Google Scholar]
- 17. Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA 98:10739–10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown DD, Cai L. 2007. Amphibian metamorphosis. Dev Biol 306:20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buchholz DR, Hsia SC, Fu L, Shi YB. 2003. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23:6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB. 2004. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol 24:9026–9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchholz DR, Paul BD, Fu L, Shi YB. 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- 22. Shi YB. 2009. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid 19:987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakajima K, Yaoita Y. 2003. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn 227:246–255 [DOI] [PubMed] [Google Scholar]
- 24. Denver RJ, Hu F, Scanlan TS, Furlow JD. 2009. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol 326:155–168 [DOI] [PubMed] [Google Scholar]
- 25. Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. 2008. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor β. J Biol Chem 283:2275–2285 [DOI] [PubMed] [Google Scholar]
- 26. Schreiber AM, Mukhi S, Brown DD. 2009. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol 331:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bilesimo P, Jolivet P, Alfama G, Buisine N, Le Mevel S, Havis E, Demeneix BA, Sachs LM. 2011. Specific histone lysine 4 methylation patterns define TR-binding capacity and differentiate direct T3 responses. Mol Endocrinol 25:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuura K, Fujimoto K, Fu L, Shi YB. 2012. Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology 153:961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Matsuda H, Shi YB. 2008. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology 149:5610–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomita A, Buchholz DR, Shi YB. 2004. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol 24:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi YB. 2002. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol 22:8527–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato Y, Buchholz DR, Paul BD, Shi YB. 2007. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mech Dev 124:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda H, Paul BD, Choi CY, Hasebe T, Shi YB. 2009. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol Cell Biol 29:745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paul BD, Buchholz DR, Fu L, Shi YB. 2005. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J Biol Chem 280:27165–27172 [DOI] [PubMed] [Google Scholar]
- 35. Paul BD, Fu L, Buchholz DR, Shi YB. 2005. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol 25:5712–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paul BD, Buchholz DR, Fu L, Shi YB. 2007. SRC-p300 coactivator complex is required for thyroid hormone induced amphibian metamorphosis. J Biol Chem 282:7472–7481 [DOI] [PubMed] [Google Scholar]
- 37. Havis E, Sachs LM, Demeneix BA. 2003. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports 4:883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. 2011. The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ishizuya-Oka A, Shi YB. 2011. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci 1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. 2011. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 29:154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. 2009. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J 23:2568–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nieuwkoop PD, Faber J. 1965. Normal table of Xenopus laevis. Amsterdam: North Holland Publishing [Google Scholar]
- 43. Yaoita Y, Nakajima K. 1997. Induction of apoptosis and CPP32 expression by thyroid hormone in a myoblastic cell line derived from tadpole tail. J Biol Chem 272:5122–5127 [DOI] [PubMed] [Google Scholar]
- 44. Nakajima K, Takahashi A, Yaoita Y. 2000. Structure, expression, and function of the Xenopus laevis caspase family. J Biol Chem 275:10484–10491 [DOI] [PubMed] [Google Scholar]
- 45. Das B, Heimeier RA, Buchholz DR, Shi YB. 2009. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem 284:34167–34178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandelin A, Wasserman WW. 2005. Prediction of nuclear hormone receptor response elements. Mol Endocrinol 19:595–606 [DOI] [PubMed] [Google Scholar]
- 47. Wong J, Shi YB. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- 48. Buchholz DR, Ishizuya-Oka A, Shi YB. 2004. Spatial and temporal expression pattern of a novel gene in the frog Xenopus laevis: correlations with adult intestinal epithelial differentiation during metamorphosis. Gene Expr Patterns 4:321–328 [DOI] [PubMed] [Google Scholar]
- 49. Friedman JE, Watson JA, Jr, Lam DW, Rokita SE. 2006. Iodotyrosine deiodinase is the first mammalian member of the NADH oxidase/flavin reductase superfamily. J Biol Chem 281:2812–2819 [DOI] [PubMed] [Google Scholar]
- 50. Morvan Dubois G, Sebillot A, Kuiper GG, Verhoelst CH, Darras VM, Visser TJ, Demeneix BA. 2006. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology 147:4941–4949 [DOI] [PubMed] [Google Scholar]
- 51. Ranjan M, Wong J, Shi YB. 1994. Transcriptional repression of Xenopus TR β gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem 269:24699–24705 [PubMed] [Google Scholar]
- 52. Wong J, Shi YB, Wolffe AP. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR β A gene by the thyroid hormone receptor. Genes Dev 9:2696–2711 [DOI] [PubMed] [Google Scholar]
- 53. Buchholz DR, Paul BD, Shi YB. 2005. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem 280:41222–41228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.