Abstract
Injury-induced bleeding is stopped by a hemostatic plug formation that is controlled by a complex nonlinear and spatially heterogeneous biochemical network of proteolytic enzymes called blood coagulation. We studied spatial dynamics of thrombin, the central enzyme of this network, by developing a fluorogenic substrate-based method for time- and space-resolved imaging of thrombin enzymatic activity. Clotting stimulation by immobilized tissue factor induced localized thrombin activity impulse that propagated in space and possessed all characteristic traits of a traveling excitation wave: constant spatial velocity, constant amplitude, and insensitivity to the initial stimulation once it exceeded activation threshold. The parameters of this traveling wave were controlled by the availability of phospholipids or platelets, and the wave did not form in plasmas from hemophilia A or C patients who lack factors VIII and XI, which are mediators of the two principal positive feedbacks of coagulation. Stimulation of the negative feedback of the protein C pathway with thrombomodulin produced nonstationary patterns of wave formation followed by deceleration and annihilation. This indicates that blood can function as an excitable medium that conducts traveling waves of coagulation.
Introduction
Blood coagulation is a space- and time-dependent process of plasma jellification that leads to formation of a plug preventing blood loss upon vascular injury. It is an intricate network of serine proteases, cofactors, and inhibitors, which are arranged into a proteolytic cascade with at least six positive and negative feedback loops. The presence of nonlinear positive feedbacks in this spatially heterogeneous reaction-diffusion system led to a prediction (1,2) that it can function as an excitable medium—a spatially distributed, dissipative, nonlinear system, in which complex spatiotemporal phenomena such as traveling waves or Turing structures can be observed. Excitable media play prominent roles in chemistry, physics, engineering, and human sciences; two classic examples in biology include neural impulse propagation (3) and morphogenesis (4).
Although the importance of positive and negative feedback loops in the spatial propagation of coagulation has been confirmed by experiments and theoretical analyses (5–7), the hypothetical traveling wave of thrombin has not been directly observed. Fibrinogen, the primary substrate for thrombin, is quickly depleted before the maximal thrombin concentration is reached. Indeed, plasma becomes fully clotted when only 5% of the total thrombin is generated. Although the clot density may be slightly predictive of thrombin activity in a 200-μm zone near thrombogenic cell monolayers (8), it has not been possible to reconstruct the spatiotemporal thrombin distribution inside the growing clot, nor to predict the shape of possible traveling thrombin waves.
In this study, we designed a fluorogenic substrate-based four-dimensional-biochemistry approach to measure distribution of thrombin as a function of space and time. Using this strategy, we demonstrated formation of excitation waves in blood plasma and identified the responsible mechanism.
Materials and Methods
Materials
Reagents were obtained from the following sources: 7-amino-4-methyl-coumarin (Sigma-Aldrich, St. Louis, MO); Z-Gly-Gly-Arg-AMC (Bachem, Torrance, CA); rabbit thromboplastin, factor VIII assay (Renam, Moscow, Russia); brain phosphatidylserine and egg phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL); low melting point agarose (Fluka Chemie AG, Buchs, Switzerland); corn trypsin inhibitor (Gamma, Pushchino, Russia); factor VIII (Hemophil M, Baxter Russia, Moscow, Russia); factor XI and rabbit lung thrombomodulin (Haematologic Technologies, Essex Junction, VT); Actichrome TF test (American Diagnostica, Stamford, CT); factor VIII-deficient plasma (George King Biomedical, Overland Park, KS); and factor XI-deficient plasma (HRF, Raleigh, NC). The glycoprotein IIb-IIIa antagonist Monafram was a generous gift of Prof. A. V. Mazurov (Russian Cardiology Research and Production Center, Moscow, Russia).
Experimental setup
Experiments were performed with a specially designed videomicroscopy system (Fig. 1 A) that allows for the simultaneous observation of spatial fibrin clot propagation and thrombin generation. The plasma sample was placed in the experimental chamber, and clotting was activated by bringing an activator with a tissue factor (TF)-coated surface in contact with plasma. The chamber was maintained at 37°C and illuminated with red (625 nm) or ultraviolet (UV) (365 nm) LEDs (Optotech, Hsinchu, Taiwan). Clot growth was detected with red light scattering, and AMC fluorescence was excited with UV LEDs. Fluorescence and scattered red light passing through the macro lens were detected by a charge-coupled device system (Apogee Imaging Systems, Roseville, CA). Images under red and blue light were acquired once per minute. Data analysis is described in detail in the Supporting Material.
Figure 1.
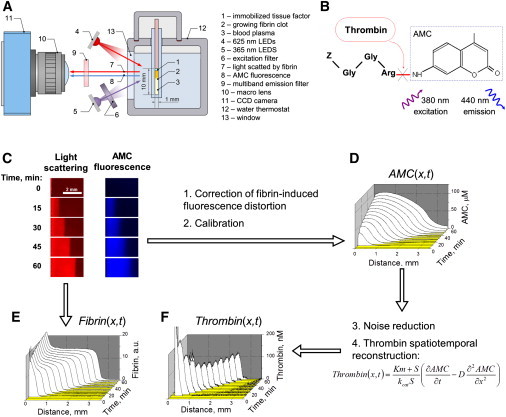
Spatiotemporally resolved imaging of thrombin activity in blood plasma from a healthy individual reveals a propagating wave. (A) Overall experimental design: A layer of immobilized tissue factor (1) induces fibrin clot propagation (2) in nonstirred plasma (3). The sample is illuminated in turn by red (4) or UV LEDs (5) through an excitation filter (6). Light scattered by fibrin (7) and fluorescence of the thrombin-generated AMC (8) pass through a multiband emission filter (9) and macro lens (10) and are recorded by a charge-coupled device (11). (B) Fluorogenic substrate Z-Gly-Gly-Arg-AMC is cleaved by thrombin to yield fluorescent AMC. (C) Time-lapse images: Light scattering from the growing fibrin clot (red) or AMC fluorescence (blue). (D) AMC concentration distribution obtained from fluorescence (C). (E) Fibrin concentration distribution obtained from light scattering (C). (F) Thrombin distribution as a function of space and time obtained from the AMC distribution (E) by solving a reverse reaction-diffusion problem. Plasma is supplemented with 10 μM phospholipids; activation is with 90 pmol TF/m2.
Blood collection and plasma preparation
Before the procedure, all volunteers provided written informed consent. Experiments were performed with approval from the Ethical Committees of the National Research Center for Hematology and Center for Theoretical Problems of Physicochemical Pharmacology. Normal plasma pools (3–7 plasma samples) were obtained from freshly drawn human blood from healthy volunteers. Blood was collected into 3.8% sodium citrate (pH 5.5) at a 9:1 volume ratio and centrifuged at 1600 × g for 15 min. The supernatant was additionally centrifuged for 5 min at 10,000 × g to obtain platelet-free plasma, which was frozen and kept at −70°C. Before each experiment, plasma samples were thawed in a water bath. For pH stabilization at 7.2–7.4, plasma was incubated with 10% lactic acid for 1 h at 37°C (9). Commercially available coagulation factor-deficient plasma samples were similarly treated after thawing.
Spatial clot growth experiments in platelet-free plasma
Plasma was supplemented with corn trypsin inhibitor (0.2 mg/ml), Z-Gly-Gly-Arg-AMC (800 μM), 10 μM lipid vesicles (phosphatidylserine/phosphatidylcholine, at a 20:80 molar ratio), and thrombomodulin at the indicated concentrations. Samples were incubated for 10 min at 37°C and then supplemented with CaCl2 (20 mM), immediately followed by clotting initiation.
Spatial clot growth experiments in platelet-rich plasma
Blood was collected directly into citrate with corn trypsin inhibitor (final concentration 0.2 mg/ml) to minimize contact activation. Platelet-rich plasma was obtained by centrifugation for 8 min at 100 × g. The platelet count was adjusted to 250,000 platelets/μL, and the pH level was stabilized at 7.4 by the addition of 20 mM Hepes. To prevent clot retraction, the glycoprotein IIb/IIIa-inhibiting antibody fragment Monafram (25 μg/mL) was used, and experiments were performed in 0.5% low melting point agarose. Samples were prepared as described previously. After recalcification, plasma was prewarmed to 42°C for 2 min. Agarose solution was added, and the mixture was incubated in the experimental cuvette for 3 min to form a gel. The experiment was started as described previously.
Spatial clot growth experiments in defibrinated plasma
Platelet-free plasma (prepared as described previously) was defibrinated by incubation with 0.02 NIH thrombin units of ancistron (Tekhnologiya-Standart, Barnaul, Russia) at 37°C for 1 h, followed by fibrin clot removal. The experiments were performed in an agarose gel, as described previously.
Results
Method description
To study spatial dynamics of blood coagulation, we developed a fluorogenic substrate-based videomicroscopy method for time- and space-resolved imaging of thrombin activity (Fig. 1 A). Clotting was initiated by a layer of immobilized TF, a transmembrane glycoprotein that activates coagulation on the damaged vascular wall surface. Clotting propagated from the activation surface into the bulk of plasma. The fluorogenic thrombin-specific peptide substrate Z-Gly-Gly-Arg-AMC was added to the plasma, so that its cleavage by thrombin produced a fluorescent molecule of AMC (Fig. 1 B). The growing fibrin clot was sequentially illuminated by red or UV light to image light scattered by the fibrin clot or spatiotemporal distribution of AMC fluorescence, respectively (Fig. 1 C). Fibrin concentration profiles were obtained from the light scattering intensity (see Fig. 1 E), and the AMC distribution (Fig. 1 D) was used to calculate thrombin (Fig. 1 F). The central idea for our approach is derived from the standard mass-balance equation for AMC, which infers that if the rate laws of fluorophore production and diffusion are known the experimentally measured AMC distribution can then be used to reconstruct the source term in this equation, i.e., thrombin activity as a function of space and time. The basic reaction-diffusion equation
| (1) |
was transformed
| (2) |
Thus, the time- and space-resolved thrombin activity distribution was calculated by numerically solving an inverse reaction-diffusion problem (10) (see the Supporting Material for the detailed algorithm description) modified to account for AMC binding in plasma and fluorescence distortion caused by the fibrin clot (Fig. S1 and Fig. S2 in the Supporting Material).
Thrombin propagation determines formation of fibrin clot
Fibrin clot initiated by a TF-covered surface steadily propagated in normal plasma at a constant spatial velocity of ∼20–50 μm/min (Fig. 1 E) and was apparently driven by an impulse-like wave of thrombin with a constant thrombin peak amplitude of 50–100 nM (Fig. 1 F and Movie S1). To investigate if clot formation regulates thrombin generation via reversible inhibition of thrombin by fibrin, or via protection of thrombin from inhibition by antithrombin III, or via acceleration of factor XI activation, we depleted fibrinogen using a fibrin-cleaving protease from snake venom. In defibrinated plasma, thrombin spatial propagation was qualitatively similar (Fig. S3), indicating that thrombin wave propagation is an independent phenomenon not determined by formation of polymerized fibrin clot.
Thrombin wave reveals properties of a traveling wave in an excitable medium
An important aspect of traveling waves in active media is the formation of identical waves after a relatively broad range of stimuli because the traveling wave is regulated by properties of active media and not by the history of its activation. To investigate the effect of initial stimulus on the properties of propagating thrombin wave, we prepared activators of different potency by changing the density of immobilized TF. The waves obtained by using 90 pmol/m2 (Fig. 2 A) or 4 pmol/m2 (Fig. 2 B) of TF were very similar (Fig. 2, C and D). It should be noted that the lowest TF density corresponds to just a few TF molecules per squared micron; this is, however, only by an order of magnitude lower than TF density on highly active fibroblasts (6), and comparable to macrophages and endothelial cells (11). Thus, thrombin activity waves travel continuously at a constant spatial velocity, have a constant shape and nondecaying amplitude, and are independent of the initial conditions once the activation signal exceeds the threshold: these are necessary and sufficient traits of a traveling wave in an excitable medium (12).
Figure 2.
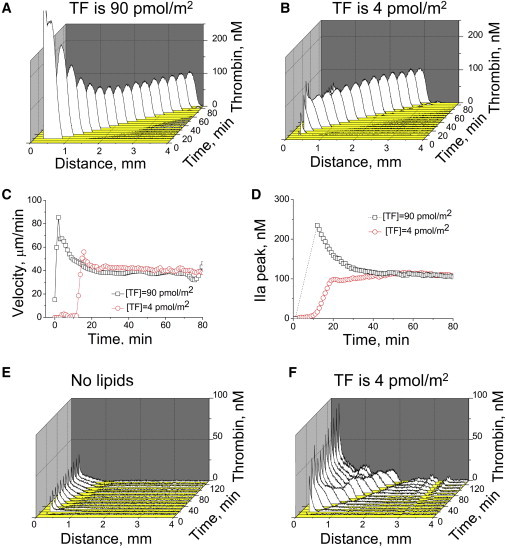
Propagating thrombin wave possesses characteristic properties of a traveling wave in an excitable medium. (A and B) Spatiotemporal thrombin distribution after clotting stimulation in normal plasma supplemented with 10 μM phospholipids with different TF densities. (C and D) Thrombin spatial velocity (A) and peak amplitude (B) for these two experiments. At the p = 0.05 level (n = 8 experiments with plasma from different donors), the thrombin peak height and velocity are not different for these two activation levels. (E and F) Typical thrombin profiles in platelet-free plasma without phospholipid supplementation (E) or in platelet-rich plasma (F). A typical experiment (out of n = 3) is shown. Stimulation is with 4 pmol TF/m2.
In contrast, this wave was sensitive to the properties of the medium. It did not propagate steadily in platelet-free plasma that was not supplemented with phospholipids (Fig. 2 E; Fig. S4) or platelets (Fig. 2 F), able to accelerate membrane-dependent reactions comprising the positive feedback loops of blood coagulation. Thrombin peak amplitude was particularly sensitive to phospholipid concentration, whereas the spatial propagation velocity of this wave was not.
Positive feedback loops are essential for wave propagation
Excitation wave formation requires the existence of positive feedback loops that support self-sustained propagation independently of the activator. Blood coagulation possesses at least one such feedback: factor XI activation with thrombin (13) as shown in Fig. 3 A. Other positive feedbacks, such as factor V, VII, and VIII activation, do not lead to formation of independently active enzymes and are therefore incapable of sustaining indefinite propagation in space. Severe factor XI deficiency, or hemophilia type C, is a rare bleeding disease. The mechanism of this bleeding is controversial, because of the apparently complementary role of factor XI in the coagulation cascade: it is unclear why humans require the minute quantities of factor IXa produced by thrombin-activated factor XIa, if there is an abundance of factor IXa produced by VIIa-TF (6). One possible explanation in view of the findings of this study could be that factor XI activation by thrombin forms an excitation wave that propagates clotting far from the activation site than could be necessary for large wound sealing.
Figure 3.
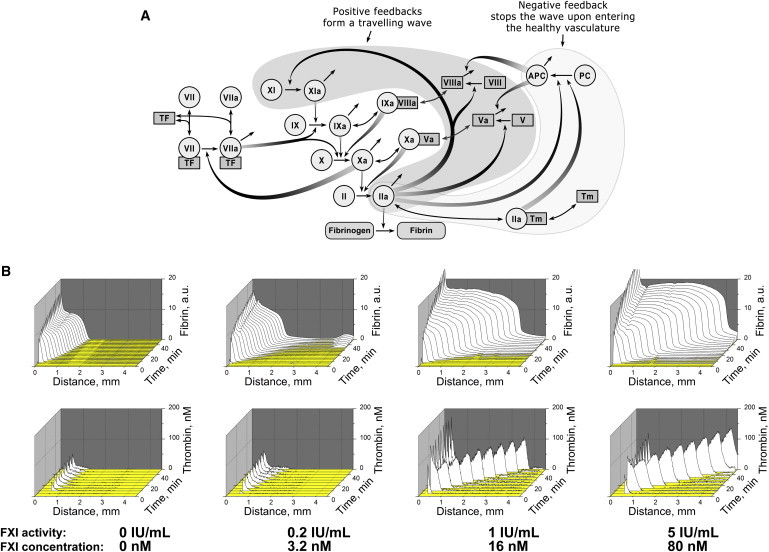
Traveling wave of thrombin activity is determined by positive feedbacks in the coagulation network. (A) The coagulation network contains positive feedbacks (filled with gray) that can support self-sustained propagation of the thrombin impulse. Factor XI, located at the top of the cascade, can be activated by thrombin in a feedback that is typical for excitable media. (B) Fibrin clot (upper row) and thrombin formation (lower) in factor XI-deficient plasma supplemented with 5 μM phospholipids and different concentrations of factor XI; a typical experiment is shown (out of n = 2). See also Figs. S5–S8.
To test this hypothesis experimentally, we performed studies with factor XI-deficient plasma from a hemophilia C patient (Fig. 3 B). A traveling wave did not form in this plasma, but was generated when a normal concentration of factor XI was added. Factor VIII deficiency, a severe bleeding disorder known as hemophilia type A, also prevented thrombin wave formation (Fig. S5). Interestingly, computer simulation analysis suggested that factor XI deficiency is in a way more fundamental for the traveling wave phenomenon far from the activator, because factor XIa is an enzyme that is indispensable for autocatalytic thrombin production far from the activator, whereas factor VIIIa is only a cofactor (Figs. S6–S8). Although this might seem paradoxical, as factor VIII is well known to be much more important clinically, however this actually agrees with the previous observation that factor VIII is important for clot formation at smaller distances (1–2 mm) from the activator, whereas factor XI becomes essential and indispensable at later stages for larger clots (6), i.e., exactly when the traveling wave is observed.
Protein C pathway is responsible for thrombin wave limitation in space
Our previous studies suggested that different modules in the coagulation network are responsible for specific tasks such as activation threshold formation, spatial propagation, and termination of this propagation (14). In particular, stimulation of the anticoagulant protein C pathway with naturally occurring allosteric regulator of thrombin thrombomodulin (Fig. 3 A) was previously found to regulate clot size (6). To study the possible effect of this pathway on the traveling wave of thrombin, we performed experiments with different thrombomodulin concentrations. Stimulation of the anticoagulant protein C pathway led to nonstationary patterns of wave formation followed by its deceleration and annihilation (Fig. 4, A and B, and Movie S2). Computer simulation is shown in Fig. S9. Clot propagation was initiated normally and then abruptly stopped at different distances from the activator. Several interesting and unusual patterns of traveling wave propagation were observed: at low thrombomodulin concentration, the initial velocity was steady, followed by deceleration and thrombin peak decrease, whereas at higher concentrations, thrombin peak ran for some distance, and then decelerated and stopped. Existence of various modes of wave propagation and pattern formation is another known feature of active media.
Figure 4.
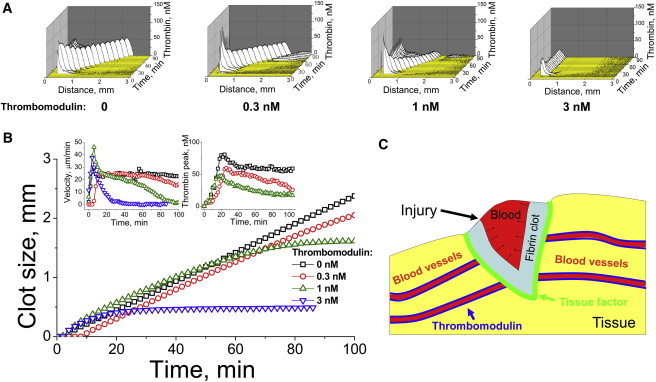
Traveling wave propagation is stopped by thrombomodulin: possible implications for confining a hemostatic plug to the wound. (A) Spatiotemporally resolved distribution of thrombin in normal plasma supplemented with different concentrations of thrombomodulin, as indicated in the panels. Plasma is supplemented with 10 μM phospholipids. Activation is with 4 pmol TF/m2. A typical experiment (out of n = 5) is shown. (B) Clot size (main plot), spatial velocity (left inset), and thrombin peak amplitude (right inset) as functions of time for different thrombomodulin concentrations. (C) Hypothetical function of the traveling wave of thrombin in vivo. In a sufficiently large wound, it is necessary to turn all of the blood into a gel, and to spread the clot from the TF-containing injury site into the bulk of the blood. This task is possible, because thrombin propagation can be self-sustained due to factor XI feedback activation, which explains the bleeding upon large injuries in hemophilia C. Abundant thrombomodulin in the healthy blood vessel endothelium prevents the traveling wave from entering the healthy vasculature.
Normally TM is localized at the undamaged vessel walls. To compare effects of soluble and immobilized TM we performed additional computer simulation (Fig. S10) showing that TM immobilized far from a TF-coated surface can stop thrombin propagation as well.
Discussion
In summary, we observed that the contact of plasma with TF results in TF-dependent thrombin generation in a 0.5- to 1-mm area near the TF-expressing surface. Within 10–40 min, a thrombin wave was formed that moved away from the area of activation at a rate and height that were independent of the initial activating signal. Our main finding is that blood can function as an excitable medium to support thrombin wave propagation. This phenomenon is easy to observe and reproduce mathematically and experimentally, and may serve as an example and research tool for synergetics research.
How are the observed time and space scales related to the (patho)physiological ones? The traveling wave of our study requires ∼1–1.5 mm to form, and it has a wavefront width of 0.2–0.3 mm. Such a wave can be imagined in a large wound (Fig. 4 C), where it can efficiently spread fibrin formation. This is in line with the usual clinical presentation of hemophilia C in large-scale wounds or surgery (15). In case of hemophilia C, absence of the travelling wave of thrombin, that can form a large-scale fibrin clot, could lead to severe bleeding. Therefore, the patients do not have problems with small wounds as it does not require rapid long-distance thrombin propagation. Thrombomodulin present in abundance in the normal endothelium would prevent this wave from entering healthy vessels. Thus, the fact that blood can function as an excitable medium to support thrombin wave propagation not only provides a new, to our knowledge, biological nonlinear dynamic system, but also appears to be important for fulfillment of the physiological function of the coagulation system.
We proposed a possible mode for the thrombin traveling wave in vivo, which should help provide a novel, to our knowledge, understanding of the role of thrombin-induced factor XI activation in blood clotting (Fig. 4 C). On the basis of our experiments, computer simulations, and the clinical presentation of hemophilia C (i.e., uncontrollable bleeding, usually in cases of large-scale wounds or surgery (15)), we speculate that the excitation wave of thrombin, formed as a result of the positive feedback loop of factor XI activation, propagates the clotting process across large wounds and far from the TF-expressing surface, resulting in efficient wound sealing. However, when thrombomodulin is abundant (e.g., in normal endothelium), the wave is prevented from entering healthy vessels.
Factor XI activation with thrombin reportedly can produce additional thrombin to protect the fibrin clot from fibrinolysis (16) and to provide sustained thrombin generation (17). However, to achieve these effects, it would be evolutionarily simpler to induce more rapid thrombin activation, instead of introducing a new positive feedback loop. Rapid and steady spatial signal propagation across significant distances in a heterogeneous system cannot be resolved in a way other than through creation of an excitable medium with traveling waves, as evidenced by neural impulse propagation. In this study, we experimentally confirm this role of factor XI, which we previously proposed on the basis of computer simulation results (1,2).
Although steady propagation of a traveling wave can be beneficial, it also can be a potentially dangerous dynamic factor. Factor XI plays a significant role in thrombosis (18), which is caused by intravascular formation of platelet-fibrin clots that obstruct blood flow through blood vessels. Thrombosis is a ubiquitous complication and cause of numerous diseases and conditions, such as atherosclerosis, trauma, stroke, infarction, cancer, and sepsis. Up to 70% of sudden cardiac deaths are due to thrombosis (19), and sudden cardiac deaths kill ∼400,000 people annually in the United States alone (20). Thrombin-dependent factor XI activation feedback may also explain why factor XI is a potential target of antithrombotic drugs (21).
Acknowledgments
The authors thank Prof. A. V. Mazurov for his generous gift of Monafram. We are indebted to Dr. V. I. Sarbash for his contribution to the construction of the experimental setup, and to Dr. O. A. Fadeeva who prepared and characterized activators. We thank Mr. James Kurasawa and Dr. Andrey Sarafanov, Centers for Biologics Evaluation and Research, Food and Drug Administration (CBER, FDA) for providing us with anti-fVIII antibody, Naveen Jha (CBER, FDA) for his help in additional experiment performing, and John W. Weisel and Rustem I. Litvinov (University of Pennsylvania) for critical reading of the manuscript and valuable suggestions.
The study was supported by the Russian Foundation for Basic Research grants 10-01-91055, 11-04-00303, 11-04-12080, 12-04-00652, 12-04-00438, 12-04-32095, 12-04-33055 and by the Russian Academy of Sciences Presidium Basic Research Programs Molecular and Cellular Biology, Basic Science for Medicine, Integrative Physiology, and Molecular Mechanisms of Physiologic Functions.
Conflict-of-interest disclosure: N.M.D., S.S.K., P.I.S., N.P.S., M.A.P., and F.I.A. are employees or founders of HemaCore LLC, which holds several patents and patent applications on the diagnostic use of coagulation assays in spatially distributed systems, which are currently developed under the trade name of Thrombodynamics. M.V.O. is an employee of the U. S. Food and Drug Administration. The findings and conclusions in this presentation have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Supporting Material
References
- 1.Ataullakhanov F.I., Guria G.T., Volkova R.I. Spatiotemporal dynamics of clotting and pattern formation in human blood. Biochim. Biophys. Acta. 1998;1425:453–468. doi: 10.1016/s0304-4165(98)00102-0. [DOI] [PubMed] [Google Scholar]
- 2.Zarnitsina V.I., Pokhilko A.V., Ataullakhanov F.I. A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. II. Results. Thromb. Res. 1996;84:333–344. doi: 10.1016/s0049-3848(96)00197-1. [DOI] [PubMed] [Google Scholar]
- 3.Rinzel J., Keller J.B. Traveling wave solutions of a nerve conduction equation. Biophys. J. 1973;13:1313–1337. doi: 10.1016/S0006-3495(73)86065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loose M., Fischer-Friedrich E., Schwille P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science. 2008;320:789–792. doi: 10.1126/science.1154413. [DOI] [PubMed] [Google Scholar]
- 5.Ovanesov M.V., Ananyeva N.M., Saenko E.L. Initiation and propagation of coagulation from tissue factor-bearing cell monolayers to plasma: initiator cells do not regulate spatial growth rate. J. Thromb. Haemost. 2005;3:321–331. doi: 10.1111/j.1538-7836.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 6.Panteleev M.A., Ovanesov M.V., Ataullakhanov F.I. Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively. Biophys. J. 2006;90:1489–1500. doi: 10.1529/biophysj.105.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroe D.M., Hoffman M., Roberts H.R. Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul. Fibrinolysis. 1996;7:459–464. doi: 10.1097/00001721-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Campbell R.A., Overmyer K.A., Wolberg A.S. Cellular procoagulant activity dictates clot structure and stability as a function of distance from the cell surface. Arterioscler. Thromb. Vasc. Biol. 2008;28:2247–2254. doi: 10.1161/ATVBAHA.108.176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinauridze E.I., Volkova R.I., Ataullakhanov F.I. Dynamics of clot growth induced by thrombin diffusing into nonstirred citrate human plasma. Biochim. Biophys. Acta. 1998;1425:607–616. doi: 10.1016/s0304-4165(98)00116-0. [DOI] [PubMed] [Google Scholar]
- 10.Kondratovich A.Y., Pokhilko A.V., Ataullakhanov F.I. Spatiotemporal dynamics of contact activation factors of blood coagulation. Biochim. Biophys. Acta. 2002;1569:86–104. doi: 10.1016/s0304-4165(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 11.Balandina A.N., Shibeko A.M., Ataullakhanov F.I. Positive feedback loops for factor V and factor VII activation supply sensitivity to local surface tissue factor density during blood coagulation. Biophys. J. 2011;101:1816–1824. doi: 10.1016/j.bpj.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaikin A.N., Zhabotinsky A.M. Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature. 1970;225:535–537. doi: 10.1038/225535b0. [DOI] [PubMed] [Google Scholar]
- 13.Gailani D., Broze G.J., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 14.Panteleev M.A., Balandina A.N., Ataullakhanov F.I. Task-oriented modular decomposition of biological networks: trigger mechanism in blood coagulation. Biophys. J. 2010;98:1751–1761. doi: 10.1016/j.bpj.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seligsohn U. Factor XI deficiency in humans. J. Thromb. Haemost. 2009;7(Suppl 1):84–87. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 16.von dem Borne P.A., Meijers J.C., Bouma B.N. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86:3035–3042. [PubMed] [Google Scholar]
- 17.Kravtsov D.V., Matafonov A., Gailani D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber A., Hanson S.R. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 19.Davies M.J. Pathophysiology of acute coronary syndromes. Indian Heart J. 2000;52:473–479. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) State-specific mortality from sudden cardiac death—United States, 1999. MMWR Morb. Mortal. Wkly. Rep. 2002;51:123–126. [PubMed] [Google Scholar]
- 21.Müller F., Gailani D., Renné T. Factor XI and XII as antithrombotic targets. Curr. Opin. Hematol. 2011;18:349–355. doi: 10.1097/MOH.0b013e3283497e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemker H.C., Giesen P.L., Béguin S. The thrombogram: monitoring thrombin generation in platelet-rich plasma. Thromb. Haemost. 2000;83:589–591. [PubMed] [Google Scholar]
- 23.Reference deleted in proof.
- 24.Hemker H.C., Béguin S. Thrombin generation in plasma: its assessment via the endogenous thrombin potential. Thromb. Haemost. 1995;74:134–138. [PubMed] [Google Scholar]
- 25.Hairer E., Norsett S.P., Wanner G. Springer; Berlin: 1993. Solving Ordinary Differential Equations I: Nonstiff Problems. [Google Scholar]
- 26.Reference deleted in proof.
- 27.Reference deleted in proof.
- 28.Meems H., Meijer A.B., Gilbert G.E. Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity. Blood. 2009;114:3938–3946. doi: 10.1182/blood-2009-01-197707. [DOI] [PubMed] [Google Scholar]
- 29.Butenas S., Undas A., Mann K.G. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb. Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 30.Butenas S., Mann K.G. Blood coagulation. Biochemistry (Mosc.) 2002;67:3–12. doi: 10.1023/a:1013985911759. [DOI] [PubMed] [Google Scholar]
- 31.Colman R.W. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. [Google Scholar]
- 32.Krishnaswamy S. The interaction of human factor VIIa with tissue factor. J. Biol. Chem. 1992;267:23696–23706. [PubMed] [Google Scholar]
- 33.Nemerson Y., Gentry R. An ordered addition, essential activation model of the tissue factor pathway of coagulation: evidence for a conformational cage. Biochemistry. 1986;25:4020–4033. doi: 10.1021/bi00362a006. [DOI] [PubMed] [Google Scholar]
- 34.Butenas S., Mann K.G. Kinetics of human factor VII activation. Biochemistry. 1996;35:1904–1910. doi: 10.1021/bi951768c. [DOI] [PubMed] [Google Scholar]
- 35.Rao L.V., Williams T., Rapaport S.I. Studies of the activation of factor VII bound to tissue factor. Blood. 1996;87:3738–3748. [PubMed] [Google Scholar]
- 36.Komiyama Y., Pedersen A.H., Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990;29:9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- 37.Warn-Cramer B.J., Bajaj S.P. Intrinsic versus extrinsic coagulation. Kinetic considerations. Biochem. J. 1986;239:757–762. doi: 10.1042/bj2390757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gailani D., Ho D., Walsh P.N. Model for a factor IX activation complex on blood platelets: dimeric conformation of factor XIa is essential. Blood. 2001;97:3117–3122. doi: 10.1182/blood.v97.10.3117. [DOI] [PubMed] [Google Scholar]
- 39.Baugh R.J., Krishnaswamy S. Role of the activation peptide domain in human factor X activation by the extrinsic Xase complex. J. Biol. Chem. 1996;271:16126–16134. doi: 10.1074/jbc.271.27.16126. [DOI] [PubMed] [Google Scholar]
- 40.Scandura J.M., Walsh P.N. Factor X bound to the surface of activated human platelets is preferentially activated by platelet-bound factor IXa. Biochemistry. 1996;35:8903–8913. doi: 10.1021/bi9525031. [DOI] [PubMed] [Google Scholar]
- 41.Rawala-Sheikh R., Ahmad S.S., Walsh P.N. Kinetics of coagulation factor X activation by platelet-bound factor IXa. Biochemistry. 1990;29:2606–2611. doi: 10.1021/bi00462a025. [DOI] [PubMed] [Google Scholar]
- 42.Panteleev M.A., Saenko E.L., Ataullakhanov F.I. Kinetics of factor X activation by the membrane-bound complex of factor IXa and factor VIIIa. Biochem. J. 2004;381:779–794. doi: 10.1042/BJ20031748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baugh R.J., Broze G.J., Jr., Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J. Biol. Chem. 1998;273:4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 44.Tracy P.B., Eide L.L., Mann K.G. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J. Biol. Chem. 1985;260:2119–2124. [PubMed] [Google Scholar]
- 45.van Dieijen G., Tans G., Hemker H.C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J. Biol. Chem. 1981;256:3433–3442. [PubMed] [Google Scholar]
- 46.Keuren J.F., Wielders S.J., Lindhout T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 2005;25:1499–1505. doi: 10.1161/01.ATV.0000167526.31611.f6. [DOI] [PubMed] [Google Scholar]
- 47.Higgins D.L., Lewis S.D., Shafer J.A. Steady state kinetic parameters for the thrombin-catalyzed conversion of human fibrinogen to fibrin. J. Biol. Chem. 1983;258:9276–9282. [PubMed] [Google Scholar]
- 48.Hill-Eubanks D.C., Lollar P. von Willebrand factor is a cofactor for thrombin-catalyzed cleavage of the factor VIII light chain. J. Biol. Chem. 1990;265:17854–17858. [PubMed] [Google Scholar]
- 49.Monkovic D.D., Tracy P.B. Activation of human factor V by factor Xa and thrombin. Biochemistry. 1990;29:1118–1128. doi: 10.1021/bi00457a004. [DOI] [PubMed] [Google Scholar]
- 50.Oliver J.A., Monroe D.M., Hoffman M. Thrombin activates factor XI on activated platelets in the absence of factor XII. Arterioscler. Thromb. Vasc. Biol. 1999;19:170–177. doi: 10.1161/01.atv.19.1.170. [DOI] [PubMed] [Google Scholar]
- 51.Yang L., Manithody C., Rezaie A.R. Activation of protein C by the thrombin-thrombomodulin complex: cooperative roles of Arg-35 of thrombin and Arg-67 of protein C. Proc. Natl. Acad. Sci. USA. 2006;103:879–884. doi: 10.1073/pnas.0507700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bush L.A., Nelson R.W., Di Cera E. Murine thrombin lacks Na+ activation but retains high catalytic activity. J. Biol. Chem. 2006;281:7183–7188. doi: 10.1074/jbc.M512082200. [DOI] [PubMed] [Google Scholar]
- 53.Panteleev M.A., Zarnitsina V.I., Ataullakhanov F.I. Tissue factor pathway inhibitor: a possible mechanism of action. Eur. J. Biochem. 2002;269:2016–2031. doi: 10.1046/j.1432-1033.2002.02818.x. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad S.S., Rawala-Sheikh R., Walsh P.N. Comparative interactions of factor IX and factor IXa with human platelets. J. Biol. Chem. 1989;264:3244–3251. [PubMed] [Google Scholar]
- 55.Ahmad S.S., Scandura J.M., Walsh P.N. Structural and functional characterization of platelet receptor-mediated factor VIII binding. J. Biol. Chem. 2000;275:13071–13081. doi: 10.1074/jbc.275.17.13071. [DOI] [PubMed] [Google Scholar]
- 56.Koppelman S.J., Hackeng T.M., Bouma B.N. Inhibition of the intrinsic factor X activating complex by protein S: evidence for a specific binding of protein S to factor VIII. Blood. 1995;86:1062–1071. [PubMed] [Google Scholar]
- 57.Hackeng T.M., van ’t Veer C., Bouma B.N. Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with factors Va and Xa. J. Biol. Chem. 1994;269:21051–21058. [PubMed] [Google Scholar]
- 58.Scandura J.M., Ahmad S.S., Walsh P.N. A binding site expressed on the surface of activated human platelets is shared by factor X and prothrombin. Biochemistry. 1996;35:8890–8902. doi: 10.1021/bi9525029. [DOI] [PubMed] [Google Scholar]
- 59.Tracy P.B., Nesheim M.E., Mann K.G. Platelet factor Xa receptor. Methods Enzymol. 1992;215:329–360. doi: 10.1016/0076-6879(92)15075-n. [DOI] [PubMed] [Google Scholar]
- 60.Pieters J., Willems G., Lindhout T. Inhibition of factor IXa and factor Xa by antithrombin III/heparin during factor X activation. J. Biol. Chem. 1988;263:15313–15318. [PubMed] [Google Scholar]
- 61.Rezaie A.R. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by a template mechanism. Evidence that calcium alleviates Gla domain antagonism of heparin binding to factor Xa. J. Biol. Chem. 1998;273:16824–16827. doi: 10.1074/jbc.273.27.16824. [DOI] [PubMed] [Google Scholar]
- 62.Ellis V., Scully M., Kakkar V. Inhibition of human factor Xa by various plasma protease inhibitors. Biochim. Biophys. Acta. 1982;701:24–31. [PubMed] [Google Scholar]
- 63.España F., Berrettini M., Griffin J.H. Purification and characterization of plasma protein C inhibitor. Thromb. Res. 1989;55:369–384. doi: 10.1016/0049-3848(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 64.Ellis V., Scully M.F., Kakkar V.V. Inhibition of prothrombinase complex by plasma proteinase inhibitors. Biochemistry. 1984;23:5882–5887. doi: 10.1021/bi00319a030. [DOI] [PubMed] [Google Scholar]
- 65.Downing M.R., Bloom J.W., Mann K.G. Comparison of the inhibition of thrombin by three plasma protease inhibitors. Biochemistry. 1978;17:2649–2653. doi: 10.1021/bi00606a030. [DOI] [PubMed] [Google Scholar]
- 66.Derechin V.M., Blinder M.A., Tollefsen D.M. Substitution of arginine for Leu444 in the reactive site of heparin cofactor II enhances the rate of thrombin inhibition. J. Biol. Chem. 1990;265:5623–5628. [PubMed] [Google Scholar]
- 67.Lollar P., Parker E.T., Fay P.J. Coagulant properties of hybrid human/porcine factor VIII molecules. J. Biol. Chem. 1992;267:23652–23657. [PubMed] [Google Scholar]
- 68.Solymoss S., Tucker M.M., Tracy P.B. Kinetics of inactivation of membrane-bound factor Va by activated protein C. Protein S modulates factor Xa protection. J. Biol. Chem. 1988;263:14884–14890. [PubMed] [Google Scholar]
- 69.Wuillemin W.A., Eldering E., Hack C.E. Modulation of contact system proteases by glycosaminoglycans. Selective enhancement of the inhibition of factor XIa. J. Biol. Chem. 1996;271:12913–12918. doi: 10.1074/jbc.271.22.12913. [DOI] [PubMed] [Google Scholar]
- 70.Meijers J.C., Vlooswijk R.A., Bouma B.N. Inhibition of human blood coagulation factor XIa by C-1 inhibitor. Biochemistry. 1988;27:959–963. doi: 10.1021/bi00403a018. [DOI] [PubMed] [Google Scholar]
- 71.Heeb M.J., Gruber A., Griffin J.H. Identification of divalent metal ion-dependent inhibition of activated protein C by alpha 2-macroglobulin and alpha 2-antiplasmin in blood and comparisons to inhibition of factor Xa, thrombin, and plasmin. J. Biol. Chem. 1991;266:17606–17612. [PubMed] [Google Scholar]
- 72.Heeb M.J., Bischoff R., Griffin J.H. Inhibition of activated protein C by recombinant alpha 1-antitrypsin variants with substitution of arginine or leucine for methionine358. J. Biol. Chem. 1990;265:2365–2369. [PubMed] [Google Scholar]
- 73.Panteleev M.A., Ananyeva N.M., Saenko E.L. Factor VIIIa regulates substrate delivery to the intrinsic factor X-activating complex. FEBS J. 2006;273:374–387. doi: 10.1111/j.1742-4658.2005.05070.x. [DOI] [PubMed] [Google Scholar]
- 74.Baerga-Ortiz A., Rezaie A.R., Komives E.A. Electrostatic dependence of the thrombin-thrombomodulin interaction. J. Mol. Biol. 2000;296:651–658. doi: 10.1006/jmbi.1999.3447. [DOI] [PubMed] [Google Scholar]
- 75.Galvin J.B., Kurosawa S., Esmon N.L. Reconstitution of rabbit thrombomodulin into phospholipid vesicles. J. Biol. Chem. 1987;262:2199–2205. [PubMed] [Google Scholar]
- 76.Aritomi M., Watanabe N., Maruyama I. Recombinant human soluble thrombomodulin delivers bounded thrombin to antithrombin III: thrombomodulin associates with free thrombin and is recycled to activate protein c. Thromb. Haemost. 1993;70:418–422. [PubMed] [Google Scholar]
- 77.Rezaie A.R., Cooper S.T., Esmon C.T. Protein C inhibitor is a potent inhibitor of the thrombin-thrombomodulin complex. J. Biol. Chem. 1995;270:25336–25339. doi: 10.1074/jbc.270.43.25336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


