Abstract
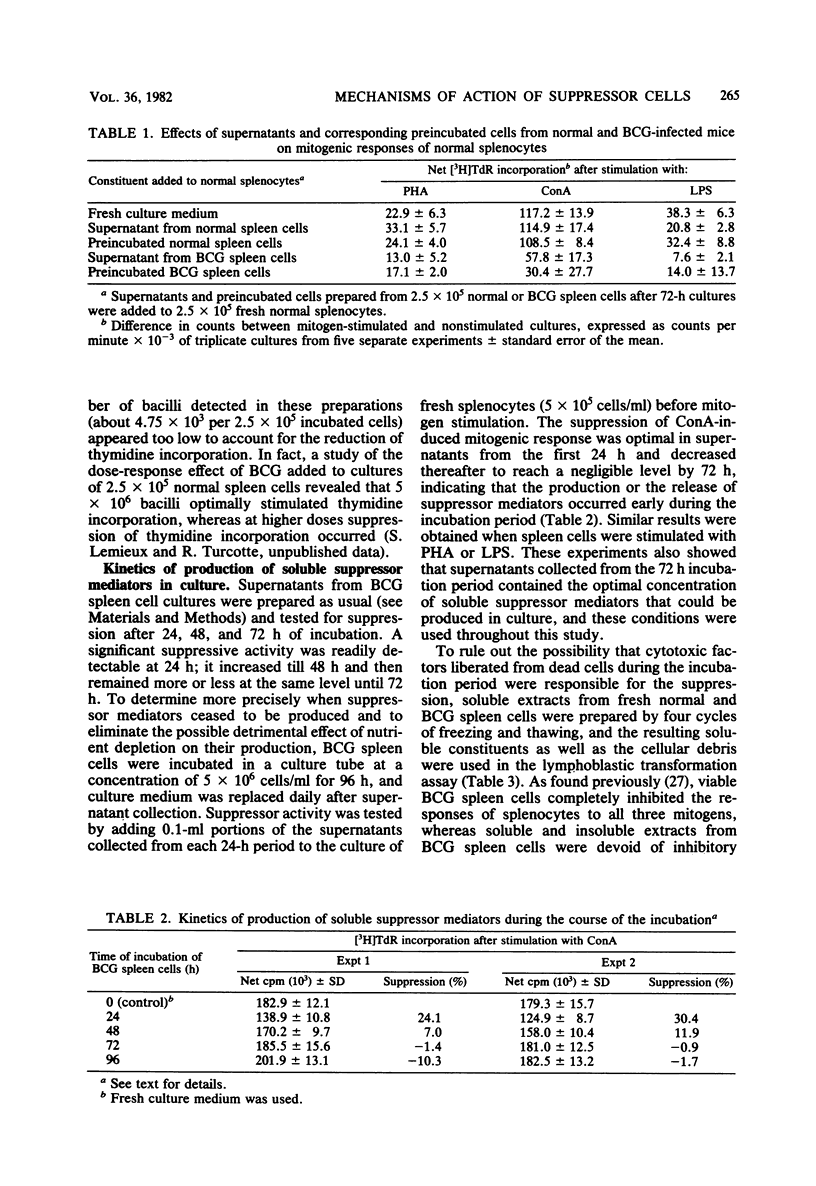
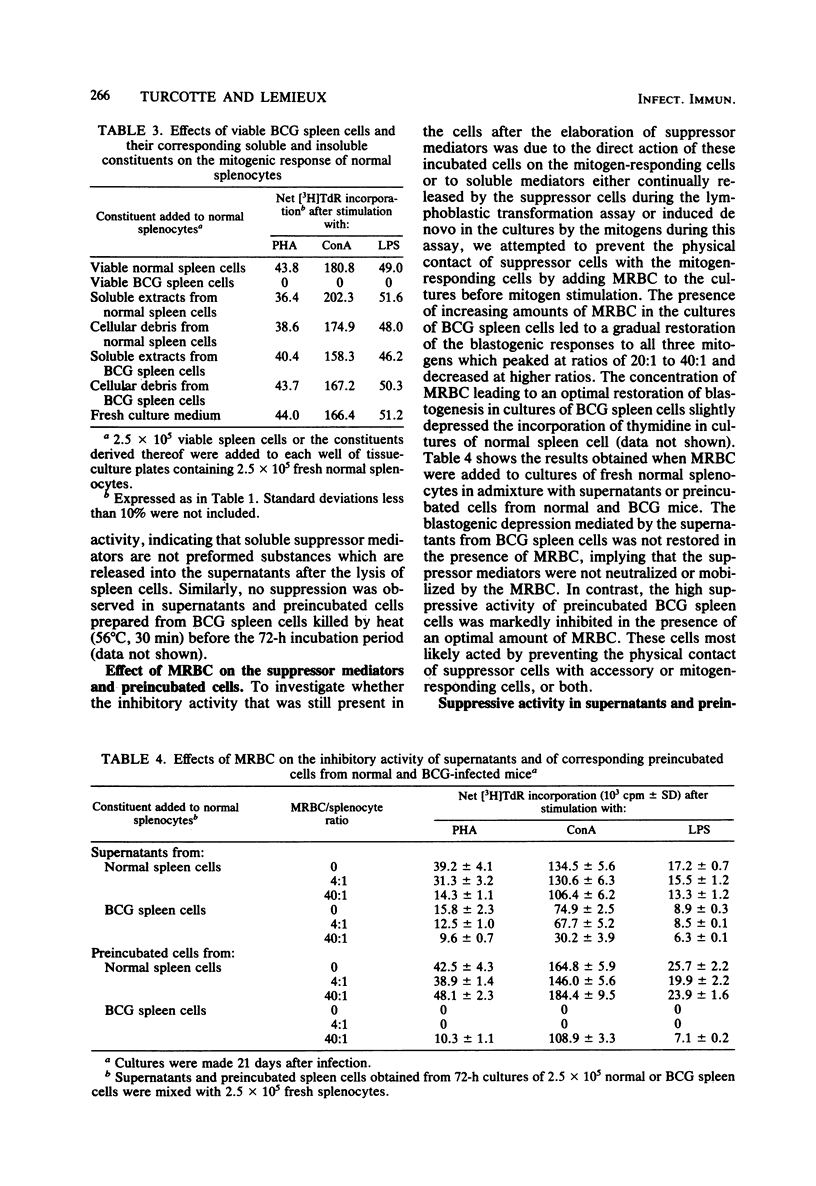
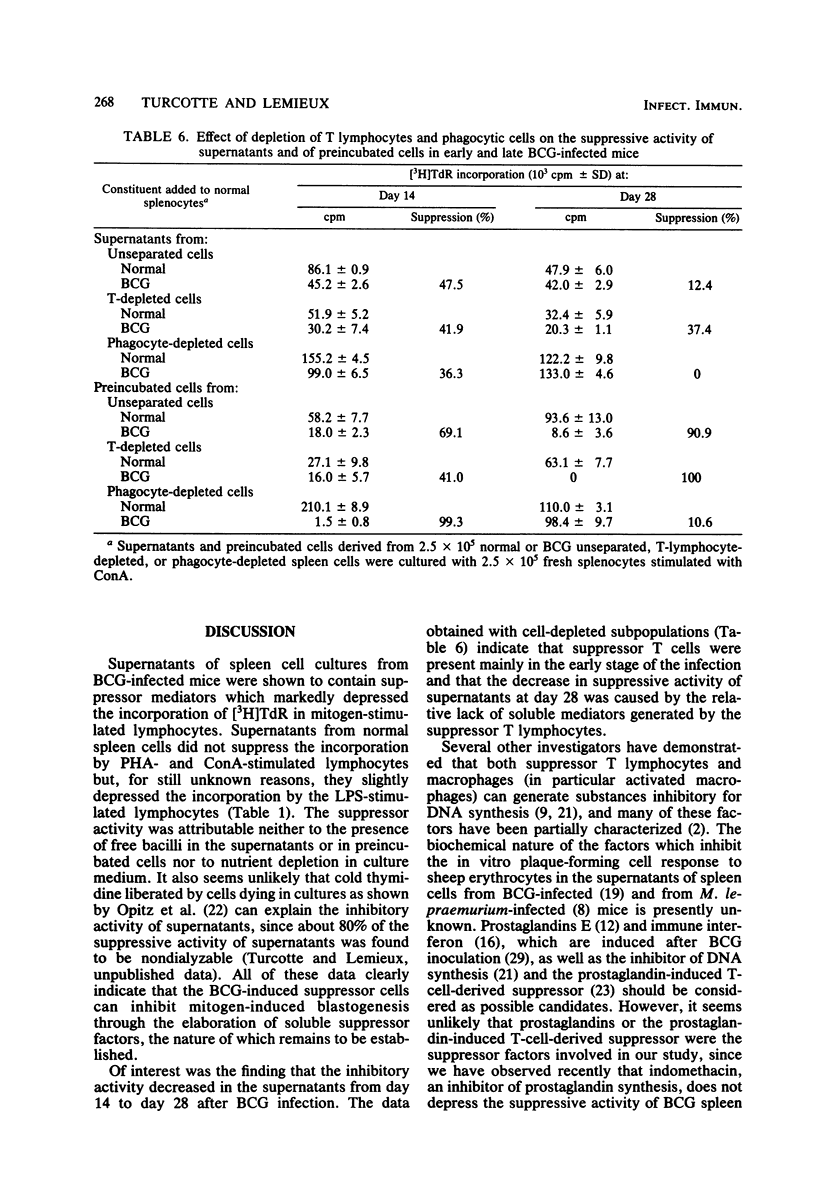
Spleen cells from Mycobacterium bovis BCG-infected C57B1/6 mice when cultured in vitro for 72 h elicited soluble suppressor mediators capable of nonspecifically suppressing the mitogen-induced blastogenesis of normal splenocytes. Maximal production of suppressor mediators occurred during the first 24 h in culture, and their production ceased after 72 h. Attempts to isolate the mediators from fresh nonincubated splenocytes failed. After incubation, a strong residual suppressive activity was constantly detected in cell preparations used for production of suppressor factors. Supernatants prepared from cultures of spleen cells of mice infected 14 days earlier possessed higher suppressive activity than did those obtained 28 days after infection. In contrast, the residual cellular suppressive activity increased during the course of the infection. Although the activity of soluble factors was not inhibited, the residual activity of incubated cells was highly depressed by the presence of mouse erythrocytes in the cultures. Thus, the incubated cells appear to act through a direct cell-to-cell contact with the mitogen-responding cells. Finally, the results of depletion experiments suggest that the two populations of BCG-induced suppressor cells, namely, T lymphocytes and macrophage-like cells, are able to elicit suppressor mediators and to retain thereafter suppressive activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L. Suppression of phytohemagglutinin and lipopolysaccharide responses in mouse spleen cells by Bacillus Calmette-Guerin. J Reticuloendothel Soc. 1979 Oct;26(4):349–356. [PubMed] [Google Scholar]
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Badger A. M., Cooperband S. R., Green J. A. Culture conditions affecting induction and release of lymphocyte produced proliferation inhibitory factor (PIF). Cell Immunol. 1973 Jul;8(1):12–27. doi: 10.1016/0008-8749(73)90089-0. [DOI] [PubMed] [Google Scholar]
- Baird L. G., Kaplan A. M. Macrophage regulation of mitogen-induced blastogenesis. I. Demonstration of inhibitory cells in the spleens and peritoneal exudates of mice. Cell Immunol. 1977 Jan;28(1):22–35. doi: 10.1016/s0008-8749(77)80003-8. [DOI] [PubMed] [Google Scholar]
- Bennett J. A., Marsh J. C. Relationship of Bacillus Calmette-Guérin-induced suppressor cells to hematopoietic precursor cells. Cancer Res. 1980 Jan;40(1):80–85. [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Active suppression masks an underlying enhancement of antibody production in vitro by spleen cells from BCG-infected mice. Immunology. 1980 Jul;40(3):303–309. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Calderon J., Williams R. T., Unanue E. R. An inhibitor of cell proliferation released by cultures of macrophages. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4273–4277. doi: 10.1073/pnas.71.11.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doft B. H., Merchant B., Johannessen L., Chaparas S. D., Sher N. A. Contrasting effects of BCG on spleen and lymph node antibody responses in nude and normal mice. J Immunol. 1976 Nov;117(5 Pt 1):1638–1643. [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Holzman R. S., Valentine F. T., Lawrence H. S. Preparation and properties of cloning inhibitory factor. II. Factors affecting its production and assay. Cell Immunol. 1973 Aug;8(2):259–269. doi: 10.1016/0008-8749(73)90115-9. [DOI] [PubMed] [Google Scholar]
- Ito M., Ralph P., Moore M. A. Suppression of spleen natural killing activity induced by BCG. Clin Immunol Immunopathol. 1980 May;16(1):30–38. doi: 10.1016/0090-1229(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Muchmore A. V., Chused T. M., Holden H. T., Herberman R. B. Inhibition of proliferation of lymphoma cells and T lymphocytes by suppressor cells from spleens of tumor-bearing mice. J Immunol. 1975 Jan;114(1 Pt 1):206–210. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Klimpel G. R. Soluble factors from BCG-induced suppressor cells inhibit in vitro PFC responses but not cytotoxic responses. Cell Immunol. 1979 Sep 15;47(1):218–226. doi: 10.1016/0008-8749(79)90331-9. [DOI] [PubMed] [Google Scholar]
- Namba Y., Jegasothy B. V., Waksman B. H. Regulatory substances produced by lymphocytes. V. Production of inhibitor of DNA synthesis (IDS) by proliferating T lymphocytes. J Immunol. 1977 Apr;118(4):1379–1384. [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Jackson R. C., Lemke H., Huget R., Flad H. D. Biochemical characterization of a factor released by macrophages. Cell Immunol. 1975 Jul;18(1):70–75. doi: 10.1016/0008-8749(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Nowowiejski I., Webb D. R. Partial characterization of a prostaglandin-induced suppressor factor. Cell Immunol. 1980 Mar 1;50(1):82–93. doi: 10.1016/0008-8749(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P. J., Brooks C. G. Macrophage-like suppressor cells in rats. I. Inhibition of natural macrophage-like suppressor cells by red blood cells. Cell Immunol. 1980 Jul 1;52(2):370–380. doi: 10.1016/0008-8749(80)90358-5. [DOI] [PubMed] [Google Scholar]
- van 't Hull E., Schellekens H., Löwenberg B., de Vries M. J. Influence of interferon preparations on the proliferative capacity of human and mouse bone marrow cells in vitro. Cancer Res. 1978 Apr;38(4):911–914. [PubMed] [Google Scholar]


