Abstract
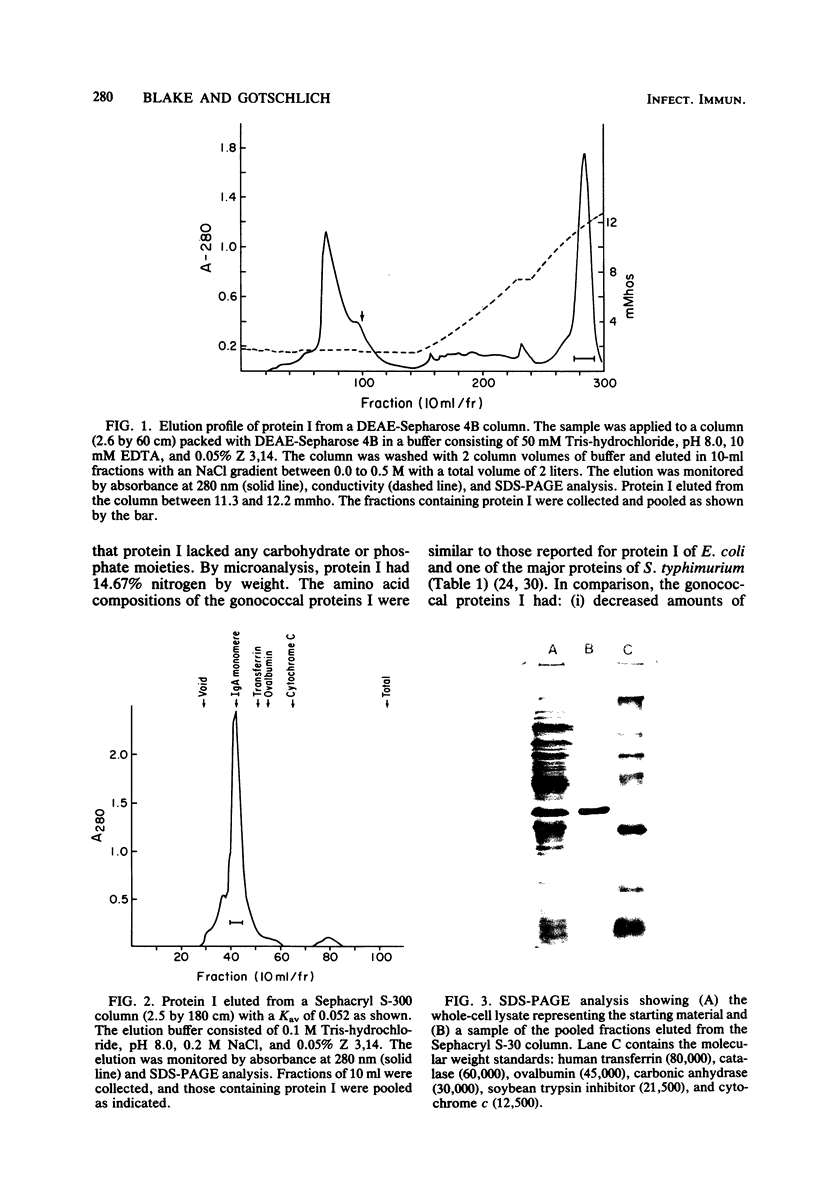
A procedure is described to isolate the major outer membrane protein (protein I) from Neisseria gonorrhoeae in large quantities. The method involves precipitation of protein I by hexadecyltrimethylammonium bromide (CTB) at low ionic strength. CTB is lethal for the gonococci and solubilizes most other proteins. Protein I is brought into solution by raising the ionic strength, and the nucleic acids are subsequently removed by 20% ethanol precipitation. The CTB is removed by precipitating protein I with ethanol and replaced by N-tetradecyl-N,N-dimethyl-3-ammonia-1-propanesulfonate, a dipolar ionic detergent. Further purification is accomplished by ion-exchange and molecular sieve chromatography. Two species of protein I (34,000 daltons [34K] and 32K) were purified by these methods. The purified proteins reacted with antisera prepared against the homologous organisms. The 34K proteins I generated proteolytic fragments upon treatment with trypsin and chymotrypsin similar to those generated by 34K protein in intact gonococci. The amino acid compositions of the three proteins were much like those of other major proteins of gram-negative organisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Ishii J., Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980 Aug 21;56(1):19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Henning U. Primary structure of major outer membrane protein I of Escherichia coli B/r. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5014–5017. doi: 10.1073/pnas.76.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Charnetzky W. T., Hurlbert R. E. Outer membrane protein composition of Yersinia pestis at different growth stages and incubation temperatures. J Bacteriol. 1980 Aug;143(2):942–949. doi: 10.1128/jb.143.2.942-949.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular composition of the outer membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch Microbiol. 1980 Sep;127(2):81–86. doi: 10.1007/BF00428010. [DOI] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Henning U., Schmidmayr W., Hindennach I. Major proteins of the outer cell envelope membrane of Escherichia coli K-12: multiple species of protein I. Mol Gen Genet. 1977 Sep 9;154(3):293–298. doi: 10.1007/BF00571285. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Major outer membrane proteins: common antigens in enterobacteriaceae species. J Gen Microbiol. 1980 Jul;119(1):123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Salit I. E., Blake M., Gotschlich E. C. Intra-strain heterogeneity of gonococcal pili is related to opacity colony variance. J Exp Med. 1980 Mar 1;151(3):716–725. doi: 10.1084/jem.151.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Wong T. P., Shockley R. K., Johnston K. H. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Apr;11(4):363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]