Abstract
The F508del mutation, the most frequent in cystic fibrosis (CF), impairs the maturation of the CFTR chloride channel. The F508del defect can be partially overcome at low temperature (27 °C) or with pharmacological correctors. However, the efficacy of correctors on the mutant protein appears to be dependent on the cell expression system. We have used a bronchial epithelial cell line, CFBE41o-, to determine the efficacy of various known treatments and to discover new correctors. Compared to other cell types, CFBE41o- shows the largest response to low temperature and the lowest one to correctors such as corr-4a and VRT-325.
A screening of a small molecule library identified 9-aminoacridine and ciclopirox, which were significantly more effective than corr-4a and VRT-325. Analysis with microarrays revealed that 9-aminoacridine, ciclopirox, and low temperature, in contrast to corr-4a, cause a profound change in cell transcriptome. These data suggest that 9-aminoacridine and ciclopirox act on F508del-CFTR maturation as proteostasis regulators, a mechanism already proposed for the histone deacetylase inhibitor SAHA. However, we found that 9-aminoacridine, ciclopirox, and SAHA, in contrast to corr-4a, VRT-325, and low temperature, do not increase chloride secretion in primary bronchial epithelial cells from CF patients. These conflicting data appeared to be correlated with different gene expression signatures generated by these treatments in the cell line and in primary bronchial epithelial cells. Our results suggest that F508del-CFTR correctors acting by altering the cell transcriptome may be particularly active in heterologous expression systems but markedly less effective in native epithelial cells.
Keywords: cystic fibrosis, drug discovery, high-throughput screening, pharmacological chaperone
INTRODUCTION
Cystic fibrosis, one of the most frequent genetic diseases among people of caucasian origin, is caused by mutations in the gene coding for CFTR, a protein with Cl− channel function (26, 32). CFTR belongs to the superfamily of ATP-binding cassette (ABC) transporters, which includes multidrug resistance proteins (32). Similarly to many other ABC transporters, CFTR has twelve transmembrane segments and two nucleotide binding domains (NBD1 and NBD2). In addition, CFTR has an additional domain, called R, which is a substrate for the cAMP-dependent protein kinase A. CFTR channel opening requires phosphorylation of the R domain and binding of two ATP molecules at the interface between NBD1 and NBD2 (32). CFTR represents a major route for Cl− transport in epithelial cells of the airways and other organs. Defective CFTR function severely impairs mucociliary transport in the respiratory system thus favoring the colonization of airway surfaces by pathogenic bacteria.
Nearly 1,500 mutations have been identified among CF patients but the most frequent molecular defect (70-90 %) is the absence of phenylalanine 508 (F508del) in NBD1 (26). F508del causes a major trafficking defect in the CFTR protein, which is largely unable to exit the endoplasmic reticulum and is rapidly degraded by the proteasome complex (18, 39, 44). Although a small fraction of F508del-CFTR reaches the plasma membrane, its half-life on the cell surface is significantly shorter than that of the wild type protein (24, 39). The F508del mutation is also responsible for a decreased channel activity (gating defect). Even under conditions inducing maximal phosphorylation, the time spent by F508del-CFTR in the Cl−-conducting state is nearly 3-fold shorter than the wild type protein (14).
An intensive research is being conducted to find drugs able to restore the function of mutant CFTR in order to treat CF patients (40). Chemical compounds called potentiators are able to ameliorate the gating defect of F508del and of other mutations (e.g. G551D). Other compounds, labeled as CFTR correctors, are potentially able to improve the trafficking of the F508del mutant. Several potentiators have been identified by various strategies, including high-throughput screening (30, 40, 43). Their activity is independent of cell background and probably based on direct interaction with CFTR itself (31). A potentiator, VX-770, is already in phase 3 clinical trials on CF patients with CFTR channel gating defect (1, 37). Instead, the identification of correctors (29, 38) appears much more problematic (31). Indeed, F508del-CFTR is detected by multiple cellular checkpoints as a misfolded protein and tagged for degradation (44). Because of this redundant quality control, it is not clear whether a single pharmacological molecule will be effective in rescuing the mutant protein.
F508del correctors may work as pharmacological chaperones, i.e. by directly binding the mutant protein and favoring its folding and maturation. Alternatively, correctors may also work as proteostasis regulators by modifying the cell proteome favoring mutant CFTR maturation (27, 41). The histone deacetylase inhibitor SAHA, which modifies the cell transcriptome by altering the expression of several genes, has been recently found to rescue F508del-CFTR (15).
Identification of effective potentiators and correctors by high-throughput screening requires the use of cell lines since primary epithelial cells are not suitable for large scale studies. However, cell lines may have intrinsic limitations due to differences in the proteome relative to primary cells. These cell-dependent differences may affect the efficacy of compounds, particularly of correctors.
In a previous study, we have compared the effect of potentiators and correctors in two different cell models, FRT and A549 cells, expressing F508del-CFTR. While potentiators appeared equally active in the two systems, correctors were strongly cell type-dependent, with most compounds being active in only one of the two cell lines (31). Our results suggest that activity of correctors in cell lines used for heterologous expression are not predictive of efficacy in primary airway epithelial cells of CF patients. We asked whether a better cell model can be identified.
In the present study, we have evaluated CFBE41o-, a cell line derived by immortalization from the bronchial epithelium of a CF patient homozygous for F508del mutation (11, 22). This cell line has been subsequently engineered to express detectable amounts of wild type and F508del-CFTR (2). We wondered whether CFBE41o- cells may be useful to identify novel correctors by small molecule screening. We have found that CFBE41o- cells show a high level of F508del-CFTR rescue by incubation at low temperature and, in contrast, a low sensitivity to known pharmacological correctors. In addition to low temperature, CFBE41o- cells are also very responsive to small molecules acting by modification of the cell transcriptome, including SAHA, 9-aminoacridine, and ciclopirox. However, these treatments are ineffective in primary airway epithelial cells. Our results suggest that the potential activity of proteostasis regulators on F508del rescue may be overestimated in recombinant cell expression systems.
MATERIALS AND METHODS
Cell culture
The CF bronchial epithelial cells CFBE41o-, with stable expression of F508del-CFTR or wild type CFTR, were obtained by Dr. J.P. Clancy (2). A549 and FRT cells were stable transfected with a plasmid coding for F508del-CFTR and carrying the resistance gene for zeocin. The three cell types were also transfected with the halide-sensitive yellow fluorescent protein (HS-YFP) YFP-H148Q/I152L (8). The culture media were: Coon’s modified Ham’s F-12 medium for FRT cells, DMEM/Ham’s F12 (1:1) for A549 cells, and MEM for CFBE41o-. All media were supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For fluorescence assays of CFTR activity, FRT, A549 and CFBE41o- cells were plated (50,000 cells/well) on clear-bottom 96-well black microplates (Corning Life Sciences, Acton, MA). For short-circuit current experiments, 500,000 CFBE41o- cells were seeded into Snapwell permeable supports (Corning Life Sciences).
Primary human bronchial epithelial cells from F508del/F508del CF patients were cultured as previously described (9, 28). Briefly, cells were initially cultured on plastic in proliferative serum-free medium, LHC9/RPMI 1640, and then cultured on Snapwell supports for 10-12 days with a differentiating medium.
HS-YFP Assay for CFTR Activity
Measurements of CFTR activity were carried out on CFBE41o-, FRT, and A549 cells expressing mutant F508del-CFTR and the HS-YFP 48 h after plating on microplates. Twenty-four hours after plating, the cells were incubated with test compounds at 37°C for 20 to 24 h. At the time of assay, cells were washed with PBS (containing 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, and 0.5 mM MgCl2) and stimulated for 30 min with forskolin (20 μM) plus genistein (50 μM). Then, cells were transferred to a microplate reader (FluoStar Galaxy; BMG Labtech GmbH, Offenburg, Germany) for CFTR activity determination. The plate reader was equipped with high-quality excitation (HQ500/20X: 500 ± 10 nm) and emission (HQ535/30M: 535 ± 15 nm) filters for YFP (Chroma Technology Corp., Brattleboro, VT). Each assay consisted of a continuous 14-s fluorescence reading with 2 s before and 12 s after injection of an iodide-containing solution (PBS with Cl− replaced by I−; final I− concentration in the well: 100 mM). Data were normalized to the initial background-subtracted fluorescence. To determine fluorescence quenching rate (QR) associated with I− influx, the final 11 s of the data for each well were fitted with an exponential function to extrapolate initial slope (dF/dt).
Transepithelial Cl− current measurements
Experiments on CFBE41o- cells were performed 5-6 days after seeding, by mounting the Snapwell inserts in a self-contained Ussing chamber system (vertical diffusion chamber; Corning Life Sciences). Transepithelial currents were measured using a transepithelial Cl− gradient. Accordingly, the basolateral solution contained (in mM): 126 NaCl, 0.38 KH2PO4, 2.1 K2HPO4, 1 MgSO4, 1 CaCl2, 24 NaHCO3, and 10 glucose. The apical solution had similar composition except for a lower NaCl concentration (63 mM NaCl) and the presence of 63 mM sodium gluconate. During experiments, solutions in both chambers were continuously bubbled with a mixture of 5% CO2 in air. The hemichambers were connected to DVC-1000 voltage clamps (World Precision Instruments, Inc., Sarasota, FL) via Ag/AgCl electrodes and 1 M KCl agar bridges. Transepithelial currents were digitized using PowerLab 4/25 data acquisition systems and stored on a personal computer. All measurements were done at 37°C. In contrast to HS-YFP experiments, stimulation of F508del-CFTR in transepithelial current recordings was done with CPT-cAMP and felodipine because currents were more stable than with the forskolin plus genistein cocktail. Conditions for recordings on primary bonchial epithelial cells were similar except that some experiments were performed with the high NaCl solution on both sides of the epithelium, whereas others were performed with the Cl− gradient as for CFBE41o- cells.
Immunoprecipitation and Western Blots
Cells grown on 100 mm diameter dishes were lysed in lysis buffer (20 mM Hepes pH 7, 150 mM NaCl, 1 mM EGTA, 1% Igepal) containing Complete Protease Inhibitor Cocktail (Roche, NJ). After preclearing of lysates with pansorbin (Calbiochem), CFTR was immunoprecipitated in RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing 2 mM Mg-ATP, using a mouse monoclonal anti-CFTR C-terminal antibody (24-1, R&D System) and pansorbin. The immunoprecipitates were subjected to SDS-PAGE and analyzed by Western blotting. Proteins were immunodetected by a mouse monoclonal anti-CFTR antibody (M3A7, Millipore) followed by HRP-conjugated anti-mouse IgG, and visualized by chemiluminescence with the LiteAblot Turbo kit (Euroclone). Direct recording of the chemiluminescence was performed using the Molecular Imager ChemiDoc XRS System and quantification using the ImageJ software (NIH).
HDAC assay
Histone deacetylase activity was measured with the Fluor-de-Lys-green fluorimetric HDAC assay kit (Enzo Life Sciences) according to manufacturer instructions.
Gene expression analysis by microarrays
Total RNAs were isolated from treated or untreated cells (CFBE41o- cells plated on petri dishes, primary bronchial epithelial cells plated on Snapwell supports) using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Oligo dT primed cDNAs were synthesized using the SuperScript® III First-Strand Synthesis SuperMix (Invitrogen, Irvine, CA) following the manufacturer’s instructions. For microarray analysis double stranded cDNAs were synthesized using the Custom SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen, Irvine, CA) and extracted with phenol-chloroform-isoamyl alcohol (25:24:1), ethanol precipitated, and used to prepare cRNAs using the Bioarray High Yield RNA Transcription Kit (Affymetrix, Santa Clara, CA) according to the manufacturer’s instructions. cRNAs were purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany), controlled by agarose gel electrophoresis and subjected to fragmentation for 35 min. at 94°C in fragmentation buffer (40mM Tris-acetate pH 8.1, 100 mM CH3COOH, 30 mM Mg(CH3COO).
Labeled cRNA was used for screenings of GeneChip Human Genome U133plus2 arrays (Affymetrix). Three to four biological replicates were analyzed for each condition. Hybridization and scanning was performed on the Affymetrix platform. Data were normalized following the GCRMA procedure (16) of Bioconductor 1.4 (http://www.bioconductor.org) (45). Statistically significant expression changes were determined using permutation tests (SAM, (http://www-stat.stanford.edu/~tibs/SAM/) implemented in MeV software (33, 36). Genes regulated at least two fold in comparison to untreated controls were considered. The delta value was set to return a median false significant number < 1. Annotations were obtained through the DAVID database (http://david.niaid.nih.gov/david/beta/index.htm) (7).
Expression of CFTR mRNA was also evaluated by real time RT-PCR as described previously (28).
Statistics
Data are shown as representative experiments or as mean ± SEM. Statistical significance of differences between groups of data was assessed with ANOVA.
RESULTS
We used engineered CFBE41o- cells derived by lentiviral transduction of F508del or wild type CFTR (2) because the expression of the endogenous CFTR gene in the parental cell line is too low to be detected by most functional assays. In short-circuit current experiments (Fig. 1), CFBE41o- cells expressing F508del-CFTR showed very little responses to cAMP agonists and potentiators such as CPT-cAMP and felodipine, respectively (Fig. 1A). After stimulation, total F508del-CFTR activity was evaluated by applying CFTRinh-172 (10 μM), a selective CFTR inhibitor (4, 25). The decrease in short-circuit current caused by the inhibitor was almost negligible (~1 μA/cm2) thus indicating that the expression level of F508del-CFTR in the plasma membrane is very low. Cell incubation for 24 hours with corr-4a (5 μM), a F508del corrector that is quite effective in other cell systems (29), caused only a non-significant 50 % increase in CFTRinh-172 effect (Fig. 1A,C). On the other hand, CFBE41o- cells were highly responsive to low temperature. Incubation at 27 °C for 24 hours markedly increased CFTR activity by more than 10-fold, as evident from the enhanced response to CPT-cAMP, felodipine, and CFTRinh-172 (Fig. 1A,C). Interestingly, low temperature incubation had also the ability to make the cells much more responsive to the corrector. Indeed, the rescue elicited by corr-4a increased significantly from 50 % at 37 °C to ~ 250 % at 27 °C (Fig. 1A,C).
Fig. 1.
Rescue of F508del-CFTR function by corr-4a and low temperature. Transepithelial Cl− currents were measured in CFBE41o- cells expressing F508del or wild type CFTR. A: representative recordings showing response of F508del-CFTR to acute application of CPT-cAMP (200 μM), felodipine (5 μM), and inh-172 (10 μM). Cells were kept for 24 hours under control conditions or treated with corr-4a (C4, 5 μM) and/or low temperature (27 °C). B: representative experiments from cells expressing wild type CFTR. CFTRinh-172 was applied after or before (red trace) stimulation with CPT-cAMP plus felodipine. C: summary of data from transepithelial Cl− current experiments. Total CFTR activity was evaluated by measuring the amplitude of the current inhibited by inh-172 (n = 19-29; **, p < 0.01).
CFBE41o- cells expressing wild type CFTR showed very large transepithelial Cl− currents under resting conditions (> 50 μA/cm2). Stimulation with CPT-cAMP and felodipine caused a relatively small (~ 20-30 %) increase in current (Fig 1B). Subsequent application of CFTRinh-172 reduced Cl− currents to nearly zero. These results indicate that a large fraction of CFTR is already active under resting conditions, a characteristic also shown by primary airway epithelial cells (5). This conclusion was supported by experiments in which CFTRinh-172 was applied before stimulation (Fig. 1B, red trace). Application of CFTRinh-172 caused a strong decrease of the current close to the zero level. Subsequent stimulation with CPT-cAMP plus felodipine elicited a total current (8.5 ± 0.7 μA/cm2, n = 6) which was much smaller than that measured in the absence of the inhibitor (71.8 ± 2.0 μA/cm2, n = 4, p < 0.01).
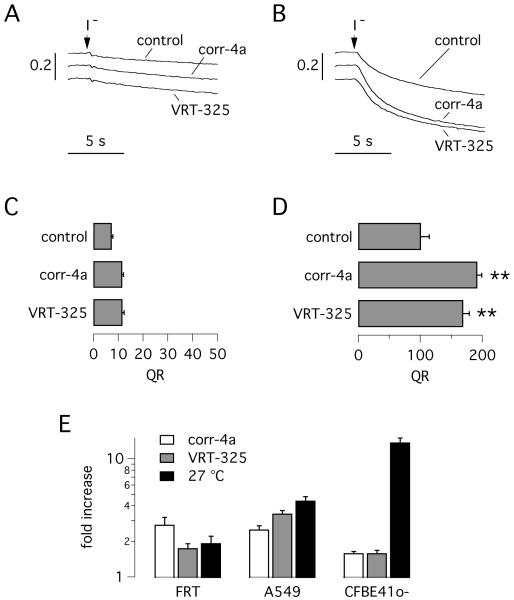
We generated a fluorescent version of CFBE41o-/F508del cells by stable transfection with the halide-sensitive yellow fluorescent protein (HS-YFP). These cells allowed to rapidly test the effect of correctors by simply measuring I− influx (29, 30, 43). The HS-YFP data confirmed the observations obtained with the short-circuit current technique. Cells kept at 37 °C had almost undetectable activity, even after maximal stimulation with forskolin (to elevate intracellular cAMP) and with the potentiator genistein (Fig. 2A,C). Treatment for 24 hours with corr-4a or another corrector, VRT-325, caused a modest (~ 60 %) F508del rescue that did not reach statistical significance (Fig. 2A,C). However, incubation of cells at low temperature strongly increased anion transport (~ 15-fold) and this effect was further enhanced by corr-4a or VRT-325 (Fig. 2B,D), thus confirming the results obtained with the short-circuit current technique.
Fig. 2.
Sensitivity of cells expressing F508del-CFTR to rescue maneuvers. F508del-CFTR activity was measured with the halide-sensitive yellow fluorescent protein (HS-YFP) assay. A,B: representative traces showing cell fluorescence decay upon extracellular addition of I−. CFBE41o- cells expressing F508del-CFTR were kept at 37 °C (A) or at 27 °C (B) for 24 hours with or without corr-4a (5 μM) or VRT-325 (10 μM). C,D: bars report the fluorescence quenching rate (QR) from experiments as those shown in A and B, respectively (n = 6-8; **, p < 0.01 vs. control). E: comparison of HS-YFP assay data obtained in FRT, A549, and CFBE41o- cells expressing F508del-CFTR. Data are reported as fold-increase of QR by rescue maneuvers (n = 10-12).
Using the HS-YFP, we also compared the sensitivity to F508del rescue maneuvers in CFBE41o-, A549, and FRT cells (Fig. 2E). The data confirm the peculiar behavior of CFBE41o- cells, which are highly responsive to low temperature rescue. In contrast, FRT cells had the minimal response to low temperature, compared to the other two cell lines (Fig. 2E).
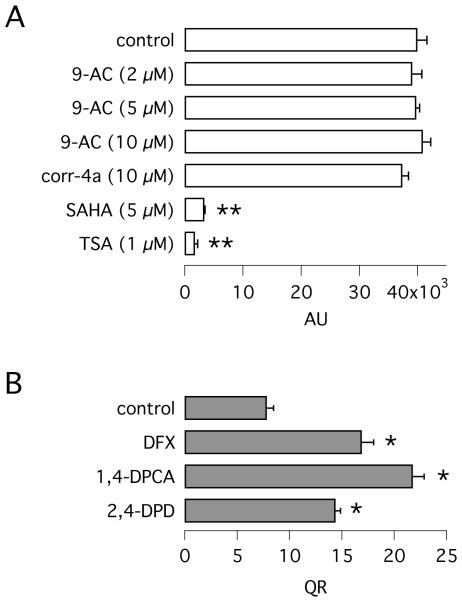
To further evaluate CFBE41o- as a cell line suitable for the identification of F508del correctors, we used the YFP assay to perform a pilot screening of 2,534 small molecules containing a large fraction of FDA-approved drugs (31). These compounds were tested by cell incubation at 37 °C for 24 hours. The screening detected essentially two active compounds: 9-aminoacridine and ciclopirox. At 5 and 20 μM, the optimal concentrations, these two compounds enhanced F508del-CFTR activity (anion transport) by 3.3- and 2.9-fold, respectively (Fig. 3A,B). For comparison, we evaluated the activity of SAHA, a histone deacetylase inhibitor, that has been recently identified in CFBE41o- cells as a F508del corrector (15). Under our conditions, SAHA (5 μM) increased anion transport by 2.2-fold (Fig. 3A,B), a result comparable to that reported previously (15). The effects of 9-aminoacridine and SAHA were confirmed with the short-circuit current technique. Incubation with 9-aminoacridine (10 μM) or SAHA (5 μM) increased total F508del-CFTR activity (i.e. the current sensitive to CFTRinh-172) by 8- and 3-fold, respectively (Fig. 3C-F). These experiments were not possible with ciclopirox since this compound decreased transepithelial resistance of CFBE41o- cells (R < 200 Ω · cm2 vs. 660 ± 40 Ω · cm2 for control; 585 ± 85 Ω · cm2 for corr-4a; 925 ± 175 Ω · cm2 for 9-aminoacridine; 625 ± 85 Ω · cm2 for 27 °C).
Fig. 3.
Rescue of F508del-CFTR by novel compounds. A,B: representative traces and summary of QR from HS-YFP assays on CFBE41o- cells expressing F508del-CFTR. Cells were treated with indicated compounds: 9-aminoacridine (9-AC, 5 μM), ciclopirox (CPX, 20 μM), SAHA (5 μM) (n = 5-12; *, p < 0.05). C-F: representative recordings of transepithelial Cl− currents (C-E) and amplitude of inh-172-sensitive current (F) from CFBE41o- cells expressing F508del-CFTR treated with and without 9-AC or SAHA (n = 6-10; *, p < 0.05).
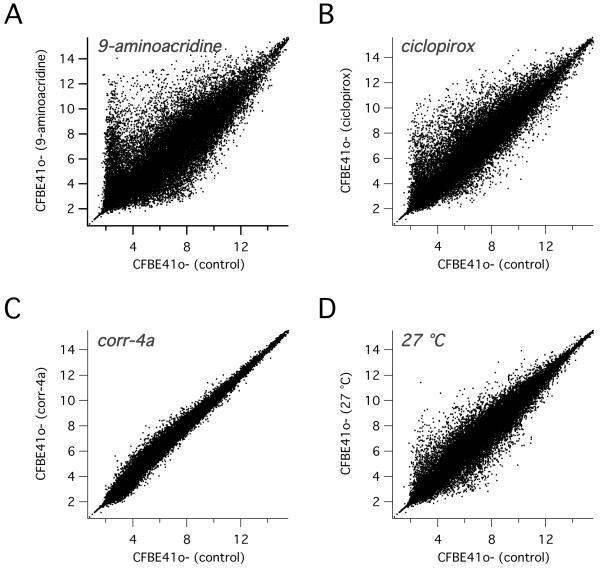
To obtain information on the mechanism of the different rescue treatments, we performed a global gene expression analysis using microarrays. We treated CFBE41o- cells for 24 hours with corr-4a (5 μM), ciclopirox (20 μM), 9-aminoacridine (5 μM), and low temperature (27 °C). After RNA extraction, we determined gene expression profile using GeneChip Human Genome U133 plus 2 arrays (Affymetrix). As shown in Fig. 4A and B, 9-aminoacridine and ciclopirox caused a profound change in gene expression, with the former compound eliciting a particularly dramatic effect. This is evident from the large scattering of data points indicating that several hundred genes are markedly upregulated or downregulated by the treatment. In contrast, incubation with corr-4a produced relatively small gene expression changes (Fig. 4C). This is also shown in Fig. 5A, which reports the fold-increase for the top 100 genes upregulated by each treatment. For 9-aminoacridine and ciclopirox, the first 100 genes were upregulated by more than 100- and 30-fold, respectively (Fig. 5A). Instead, the largest effect of corr-4a was a 8- to 4-fold increase, restricted only to the top 20 genes (see also Supplementary Tables 1S-4S). A similar pattern is also evident by looking at the genes downregulated by each treatment: 9-aminoacridine and ciclopirox, but not corr-4a, caused a dramatic decrease in the expression of several genes (Tables 5S-8S). In other words, 9-aminoacridine and ciclopirox are very pleiotropic substances, causing a global change in the cell transcriptome. Surprisingly, incubation of cells at low temperature also evoked a wide effect on gene expression, qualitatively comparable to that of 9-aminoacridine and ciclopirox (Fig. 4D, Fig. 5A, Table 3S, and Table 7S). We looked at the list of the most upregulated and downregulated genes by the four different treatments looking for a possible overlap (Fig. 5C). The only gene upregulated by all treatments was PTGS2 (i.e. cyclooxygenase 2). This finding can be explained by the behavior of PTGS2 as an inducible gene lying downstream various signaling cascades. Among the most affected genes (i.e. the 100 genes most upregulated or downregulated), we found 50 other genes being affected by more than one treatment. Concordant genes were not clearly suggestive of a common mechanism of action.
Fig. 4.
Global effects of F508del-CFTR rescue maneuvers on gene expression in CFBE41o- cells. Each dot in the graph reports the expression level for a given gene after treatment vs. the expression level in untreated cells (mean of three separate cell preparations). Data were obtained with gene expression microarrays.
Fig. 5.
Analysis of gene expression changes by F508del-CFTR rescue maneuvers. A: extent of gene expression changes by 9-aminoacridine (5 μM), ciclopirox (20 μM), low temperature, and corr-4a (5 μM). For every treatment, the top 100 upregulated genes were selected. The graph reports the fold-increase for each one of these genes. Data are from the gene expression microarrays shown in Fig. 4. B: fold increase in CFTR mRNA caused by rescue maneuvers, as determined by real time RT-PCR (n = 3; *, p < 0.05; **, p < 0.01 vs. corr-4a). C: the two panel report, among the 100 most upregulated genes (left) or downregulated genes (right), the ones common to more than one treatment.
We have inspected the identity of genes modulated by correctors and low temperature to obtain possible indications about the underlying molecular mechanisms (Suppementary Tables 1S-8S). The effect of 9-aminoacridine consists of a massive upregulation or downregulation of several genes, which seem to be largely unrelated to each other. For example, the list reported in Table 1S includes hemoglobin A1 and A2. These genes are essentially silent under resting conditions, as expected from an epithelial cell line, but become highly active after treatment with 9-aminoacridine. These characteristics suggest that 9-aminoacridine is a general inducer of gene transcription. Interestingly, CFTR transcript was also strongly upregulated by 9-aminoacridine (~ 60-fold, position 253 in the list of upregulated genes). This result was confirmed by real time RT-PCR (65-fold upregulation of CFTR transcript by 9-aminoacridine, Fig. 5B). Therefore, the rescue by 9-aminoacridine may be a consequence of the strong increase in F508del-CFTR protein synthesis and escape from the endoplasmic reticulum by mass action. However, we cannot exclude that other proteins, also affected by 9-aminoacridine, contribute to F508del rescue. Given the very strong and general effect of 9-aminoacridine on gene transcription, we hypothesized that it acts as a histone deacetylase inhibitor (HDACi) similarly to SAHA. We performed an assay using a commercially-available kit (Fig. 6A). The results show clearly that 9-aminoacridine has no HDACi activity, in contrast to positive controls SAHA and trichostatin A (TSA).
Fig. 6.
Mechanism of action of F508del-CFTR rescue maneuvers. A: histone deacetylase activity in the presence of indicated compounds (n = 3; **, p < 0.01 vs. control). 9-AC: 9-aminoacridine; TSA: trichostatin A. B: F508del-CFTR activity (QR) in CFBE41o- after treatment with deferoxamine (DFX, 200 μM), 1,4-DPCA (50 μM), or 2,4-DPD (20 μM) (n = 8-21; *, p < 0.05). Data were obtained by incubating the cells with compounds for 24 hours at 37 °C.
Ciclopirox and low temperature also have a global effect on cell transcriptome that includes upregulation of CFTR gene expression. Indeed, the mutant CFTR transcript was upregulated by 8- and 5-fold, respectively (Fig. 5B). However, compared to 9-aminoacridine, these compounds may trigger a more specific transcriptional program. Regarding low temperature, it has been reported that cells exposed to moderate hypothermia show a cold shock response that modifies the proteostasis environment (10). Indeed, the list in Table 3S includes various genes related to protein synthesis and degradation. Some of the genes induced at 27 °C may be particularly relevant to F508del-CFTR processing, such as de-ubiquitylating enzymes (e.g. USP15 and USP36, 18-fold and 10-fold increase, respectively). Among the genes downregulated by low temperature, AHSA2 (homolog of Aha1; Table 7S) was particularly interesting (> 6-fold decrease). Inhibition of Aha1 by siRNA was found in another study to result in F508del-CFTR rescue (41).
Ciclopirox is instead known as an iron-chelating agent and, consequently, an inducer of the hypoxia-inducible factor 1α (HIF-1α) cascade (23). This transcription factor is controlled by a panel of oxygen-sensing and iron-dependent enzymes, the prolyl hydroxylases (PHDs). The prolyl hydroxylation of HIF-1α is responsible for its degradation through the proteasome (20). Under hypoxic conditions, PHDs are inhibited, thus allowing stabilization of HIF-1α and induction of the corresponding transcriptional response. Therefore, the mechanism of ciclopirox as a F508del corrector may involve the triggering of a response mimicking the hypoxic condition with altered expression of several genes. Indeed, many genes reported to be induced by hypoxia in other studies, such as ATF3, BTG2, NDRG-1, BMP2, and RGS2 (3, 6, 17, 35), are present among the ones upregulated by ciclopirox (Supplementary Table 2S). To verify this link, we tested various pharmacological modulators acting on the hypoxia-induced pathway. We found that another iron chelating agent, desferoxamine (DFX), also increases the function of F508del-CFTR (Fig. 6B). Furthemore, we found that prolyl hydroxylase (PHD) inhibitors, 1,4-DPCA and 2,4-DPD are active in rescuing F508del-CFTR activity.
In contrast to the other treatments, corr-4a has little consequences on gene expression. Evaluation of the few genes upregulated or downregulated by this corrector (Tables 4S and 8S) did not reveal a signature associated with a known biological response.
We also investigated the effect of different rescue maneuvers by determining the electrophoretic mobility maturation of F508del-CFTR protein (Fig. 7). Cells lysates were processed by immunoprecipitation followed by western blot. In control conditions at 37 °C, F508del-CFTR is mostly revealed as band B (~ 150 kDa) representing the core-glycosylated form of the protein residing in the endoplasmic reticulum (Fig. 7A, third lane). Cells expressing wild type CFTR instead show the fully-glycosylated mature form of the protein, band C, migrating at ~ 180 kDa (Fig. 7A, second lane). At 37 °C, the various treatments did not significantly increase the maturation of F508del-CFTR. Actually, SAHA and 9-aminoacridine caused an increase in band B intensity alone. Improvements in F508del-CFTR maturation were mostly seen at low temperature as indicated by the increase in C/(B+C) value (Fig. 7B). At 27 °C, the relative abundance of band C was significantly increased, although the extent of rescue was still far from reaching the pattern of wild type CFTR, as also shown previously (2). Treatment with corr-4a (5 μM), VRT-325 (10 μM), ciclopirox (20 μM), or 9-aminacrine (10 μM) at low temperature further improved F508del-CFTR maturation. Instead, SAHA caused a marked inhibition of C/(B+C) value at low temperature (Fig. 7B,C). We also tested 1,4-DPCA and 2,4-DPD. These compounds did not significantly improve CFTR protein maturation (not shown).
Fig. 7.
Biochemical analysis of F508del-CFTR protein maturation. A: representative immunoprecipitation/western blot experiments on cell lysates from CFBE41o- cells expressing F508del-CFTR, wild type CFTR, or from parental (null) cells. Cells were treated with indicated compounds (5 μM corr-4a; 10 μM VRT-325; 5 μM SAHA; 20 μM ciclopirox, CPX; 5-10 μM 9-aminacrine, 9-AC) at 37 °C or 27 °C for 24 hours. The content of actin in each lysate was determined by western blot. B: summary of data expressed as the ratio of band C/(band B+ band C) intensity (n = 4-5; *, p < 0.05 vs. control; ††, p < 0.01 vs. control at 37 °C).
We checked the efficacy of different compounds and low temperature in primary bronchial epithelial cells from a homozygous F508del patient (Fig. 8). The effective treatments were corr-4a, VRT-325, and low temperature, although the extent of correction was relatively modest (1.7-, 2.1-, and 2.3-fold increase, respectively). In contrast, SAHA, 9-aminoacridine, ciclopirox, 1,4-DPCA, and 2-4-DPD were ineffective (Fig. 8A,B). Actually, SAHA and 9-aminoacridine significantly decreased the CFTR-dependent current. Such negative findings were also confirmed in experiments in which a Cl− gradient was applied to increase the driving force for Cl− secretion (Fig. 8C). We asked whether the failure of some compounds in increasing CFTR-dependent Cl− secretion was due to a decrease in transepithelial resistance. Resistance was measured with an epithelial voltohmeter. None of the treatments significantly decreased the resistance (control: 2060 ± 37 Ω · cm2; corr-4a: 2180 ± 76 Ω · cm2; 9-aminoacridine: 1905 ± 171 Ω · cm2; ciclopirox: 2177 ± 48 Ω · cm2; 27 °C: 2048 ± 37 Ω · cm2). Similar results were also obtained by calculating the resistance from the Ohm’s law using the values of short-circuit current and transepithelial potential difference measured in the Ussing chamber.
Fig. 8.
Evaluation of rescue maneuvers in primary bronchial epithelia. A: representative short-circuit current recordings performed on cultured primary bronchial epithelial cells from F508del CF patients. The experiments were performed in symmetrical Cl−. B: inh-172-sensitive current after treatment of bronchial epithelial cells with indicated conditions. Data are from the set of experiments shown in A. Each bar is the mean ± SEM of 8-21 experiments. Corr-4a (5 μM), VRT-325 (10 μM) and low temperature significantly increase the current (*, p < 0.05 vs. control). Instead, SAHA (5 μM) and 9-aminoacridine (5 μM) cause a significant inhibition. (†, p < 0.05 vs. control). C: inh-172-sensitive current from short-circuit current experiments performed with a Cl− gradient (n = 5-6; *, p < 0.05 vs. control).
We analyzed gene expression with microarrays also in primary bronchial epithelial cells (Fig. 9). The effect of low temperature and ciclopirox was qualitatively similar to that observed in CFBE41o- cells in that a large number of genes showed altered expression (1418 genes for 27 °C and 554 for ciclopirox, see Fig. 10). However, the incubation at low temperature produced a very interesting result. There was remarkable overlap of the genes affected by low temperature in both cell types (97% of the genes affected by low temperature in primary cells were also present in the CFBE41o- list; Fig. 10). This is also evident from the list of the 100 most upregulated or downregulated genes (Tables 3S and 7S). Genes involved in protein quality control and degradation (in particular USP15, USP36, and AHSA2) were strongly affected in both cell types. The extent of concordant genes was smaller for ciclopirox (64% of genes modulated by ciclopirox in primary cells were also present in the CFBE41o- list). In contrast, a striking difference was noted for 9-aminoacridine. This compound had a relatively small activity in bronchial epithelial cells (compare Fig. 4 and 9). In this respect, it is important to take into account that some differences in our results could be due to the conditions used before extraction of RNA for microarray analysis (cells on solid support for CFBE41o- vs. cells on permeable support for primary epithelial cells). Corr-4a was also characterized by a small effect on gene expression in primary cells, but this result was consistent with the findings obtained in CFBE41o- cells. Interestingly, none of the treatments increased CFTR expression in primary bronchial epithelial cells (not shown) as instead observed in CFBE41o- cells for 9-aminoacridine, ciclopirox, and low temperature.
Fig. 9.
Gene expression profiling in human bronchial epithelial cells. Cells were treated with 9-aminoacridine (5 μM), ciclopirox (20 μM), low temperature, or corr-4a (5 μM). Each dot in the graph reports the expression level for a given gene after treatment vs. the expression level in untreated cells (mean of four separate cell preparations). Data were obtained with gene expression microarrays.
Fig. 10.
Comparison of gene expression signatures in CFBE41o- and primary bronchial epithelial cells. Data are from the experiments shown in Fig. 4 and 9. The Venn diagrams report the number of genes with altered expression (2-fold increase or decrease) by each one of the indicated treatments and the number of the commonly affected genes in the two types of cells.
DISCUSSION
Identification of effective F508del correctors is a major goal of CF research given the frequency and severity of this mutation. This search could be helped by the knowledge of the relevant molecular targets and mechanisms involved in F508del-CFTR protein misprocessing and degradation, but the existing information is not yet sufficient to proceed with a rational design/search of F508del correctors. Therefore, one of the most reasonable approaches to find active compounds is the screening of chemical libraries using a functional or biochemical readout (40). However, this process may be strongly affected by the cell type used for the screening. In contrast to the search for CFTR potentiators, the screening for correctors appears to be markedly affected by the cell background (31). Using a cell line that is as close as possible to the native cells expressing F508del-CFTR in vivo seems the most logical solution.
In the present study, we have evaluated the bronchial cell line CFBE41o- that has become a popular cell model in CF research. In particular, we used an engineered version of the cell line that has increased expression of wild type or F508del-CFTR (2, 19, 39). Compared to other cell models previously used for high-throughput screenings, we found that CFBE41o- cells are poorly sensitive to F508del correctors such as corr-4a but strongly responsive to low temperature treatment. However, the incubation at 27 °C seems to shift the cells to a “permissive” state that is much more sensitive to the effect of correctors. It has been recently shown that corr-4a acts only when the F508del-CFTR protein is fully synthesized (13), and possibly by also stabilizing the protein at the plasma membrane (39). It has been also shown that F508del-CFTR is detected by the cell quality control systems and largely degraded when its synthesis is not complete (13, 44). Therefore, the poor rescue by corr-4a and other known correctors in CFBE41o- may be explained by a tighter quality control compared to other cells that instead appear less stringent. In “leaky” cells, such as FRT, a significant amount of mutant protein may escape from the degradation pathway and be amenable to the action of correctors (29). The particularly strong effect of low temperature in CFBE41o- cells, which increases the efficacy of corr-4a and VRT-325, may be due to a stabilization of the mutant protein and a slowing down of degradation. Therefore, understanding the mechanism of low temperature and mimicking it with a small molecule appears as an important priority to design novel maneuvers for F508del rescue. Indeed, such maneuvers would act at early levels, before nascent F508del-CFTR is tagged for degradation. Our gene expression analysis with microarrays reveals that the low temperature treatment has a complex effect since it causes a profound alteration in cell transcriptome. Therefore, it is probable that the corresponding change in the proteostasis environment, and not the activity/expression of a single protein, is globally responsible for F508del-CFTR rescue by low temperature. Nevertheless, as discussed in a subsequent paragraph, low temperature has also a marked effect on CFTR mRNA levels in CFBE41o- cells.
To assess the suitability of CFBE41o- cells as a model to identify correctors, we performed a pilot screening of a chemical library using the HS-YFP assay. One of the active compounds detected in the screening was 9-aminoacridine. This compound elicited a marked rescue of F508del-CFTR function in CFBE41o- cells. As for low temperature, we found that 9-aminoacridine causes a dramatic upregulation of hundreds of genes. Therefore, 9-aminoacridine appears as a non-specific inducer of gene expression. We hypothesized that this compound works as a histone deacetylase inhibitor (HDACi) similarly to SAHA, which has been found to rescue F508del-CFTR (15). However, 9-aminoacridine was completely inactive in a HDAC assay. Therefore, this compound acts on gene expression with a different mechanism. It is possible that 9-aminoacridine works as a DNA-threading agent (12, 46). The effect on chromatin structure may be responsible for the global gene transcription effect that also results in increased synthesis of the transfected F508del-CFTR.
The screening of the chemical library also detected ciclopirox as a possible corrector. Interestingly, a similar screening on A549 cells expressing F508del-CFTR also identified ciclopirox (31). The results obtained with desferoxamine and PHD inhibitors suggest that ciclopirox acts by activating the HIF-1α cascade. This biological process involves a change in expression and activity of many proteins (hypoxic response). One or more of these proteins could affect F508del-CFTR processing, probably in an indirect way. For example, it has been shown that EGLN3 causes the prolyl hydroxylation and direct degradation of the beta-adrenergic receptor (42). A similar process may also occur for CFTR.
Surprisingly, we found that 9-aminoacridine, ciclopirox, and low temperature also cause a marked upregulation of CFTR mRNA in CFBE41o- cells. This effect was also reported previously for SAHA in the same cells (15). Therefore, it is not clear how much of the rescue is due to F508del-CFTR upregulation vs. modification of proteostasis environment. The increase in mutant protein expression could cause the escape from the endoplasmic reticulum by mass action leading to an overestimation of the real effect on F505del-CFTR maturation. Indeed, SAHA, 9-aminoacridine, and ciclopirox appeared ineffective when tested in primary bronchial epithelial cells from F508del patients. The reason for the high sensitivity of F508del-CFTR expression to a variety of treatments in CFBE41o- cells is not clear but it is possible that the viral elements used to drive expression of transfected CFTR are sensitive to different types of chemical or physical cell stress as reported previously (21, 34).
Analysis of gene expression in primary bronchial epithelial cells provided useful information to understand the mechanisms of the different F508del rescue maneuvers and to explain the failure of some molecules as correctors in primary cells. Both ciclopirox and low temperature were confirmed as treatments causing a broad effect on cell transcriptome. Low temperature was particularly interesting because it caused a very similar effect in CFBE41o- and primary bronchial epithelial cells, with several genes being equally affected in both cell types. Interestingly, none of the treatments caused a change in CFTR mRNA levels in primary bronchial epithelial cells. This finding further indicates that the peculiar activity of 9-aminocridine and ciclopirox in CFBE41o- as “apparent correctors” may be explained in large part by their effect on CFTR expression. This conclusion may be true also for SAHA.
Summarizing, our results suggest that the failure of some F508del rescue maneuvers in primary bronchial epithelial cells correlates with different gene expression signatures generated by these treatments in cell lines and primary cells. For example, the most effective F508del rescue maneuvers in CFBE41o- cells are the ones associated with global effects on cell transcriptome, such as 9-aminoacridine, SAHA, ciclopirox, and low temperature. In theory, these treatments may promote a change in the cell proteome, particularly in the CFTR protein interactome (41), thus creating an environment that is more favorable for F508del-CFTR maturation. However, we found that some treatments do not have the same effect on the cell transcriptome of primary cells which may explain their lack of efficacy as correctors. In particular, the upregulation of CFTR protein expression observed in CFBE41o- but not in primary cells may lead to an overestimation of F508del-CFTR corrector activity.
Despite its global effect on cell transcriptome in general and on heterologous CFTR in particular, rescue by low temperature appears as an interesting phenomenon to investigate. Indeed, in contrast to 9-aminoacridine, ciclopirox, and SAHA, low temperature improves by itself F508del-CFTR protein maturation, synergizes with correctors, and is effective in primary cells. Rescue of F508del-CFTR at 27 °C has also another interesting feature, as discussed above. Our gene expression profiling has revealed that the gene expression signatures generated at low temperature in CFBE41o- and primary cells are very similar, thus suggesting the existence of a cell type-independent response to hypothermia. Dissection of molecular events underlying rescue by low temperature may lead to improved strategies for CF therapy. For example, genes affected by low temperature could be upregulated/silenced individually or in combination to assess their effect on F508del-CFTR. In the present study, we have payed attention to the most affected genes by each treatment. However, in future studies analysis of genes with less dramatic upregulation or downregulation may also provide useful information.
In conclusion, CFBE41o- cells are potentially an interesting cell model to identify F508del correctors. In particular, CFBE41o- are less “leaky” than other cell lines and therefore may be useful to find correctors acting at early steps of CFTR protein processing thus preventing its premature degradation. However, CFBE41o- are also particularly sensitive to treatments increasing gene expression in general and CFTR transcript in particular. This issue needs to be taken into account during the evaluation and prioritization of primary active compounds arising from screenings. Accordingly, future small molecule screenings on CFBE41o- or similar cells will need to be followed by secondary analysis to categorize putative correctors and to differentiate compounds with more specific mechanism of action from those having general effects on cell transcriptome and proteome.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J.P. Clancy for kindly providing us with CFBE41o- cells expressing wild tipe and F508del-CFTR.
GRANTS
This work was supported by funds from Cystic Fibrosis Foundation Therapeutics, Telethon Foundation (GGP10026), and Fondazione Italiana Fibrosi Cistica (FFC#2/2009 with the contribution of “Delegazione FFC di Vicenza”). N.P. is also a recipient of a grant to young investigators from the Italian Ministry of Health (GR-2008-1141326).
REFERENCES
- 1.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordoñez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued ΔF508 CFTR in CFBE41o- airway epithelial monolayers. J Physiol. 2005;569:601–615. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelte KC, Gordy LE, Joyce S, Thompson MA, Yang L, Lin PC. Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PLoS One. 2011;6:e18534. doi: 10.1371/journal.pone.0018534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTRinh-172 based on Arg347 mutagenesis. Biochem J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 5.Caci E, Folli C, Zegarra-Moran O, Ma T, Springsteel MF, Sammelson RE, Nantz MH, Kurth MJ, Verkman AS, Galietta LJ. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol. 2003;285:L180–L188. doi: 10.1152/ajplung.00351.2002. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, Liu YC, Shyu KG, Wang DL. Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis. 2008;201:281–288. doi: 10.1016/j.atherosclerosis.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 8.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 9.Galietta LJ, Musante L, Romio L, Caruso U, Fantasia A, Gazzolo A, Romano L, Sacco O, Rossi GA, Varesio L, Zegarra-Moran O. An electrogenic amino acid transporter in the apical membrane of cultured human bronchial epithelial cells. Am J Physiol. 1998;275:L917–L923. doi: 10.1152/ajplung.1998.275.5.L917. [DOI] [PubMed] [Google Scholar]
- 10.Gomes-Alves P, Neves S, Coelho AV, Penque D. Low temperature restoring effect on F508del-CFTR misprocessing: A proteomic approach. J Proteomics. 2009;73:218–230. doi: 10.1016/j.jprot.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Goncz KK, Feeney L, Gruenert DC. Differential sensitivity of normal and cystic fibrosis airway epithelial cells to epinephrine. Br J Pharmacol. 1999;128:227–233. doi: 10.1038/sj.bjp.0702772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodell JR, Ougolkov AV, Hiasa H, Kaur H, Remmel R, Billadeau DD, Ferguson DM. Acridine-based agents with topoisomerase II activity inhibit pancreatic cancer cell proliferation and induce apoptosis. J Med Chem. 2008;51:179–182. doi: 10.1021/jm701228e. [DOI] [PubMed] [Google Scholar]
- 13.Grove DE, Rosser MF, Ren HY, Naren AP, Cyr DM. Mechanisms for rescue of correctable folding defects in CFTRΔF508. Mol Biol Cell. 2009;20:4059–4069. doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, Wine JJ. ΔF508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- 15.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, Thomas PJ, Matsumura Y, Skach WR, Gentzsch M, Riordan JR, Sorscher EJ, Okiyoneda T, Yates JR, 3rd, Lukacs GL, Frizzell RA, Manning G, Gottesfeld JM, Balch WE. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igwe EI, Essler S, Al-Furoukh N, Dehne N, Brüne B. Hypoxic transcription gene profiles under the modulation of nitric oxide in nuclear run on-microarray and proteomics. BMC Genomics. 2009;10:408. doi: 10.1186/1471-2164-10-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 19.Jurkuvenaite A, Chen L, Bartoszewski R, Goldstein R, Bebok Z, Matalon S, Collawn JF. Functional stability of rescued delta F508 cystic fibrosis transmembrane conductance regulator in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:363–372. doi: 10.1165/rcmb.2008-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 21.Kretz-Remy C, Arrigo AP. The kinetics of HIV-1 long terminal repeat transcriptional activation resemble those of hsp70 promoter in heat-shock treated HeLa cells. FEBS Lett. 1994;353:339–344. doi: 10.1016/0014-5793(94)00828-0. [DOI] [PubMed] [Google Scholar]
- 22.Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the ΔF508 CFTR mutation. Am J Respir Cell Mol Biol. 1993;8:522–529. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- 23.Linden T, Katschinski DM, Eckhardt K, Scheid A, Pagel H, Wenger RH. The antimycotic ciclopirox olamine induces HIF-1alpha stability, VEGF expression, and angiogenesis. FASEB J. 2003;17:761–763. doi: 10.1096/fj.02-0586fje. [DOI] [PubMed] [Google Scholar]
- 24.Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S. The ΔF508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- 25.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Chmiel JF, Sternen DL, Cheng E, Gibson RL, Marshall SG, Cutting GR. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet Med. 2008;10:851–868. doi: 10.1097/GIM.0b013e31818e55a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 29.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, Kurth MJ, Galietta LJ, Verkman AS. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 31.Pedemonte N, Tomati V, Sondo E, Galietta LJ. Influence of cell background on pharmacological rescue of mutant CFTR. Am J Physiol. 2010;298:C866–C874. doi: 10.1152/ajpcell.00404.2009. [DOI] [PubMed] [Google Scholar]
- 32.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 33.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 34.Torgeman A, Ben-Aroya Z, Grunspan A, Zelin E, Butovsky E, Hallak M, Löchelt M, Flügel RM, Livneh E, Wolfson M, Kedar I, Aboud M. Activation of HTLV-I long terminal repeat by stress-inducing agents and protection of HTLV-I-infected T-cells from apoptosis by the viral tax protein. Exp Cell Res. 2001;271:169–179. doi: 10.1006/excr.2001.5363. [DOI] [PubMed] [Google Scholar]
- 35.Tseng WP, Yang SN, Lai CH, Tang CH. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol. 2010;223:810–818. doi: 10.1002/jcp.22104. [DOI] [PubMed] [Google Scholar]
- 36.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 39.Varga K, Goldstein RF, Jurkuvenaite A, Chen L, Matalon S, Sorscher EJ, Bebok Z, Collawn JF. Enhanced cell-surface stability of rescued ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem J. 2008;410:555–564. doi: 10.1042/BJ20071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verkman AS, Lukacs GL, Galietta LJ. CFTR chloride channel drug discovery--inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des. 2006;12:2235–2247. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 42.Xie L, Xiao K, Whalen EJ, Forrester MT, Freeman RS, Fong G, Gygi SP, Lefkowitz RJ, Stamler JS. Oxygen-regulated beta(2)-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL. Sci Signal. 2009;2:ra33. doi: 10.1126/scisignal.2000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, Galietta LJ, Verkman AS. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J Biol Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 44.Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, Cyr DM. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Carey V, Gentleman R. An extensible application for assembling annotation for genomic data. Bioinformatics. 2003;19:155–156. doi: 10.1093/bioinformatics/19.1.155. [DOI] [PubMed] [Google Scholar]
- 46.Zihlif M, Catchpoole DR, Stewart BW, Wakelin LP. Effects of DNA threading bis(9-aminoacridine-4-carboxamides) on global gene expression. Cancer Genomics Proteomics. 2009;6:317–323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.