Abstract
Plants exposed to repetitive touch or wind are generally shorter and stockier than sheltered plants. These mechanostimulus-induced developmental changes are termed thigmomorphogenesis and may confer resistance to subsequent stresses. An early response of Arabidopsis thaliana to touch or wind is the up-regulation of TCH (touch) gene expression. The signal transduction pathway that leads to mechanostimulus responses is not well defined. A role for ethylene has been proposed based on the observation that mechanostimulation of plants leads to ethylene evolution and exogenous ethylene leads to thigmomorphogenetic-like changes. To determine whether ethylene has a role in plant responses to mechanostimulation, we assessed the ability of two ethylene-insensitive mutants, etr1–3 and ein2–1, to undergo thigmomorphogenesis and TCH gene up-regulation of expression. The ethylene-insensitive mutants responded to wind similarly to the wild type, with a delay in flowering, decrease in inflorescence elongation rate, shorter mature primary inflorescences, more rosette paraclades, and appropriate TCH gene expression changes. Also, wild-type and mutant Arabidopsis responded to vibrational stimulation, with an increase in hypocotyl elongation and up-regulation of TCH gene expression. We conclude that the ETR1 and EIN2 protein functions are not required for the developmental and molecular responses to mechanical stimulation.
In response to mechanical stimuli such as wind or touch, plants undergo physiological and developmental changes that enhance resistance to subsequent mechanical stress. In general, plants that are grown in windy environments or exposed to repetitive touch stimulation are shorter, stockier, and often have altered flexibility. These changes in development in response to mechanostimulation are collectively known as thigmomorphogenesis (Mitchell, 1996; Ennos, 1997).
In Arabidopsis thaliana wind or touch stimulation results in the enhancement of expression of the touch (TCH) genes. TCH gene mRNAs accumulate very rapidly, within 10 min of touch stimulation of plants (Braam and Davis, 1990). TCH1 encodes CaM (Braam and Davis, 1990), TCH2 and TCH3 encode CaM-related proteins (Braam and Davis, 1990; Sistrunk et al., 1994; Khan et al., 1997), and TCH4 encodes a xyloglucan endotransglycosylase capable of modifying cell wall xyloglucans (Xu et al., 1995). Mechanostimulus regulation of TCH gene expression suggests that TCH protein function may contribute to the process of thigmomorphogenesis, e.g. by altering the properties of the cell wall (Antosiewicz et al., 1995; Braam et al., 1996; Xu et al., 1996).
The mechanostimulus signal transduction pathway leading to thigmomorphogenesis is not yet well defined. Changes in concentrations of cytoplasmic calcium may play the role of second messenger, transducing the mechanostimulus into an intracellular signal. Dramatic up-regulation of expression of the CaM and CaM-related TCH genes following mechanical stimulation implicates the involvement of Ca2+-binding proteins in touch responses of plants (Braam and Davis, 1990). In addition, rapid increases in concentrations of cytoplasmic calcium occur in plants subjected to touch or wind stimulation (Knight et al., 1991; Haley et al., 1995). Finally, externally applied Ca2+ (Braam, 1992) and Ca2+-channel antagonists (Polisensky and Braam, 1996) have been shown to affect the expression of touch-induced genes.
Recent evidence implicates the possible involvement of protein kinases in the mechanostimulus response pathway of plants. The activities of protein kinases have been shown to be rapidly increased following wounding or mechanical stimulation; in vitro phosphorylation assays demonstrate activation within 1 to 5 min of stimulation (Suzuki and Shinshi, 1995; Bögre et al., 1996, 1997). In addition, expression levels of genes encoding mitogen-activated protein kinases and other protein kinases are increased within 30 min following wounding or mechanical stimulation (Seo et al., 1995; Mizoguchi et al., 1996; Bögre et al., 1997). This suggests that mitogen-activated protein and other protein kinases, possibly Ca2+-dependent protein kinases (Pio-trowski et al., 1996) and ribosomal protein S6 kinase (Mizoguchi et al., 1996), are involved in the mechanostimulus signal transduction pathway leading to gene expression.
For many years a role for the phytohormone ethylene in thigmomorphogenesis has been suspected. Touch stimulation results in a rapid increase in ethylene evolution (Goeschl et al., 1966; Poovaiah, 1974; Eisinger, 1983; Pressman et al., 1983; Biro and Jaffe, 1984; Takahashi and Jaffe, 1984), coinciding with a touch-induced increase in ACC synthase activity (Biro and Jaffe, 1984), a key enzyme in ethylene biosynthesis. Mechanical impedance of maize roots also results in an increase in ethylene production, which is believed to be responsible for inducing root swelling and aerenchyma formation (Sarquis et al., 1991; He et al., 1996a). In addition, exogenous application of ethylene to plants often results in developmental and morphological changes that are similar to those occurring during thigmomorphogenesis (Goeschl et al., 1966; Brown and Leopold, 1972; Jaffe and Biro, 1979; Salveit et al., 1979; Erner and Jaffe, 1982; de Jaegher et al., 1987). For example, both exogenous ethylene treatment and touch stimulation lead to development of shorter tracheids (Biro et al., 1980; Telewski et al., 1983), alterations in cell shape (Jaffe and Biro, 1979), and changes in membrane fatty acid content (Erner and Jaffe, 1983).
Arabidopsis mutants defective in sensing or responding to ethylene are valuable tools for assessing the potential role of ethylene in plant responses to mechanical stimulation. The dominant etr1–3 (Bleecker et al., 1988; Chang et al., 1993) and recessive ein2–1 (Guzman and Ecker, 1990; Roman et al., 1995) mutants were identified because of their ethylene-insensitive growth and have been shown to be blocked in ethylene-induced gene transcription (Samac et al., 1990; Lawton et al., 1994). ETR1 is similar to the two-component His kinase receptors and most likely functions as an ethylene receptor (Bleecker et al., 1988; Chang et al., 1993; Schaller and Bleecker, 1995). The sequence and function of EIN2 have not yet been reported.
To determine whether ethylene response pathways are involved in controlling plant responses to mechanical stimuli, we examined whether etr1–3 and ein2–1 mutants display appropriate developmental alterations following mechanical stimulation. Furthermore, regulation of TCH gene expression was monitored to determine whether ethylene signaling is required for mechanostimulus-induced regulation of gene expression.
MATERIALS AND METHODS
Origin of Seed Stocks
ColO, etr1–3, and ein2–1 seeds were obtained from the Arabidopsis Stock Center (The Ohio State University, Columbus).
Treatment of Plants and Seedlings
For assessment of thigmomorphogenesis, plants were grown individually in pots under constant light at 22°C; at 14 d of age, plants were exposed to wind three times daily for 30 min using oscillating fans. Measurements were taken on plants throughout development, and final height measurements were determined when senescence was apparent (i.e. when the first silique turned brown). Measurements are reported as means ± se, and statistical significance was determined using Student's t test.
Touch stimulation was performed on 14-d-old soil-grown plants (approximately 6–10 plants per 4-inch pot) by gently touching the rosette leaves and bending the plants back and forth 10 times. To generate sterile plants, seeds were treated briefly with 95% ethanol, suspended for 14 min in 5.25% sodium hypochlorite (Clorox), rinsed three times with sterile water, and then resuspended in sterile water before plating on a growth medium consisting of 0.5× minimal salts (Sigma), 1% Suc, and 1× Gamborg's vitamins (Sigma), pH 5.7. After the seeds were plated, they were placed at 4°C in the dark for 4 d to enhance germination efficiency.
A vibration was applied by attaching the plates to a platform covering a 15-inch subwoofer speaker. The low-frequency vibration (50 Hz) was at approximately 90 dB at the speaker surface. For hypocotyl-length analysis, seedlings were grown in the dark on filter paper soaked with growth medium and were subjected to vibrational stimulation starting at the time of germination and continuing for 72 h. Measurements were made immediately after the cessation of stimulation. For vibration-induced gene expression analysis, light-grown 14-d-old seedlings grown on 0.8% agar in growth medium were subjected to 10 min of stimulation and then harvested at the indicated times following the cessation of stimulation.
RNA Analysis
Plants were harvested, placed immediately in liquid N2, and then ground to a powder with a mortar and pestle. RNA was isolated (Verwoerd et al., 1989), size separated on formaldehyde-agarose gels, and transferred to nylon membranes (Sambrook et al., 1989). Hybridization of the RNA blots was performed as described by Sambrook et al., (1989) using the radioactively labeled (Feinberg and Vogelstein, 1983) cloned cDNA fragments described by Braam and Davis (1990).
RESULTS
Growth Responses to Wind
The requirement for ethylene-regulated processes in the developmental responses of Arabidopsis to mechanical stimulation was investigated using the ethylene-insensitive mutants etr1–3 and ein2–1. The effects of wind on aspects of plant development were monitored to determine whether etr1–3 and ein2–1 plants responded similarly to the wild type.
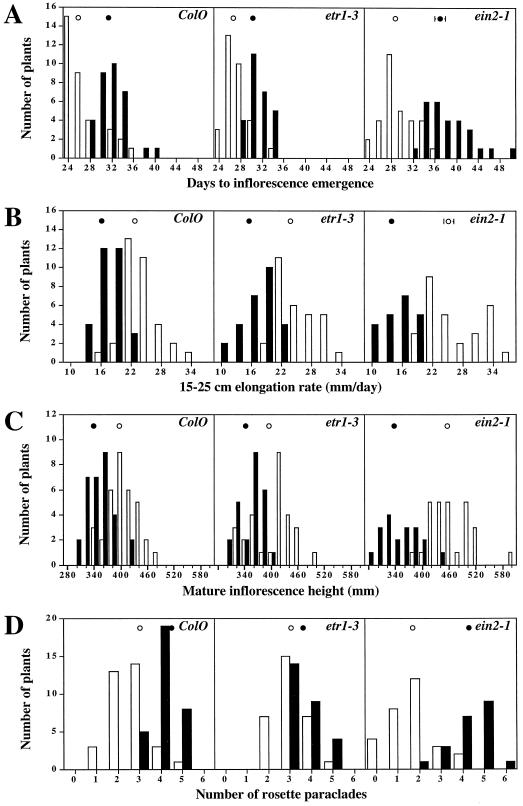
Plants treated with wind showed a delay in the initiation of the inflorescence growth relative to control plants (Fig. 1A). Inflorescence emergence of wild-type plants was delayed by almost 6 d (25.6 ± 0.6 versus 31.3 ± 0.5 d for control and wind treatments, respectively), whereas emergence of etr1–3 and ein2–1 inflorescences was delayed by 3 (26.7 ± 0.3 versus 30.3 ± 0.3 d) and 8 d (28.7 ± 0.5 versus 37.0 ± 1.0 d), respectively. Wind treatment also retarded the rate of inflorescence elongation. In wild-type plants the initial rate of inflorescence elongation from inflorescence initiation to 15 cm in height was reduced from 22.0 ± 0.5 to 19.8 ± 0.6 mm/d (P < 0.01; data not shown). A more significant reduction of growth rate was evident when the increment of growth between 15 and 25 cm in height was assessed. Wild-type plants showed a reduction in growth rate from 22.8 ± 0.6 to 16.3 ± 0.4 mm/d (P < 0.001; Fig. 1B). The growth rate reduction, as a consequence of wind treatment, was also observed for both ethylene-insensitive mutants analyzed. From inflorescence emergence to elongation to 15 cm, etr1–3 growth rate was reduced from 24.3 ± 0.4 to 23.7 ± 1.1 mm/d (not significant) and ein2–1 elongation rate was changed from 23.9 ± 0.6 to 20.2 ± 0.6 mm/d (P < 0.001; data not shown). In addition, reductions in elongation rates between 15 and 25 cm were observed for the mutants. The growth rate of etr1–3 was reduced from 23.9 ± 0.8 to 15.6 ± 0.6 mm/d (P < 0.001) and that for ein2–1 was decreased from 25.3 ± 1.0 to 13.9 ± 0.8 mm/d (P < 0.001; Fig. 1B).
Figure 1.
Effects of wind on plant growth and development. A, Histograms showing the number of days before inflorescence emergence. B, Histograms showing the rate of inflorescence elongation from 15 to 25 cm. C, Histograms showing plant height at senescence. D, Histograms showing the number of rosette paraclades visible when plants reached 25 cm in height. Open bars, Untreated plants; black bars, wind-treated plants; ○, mean for control plants; and •, mean for wind-treated plants. ses are indicated, when appropriate, at the circles.
Individual inflorescences were considered mature when the first silique turned brown, and the heights of mature inflorescences were determined. Inflorescence height of wild-type plants was reduced from 397.4 ± 5.9 mm for control plants to 339.8 ± 5.2 mm for wind-treated plants (P < 0.001; Fig. 1C). The inflorescences of the ethylene-insensitive mutants were also reduced in height when plants were exposed to wind. For the etr1–3 mutant, mature inflorescence height was reduced from 395.2 ± 8.5 to 341.9 ± 5.5 mm (P < 0.001), and for ein2–1, the reduction was from 456.5 ± 9.1 to 338.4 ± 10.0 mm (P < 0.001).
Wind treatment of plants was also found to increase the number of rosette paraclades (secondary inflorescences) that formed when the primary inflorescences reached 25 cm in height. Most of the untreated wild-type plants had two or three rosette paraclades (average 2.6 ± 0.2), whereas most wind-treated wild-type plants had four paraclades (average 4.1 ± 0.1, P < 0.001; Fig. 1D). Increases in the number of rosette paraclades were observed for the ethylene-insensitive mutants. The calculated average of rosette paraclades for etr1–3 mutants was 3.1 ± 0.1 paraclades on untreated plants and 3.6 ± 0.1 paraclades on wind-treated plants (P < 0.01; Fig. 1D). Most untreated ein2–1 mutants had one or two paraclades (average 1.7 ± 0.2), whereas most wind-treated ein2–1 plants had four or five rosette paraclades (average 4.3 ± 0.2 P < 0.001; Fig. 1D).
Touch-Induced Gene Expression
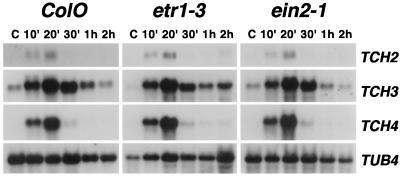
To determine whether ETR1 and EIN2 protein functions are necessary for the rapid up-regulation of expression of the TCH genes by touch stimulation, we assayed TCH mRNA levels of control and touch-stimulated wild-type etr1–3 and ein2–1 plants. Northern analysis of etr1–3 and ein2–1 revealed that the TCH mRNAs increased in abundance following touch stimulation, with similar kinetics as in wild-type plants (Fig. 2). In all three genetic backgrounds, increases in the TCH mRNAs were detectable within 10 min following touch stimulation and peaked in abundance by 20 min after stimulation (Fig. 2). Therefore, signaling through ETR1 or EIN2 is clearly not necessary for the rapid up-regulation of the TCH genes following mechanical stimulation.
Figure 2.
Touch-induced expression of TCH genes in wild-type and ethylene-insensitive mutants. RNA was isolated from ColO, etr1–3, and ein2–1 shoot tissues harvested following no stimulation (C) or at the indicated times following touch stimulation. Northern blots were probed successively with the TCH gene probes indicated at the right. TUB4 is a probe for β-tubulin (Marks et al., 1987) and is used to monitor differences of RNA levels between lanes (′ indicates minutes).
Gene Expression and Growth Responses to Vibration
Another method of delivering a mechanical stimulus to plants is to apply vibration. An advantage of a vibratory stimulus is that it can be quantified with respect to frequency and amplitude, thus making it feasible to provide an approximately reproducible stimulus.
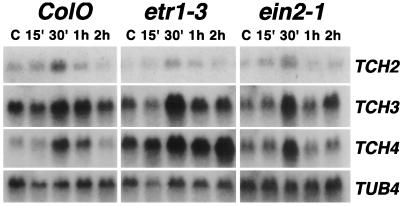
A continuous, low-frequency (50 Hz) vibration was applied to 14-d-old seedlings, and expression levels of the TCH genes were monitored. Northern analysis indicated that the TCH genes were up-regulated in expression following vibration, with maximal RNA accumulation approximately 30 min after the initiation of stimulation (Fig. 3). The increases in TCH mRNA levels were much less dramatic than the up-regulation observed in touch-stimulated plants. This suggests that vibration provides a weaker mechanical strain than touch. In general, the kinetics and magnitude of vibration-induced TCH mRNA accumulation in the ethylene-insensitive mutants were similar to that of wild-type plants (Fig. 3). However, induction of TCH4 expression appears to be more prolonged in the etr1–3 mutant.
Figure 3.
Vibration of seedlings results in expression of the TCH genes. Northern-blot analysis was conducted on RNA isolated from untreated (C) ColO, etr1–3, and ein2–1 seedlings and seedlings treated for the indicated times. The blots were probed sequentially with TCH and TUB4 gene probes as indicated at right (′ indicates minutes).
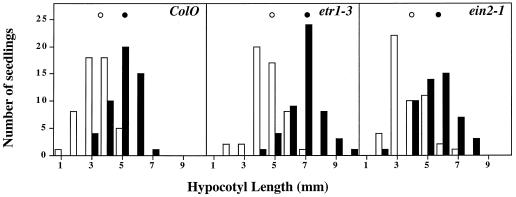
Takahashi et al. (1991) showed that treatment of germinating rice (Oryza sativa L.) or cucumber (Cucumis sativus L.) seeds with a 50-Hz vibration for 72 h results in a stimulation of the hypocotyl elongation rate. To test whether Arabidopsis plants also show a stimulatory growth response to vibration and whether any morphogenetic response to vibration requires the ETR1 or EIN2 proteins, we assessed the effects of a 50-Hz vibration on wild-type, etr1–3, and ein2–1 seedlings. Consistent with results seen for rice and cucumber, vibration of Arabidopsis seedlings for 72 h resulted in a stimulation of hypocotyl elongation (Fig. 4). At 72 h, stimulated wild-type seedling hypocotyls were 5.2 ± 0.1 mm in length, whereas untreated plant hypocotyls were approximately 3.6 ± 0.1 mm (P < 0.001). ETR1 and EIN2 are not required for this response because both ethylene-insensitive mutants showed increases in hypocotyl elongation (4.8 ± 0.1 to 7.1 ± 0.2 mm for etr1–3 and 3.9 ± 0.2 to 5.7 ± 0.2 mm for ein2–1; P < 0.001) after exposure to vibratory stimulation.
Figure 4.
Enhancement of hypocotyl elongation by vibration. Hypocotyl lengths of etiolated seedlings were measured following no treatment (open bars) or vibration (closed bars). ○, Mean for untreated plants; •, mean for vibrated plants.
DISCUSSION
Mechanical stimuli such as wind bursts or direct contact are frequently encountered by plants. Plants have evolved the ability to sense and respond to these stimuli in ways that increase their resistance to such stresses. Arabidopsis plants have been shown previously to be delayed in bolting and display overall less elongation in response to repetitive touch stimulation (Braam and Davis, 1990). In addition, a prominent and fast molecular response occurs in Arabidopsis; the TCH genes are up-regulated in expression in response to mechanical stimuli. The work described here takes a genetic approach to investigating the role of the ethylene-signaling pathway in controlling the developmental and molecular responses to mechanical stimulation. Definitive evidence demonstrates that ETR1 and EIN2, two loci that act early in ethylene signaling, are not required for plants to sense and respond to different forms of mechanical stress. The etr1–3 and ein2–1 Arabidopsis mutants show a delay in inflorescence emergence, a reduced rate of inflorescence elongation, and a decrease in the height of mature inflorescences in response to wind treatment; these responses are similar to those of wind-treated wild-type plants (Fig. 1). Slight differences in the sensitivities between genotypes may be due to the overall structure and stature of the plants, making them differentially sensitive to mechanical perturbation. The critical result is that the ethylene mutants are clearly able to respond to the mechanical stimuli.
We also investigated whether Arabidopsis displays growth-response changes when subjected to the mechanical stimulus of vibration. The elongation rate of hypocotyls of etiolated Arabidopsis, like that of etiolated rice and cucumber hypocotyls (Takahashi et al., 1991), is enhanced when seedlings are subjected to a 50-Hz vibration (Fig. 4). This mechanoresponse also occurs in plants lacking ETR1 or EIN2 function. Therefore, the developmental and growth alterations that occur in Arabidopsis plants subjected to either wind or vibration do not require the functioning of ETR1 or EIN2. ETR1 and EIN2 are not necessary for the up-regulation of TCH gene expression in response to touch or vibration (Figs. 2 and 3). Hence, ethylene is unlikely to be involved in either the molecular or developmental responses of plants to mechanical stimuli.
Previous observations support these conclusions. Plants treated with touch or shaking show a rapid (1–3 min) reduction in the rate of elongation, whereas evolution of ethylene is not detectable until 30 to 45 min following stimulation (Goeschl et al., 1966; Jaffe and Biro, 1979). Additional data that help rule out an early required role for ethylene biosynthesis in the mechanoresponse pathway is the recent finding that there is a very rapid phosphorylation of protein kinases following touch or wounding (Suzuki and Shinshi, 1995; Usami et al., 1995; Bögre et al., 1996, 1997). Furthermore, treatment of plants with some inhibitors of ethylene production or action have been shown to have no effect on thigmomorphogenesis (Takahashi and Suge, 1980; Boyer et al., 1983; Biddington, 1986) or to reduce radial expansion only, with no effect on touch-induced decreases in elongation growth (Biro and Jaffe, 1984). In contrast, however, Boyer et al. (1979, 1983, 1986) reported that ethylene action/biosynthesis inhibitors can reduce the thigmomorphogenetic effects on both radial expansion and elongation. Because Arabidopsis plants do not display a significant increase in radial expansion following mechanical stimulation, we are unable to test whether the ethylene response effectors etr1–1 and ein2–1 affect changes in radial expansion following mechanical stress.
In summary, the work presented here indicates that ETR1 and EIN2 protein functions are not required for the molecular and developmental responses of Arabidopsis to mechanical stress. A more direct mechanosignaling/transduction pathway must exist, perhaps involving the function of transmembrane proteins (He et al., 1996b; Brummell et al., 1997; Thonat et al., 1997) and/or stretch-activated channels (Falke et al., 1988; Zimmermann et al., 1997) as mechanosensors, Ca2+ as a second messenger (Knight et al., 1991; Braam, 1992; Bush, 1995; Haley et al., 1995; Polisensky and Braam, 1996), and a kinase cascade (Suzuki and Shinshi, 1995; Usami et al., 1995; Bögre et al., 1997) to signal mechanoresponses, such as TCH gene expression (Braam and Davis, 1990), oxidative burst (Yahraus et al., 1995), cell wall changes (Bradley et al., 1992; Levine et al., 1994; Xu et al., 1995), and growth alterations (Jaffe, 1973; Jaffe and Forbes, 1993; Mitchell, 1996).
ACKNOWLEDGMENTS
We would like to thank Tara Miller for assistance with preparing some of the plant materials. We would also like to thank members of the Braam laboratory for critical reading of the manuscript.
Abbreviation:
- CaM
calmodulin
Footnotes
This work was funded by the National Institutes of Health (grant no. R9 GM 46346) and the National Aeronautics and Space Administration (grant no. NSCORT NAGW-5007).
LITERATURE CITED
- Antosiewicz DM, Polisensky DH, Braam J. Cellular localization of the Ca2+ binding TCH3 protein of Arabidopsis. Plant J. 1995;8:623–636. doi: 10.1046/j.1365-313x.1995.08050623.x. [DOI] [PubMed] [Google Scholar]
- Biddington NL. The effects of mechanically-induced stress in plants. Plant Growth Regul. 1986;4:103–123. [Google Scholar]
- Biro R, Hunt E, Erner Y, Jaffe M. Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically perturbed or ethrel-treated bean plants. Ann Bot. 1980;45:655–664. [Google Scholar]
- Biro RL, Jaffe MJ. Thigmomorphogenesis: ethylene evolution and its role in the changes observed in mechanically perturbed bean plants. Physiol Planta. 1984;62:289–296. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Heberle-Bors E, Hirt H. Mechanosensors in plants. Nature. 1996;383:489–490. doi: 10.1038/383489a0. [DOI] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, Hirt H. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer N, Chapelle B, Gaspar T. Lithium inhibition of thigmomorphogenetic response in Bryonia dioica. Plant Physiol. 1979;63:1215–1216. doi: 10.1104/pp.63.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer N, de Jaegher G, Bon M-C, Gaspar T. Cobalt inhibition of thigmomorphogenesis in Bryonia dioica: possible role and mechanism of ethylene production. Physiol Plant. 1986;67:552–556. [Google Scholar]
- Boyer N, Desbiez M-O, Hofinger M, Gaspar T. Effect of lithium on thigmomorphogenesis in Bryonia dioica ethylene production and sensitivity. Plant Physiol. 1983;72:522–525. doi: 10.1104/pp.72.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J. Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: induction by calcium and heat shock. Proc Natl Acad Sci USA. 1992;89:3213–3216. doi: 10.1073/pnas.89.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Braam J, Sistrunk ML, Polisensky DH, Xu W, Purugganan MM, Antosiewicz DM, Campbell P, Johnson KA. Life in a changing world: TCH gene regulation of expression and responses to environmental signals. Physiol Plant. 1996;98:909–916. [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Brown KM, Leopold AC. Ethylene and the regulation of growth in pine. Can J For Res. 1972;3:143–145. [Google Scholar]
- Brummell DA, Catala C, Lashbrook CC, Bennett AB. A membrane-anchored E-type endo-1,4-β-glucanase is localized on Golgi and plasma membranes of higher plants. Proc Natl Acad Sci USA. 1997;94:4794–4799. doi: 10.1073/pnas.94.9.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- de Jaegher G, Boyer N, Bon M-C, Gaspar T. Thigmomorphogenesis in Bryonia dioica: early events in ethylene biosynthesis pathway. Biochem Physiol Pflanz. 1987;182:49–56. [Google Scholar]
- Eisinger W. Regulation of pea internode expansion by ethylene. Annu Rev Plant Physiol. 1983;34:225–240. [Google Scholar]
- Ennos AR. Wind as an ecological factor. Trends Ecol Evol. 1997;12:108–111. doi: 10.1016/s0169-5347(96)10066-5. [DOI] [PubMed] [Google Scholar]
- Erner Y, Jaffe MJ. Thigmomorphogenesis: the involvement of auxin and abscisic acid in growth retardation due to mechanical perturbation. Plant Cell Physiol. 1982;23:935–941. [Google Scholar]
- Erner Y, Jaffe MJ. Thigmomorphogenesis: membrane lipid and protein changes in bean plants as affected by mechanical perturbation and ethrel. Physiol Plant. 1983;58:197–203. [Google Scholar]
- Falke LC, Edwards KL, Pickard BG, Misler S. A stretch-activated anion channel in tobacco protoplasts. FEBS Lett. 1988;237:141–144. doi: 10.1016/0014-5793(88)80188-1. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. Random oligonucleotide priming of DNA for labeling. Anal Biochem. 1983;137:266–269. [Google Scholar]
- Goeschl JD, Rappaport L, Pratt HK. Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol. 1966;41:877–884. doi: 10.1104/pp.41.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ. Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA. 1995;92:4124–4128. doi: 10.1073/pnas.92.10.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CH, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 1996a;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z-H, Fujiki M, Kohorn BD. A cell wall-associated, receptor-like protein kinase. J Biol Chem. 1996b;271:19789–19793. doi: 10.1074/jbc.271.33.19789. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Biro R. Thigmomorphogenesis: the effect of mechanical perturbation on the growth of plants, with special reference to anatomical changes, the role of ethylene, and interaction with other environmental stresses. In: Mussell H, Staples RC, editors. Stress Physiology in Crop Plants. New York: John Wiley & Sons; 1979. pp. 25–69. [Google Scholar]
- Jaffe MJ, Forbes S. Thigmomorphogenesis: the effect of mechanical perturbation on plants. Plant Growth Regul. 1993;12:313–324. doi: 10.1007/BF00027213. [DOI] [PubMed] [Google Scholar]
- Khan A, Johnson KA, Braam J, James M. Comparative modeling of the three-dimensional structure of the calmodulin-related TCH2 protein from Arabidopsis. Proteins. 1997;27:144–153. doi: 10.1002/(sici)1097-0134(199701)27:1<144::aid-prot14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Potter SL, Uknes S, Ryals J. Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Marks MD, West J, Weeks DP. The relatively large beta-tubulin gene family of Arabidopsis contains a member with an unusual transcribed 5′ noncoding sequence. Plant Mol Biol. 1987;10:91–104. doi: 10.1007/BF00016147. [DOI] [PubMed] [Google Scholar]
- Mitchell CA. Recent advances in plant response to mechanical stress: theory and application. Hortscience. 1996;31:31–35. [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski M, Liss H, Weiler EW. Touch-induced protein phosphorylation in mechanosensitive tendrils of Bryonia dioica Jacq. J Plant Physiol. 1996;147:539–546. [Google Scholar]
- Polisensky DH, Braam J. Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 1996;111:1271–1279. doi: 10.1104/pp.111.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah BW. Promotion of radial growth by 2-chloroethylphosphonic acid in bean. Bot Gaz. 1974;135:289–292. [Google Scholar]
- Pressman E, Huberman M, Aloni B, Jaffe MJ. Thigmomorphogenesis: the effect of mechanical perturbation and ethrel on stem pithiness in tomato [Lycopersicon esulentum (Mill.) plants] Ann Bot. 1983;52:93–100. [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salveit ME, Pharr DM, Larson RA. Mechanical stress induces ethylene production and epinasty in Poinsettia cultivars. J Am Soc Hortic Sci. 1979;104:452–455. [Google Scholar]
- Samac DA, Hironaka CM, Yallaly PE, Shah DM. Isolation and characterization of the genes encoding basic and acidic chitinase in Arabidopsis thaliana. Plant Physiol. 1990;93:907–914. doi: 10.1104/pp.93.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sarquis JI, Jordan WR, Morgan PW. Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiol. 1991;96:1171–1177. doi: 10.1104/pp.96.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Braam J. Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell. 1994;6:1553–1565. doi: 10.1105/tpc.6.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Jaffe MJ. Thigmomorphogenesis: the relationship of mechanical perturbation to elicitor-like activity and ethylene production. Physiol Plant. 1984;61:405–411. doi: 10.1111/j.1399-3054.1984.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Suge H. Sex expression in cucumber plants as affected by mechanical stress. Plant Cell Physiol. 1980;21:303–310. [Google Scholar]
- Takahashi H, Suge H, Kato T. Growth promotion by vibration at 50 Hz in rice and cucumber seedlings. Plant Cell Physiol. 1991;32:729–732. [Google Scholar]
- Telewski F, Wakefield A, Jaffe M. Computer-assisted image analysis of tissues of ethrel-treated Pinus taeda seedlings. Plant Physiol. 1983;72:177–181. doi: 10.1104/pp.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonat C, Mathieu C, Crevecoeur M, Penel C, Gaspar T, Boyer N. Effects of a mechanical stimulation on localization of annexin-like protein in Bryonia dioica internodes. Plant Physiol. 1997;114:981–989. doi: 10.1104/pp.114.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Banno H, Ito Y, Nishihama R, Machida Y. Cutting activates a 46-kilodalton protein kinase in plants. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Campbell P, Vargheese AK, Braam J. The Arabidopsis XET-related gene family: environmental and hormonal regulation of expression. Plant J. 1996;9:879–889. doi: 10.1046/j.1365-313x.1996.9060879.x. [DOI] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Nürnberger T, Frachisse J-M, Wirtz W, Guern J, Hedrish R, Scheel D. Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc Natl Acad Sci USA. 1997;94:2751–2755. doi: 10.1073/pnas.94.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]