Abstract
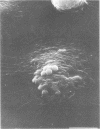
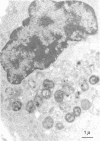
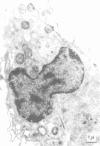
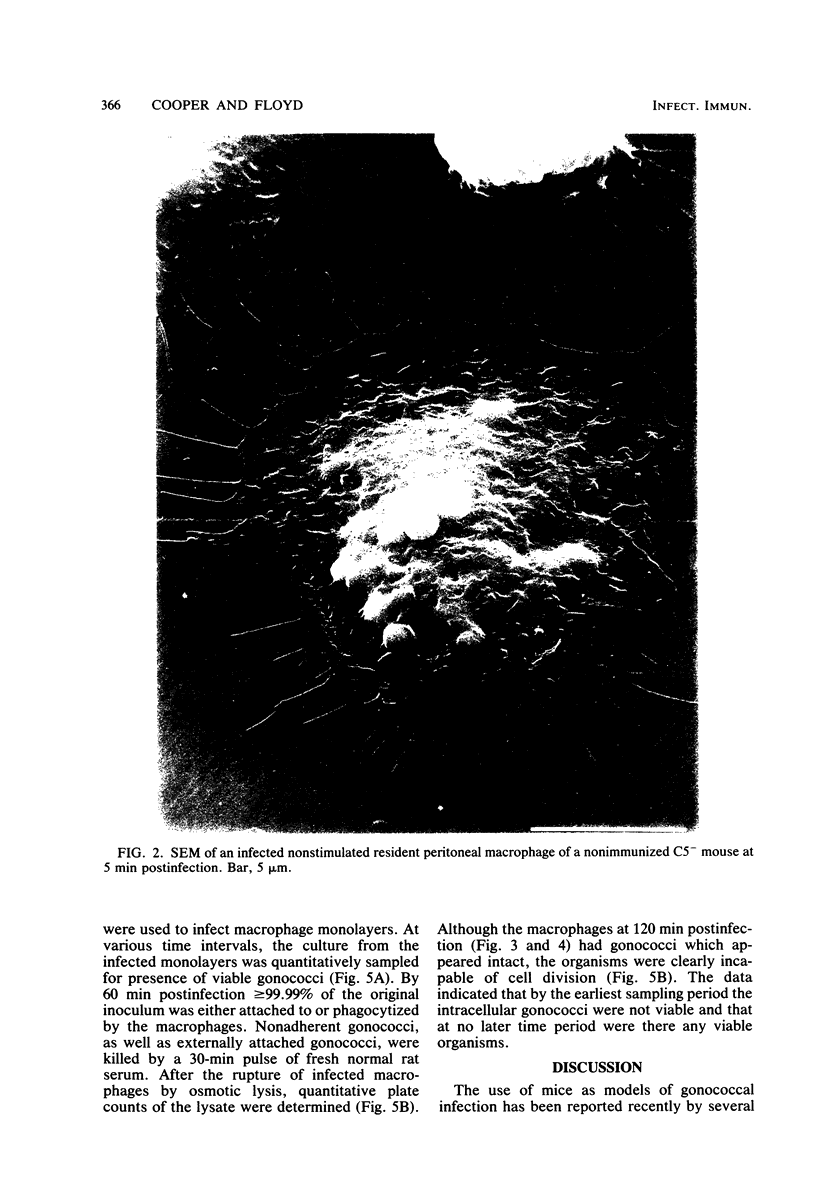
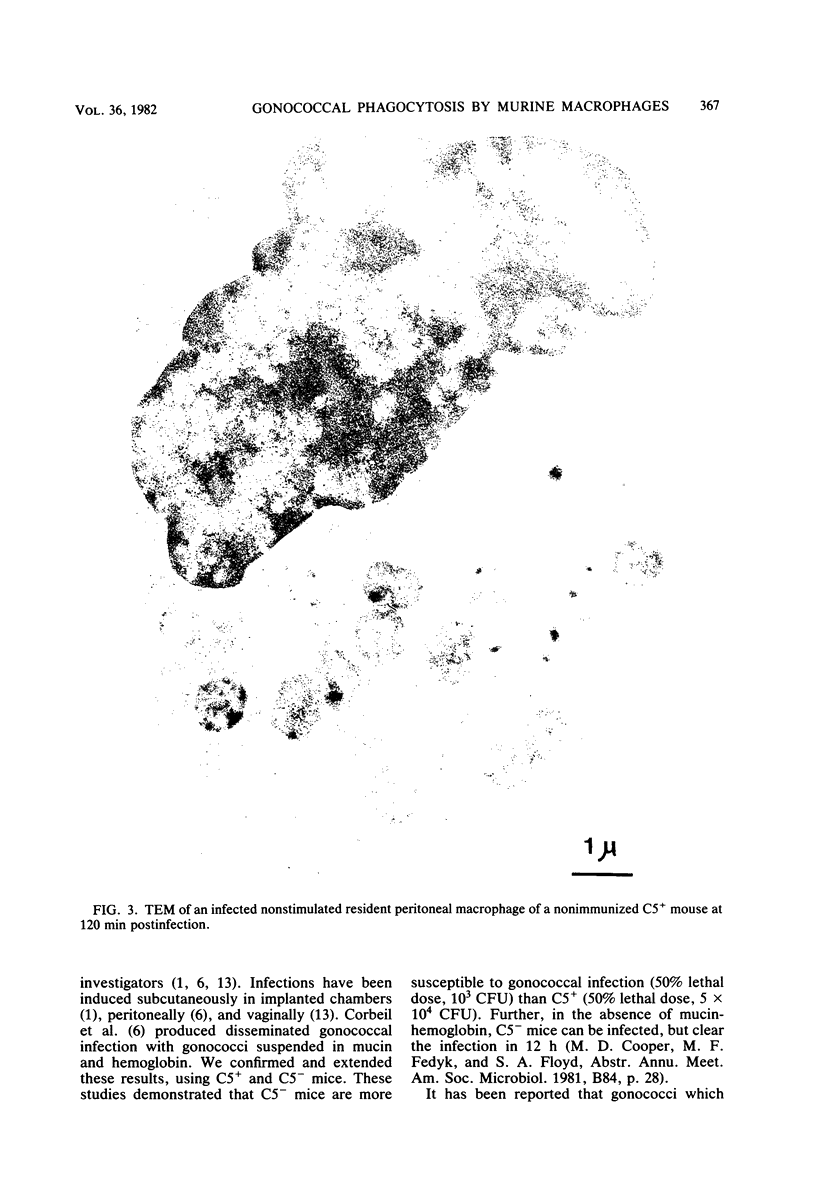
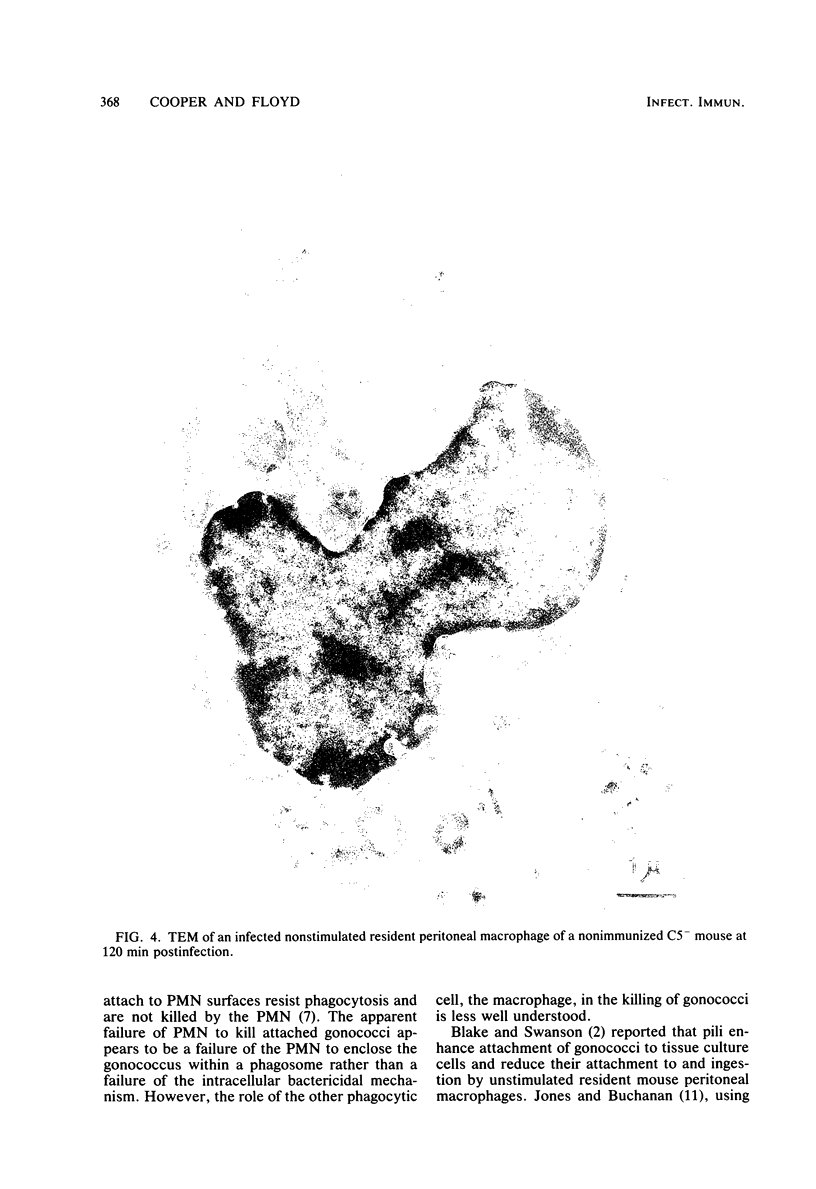
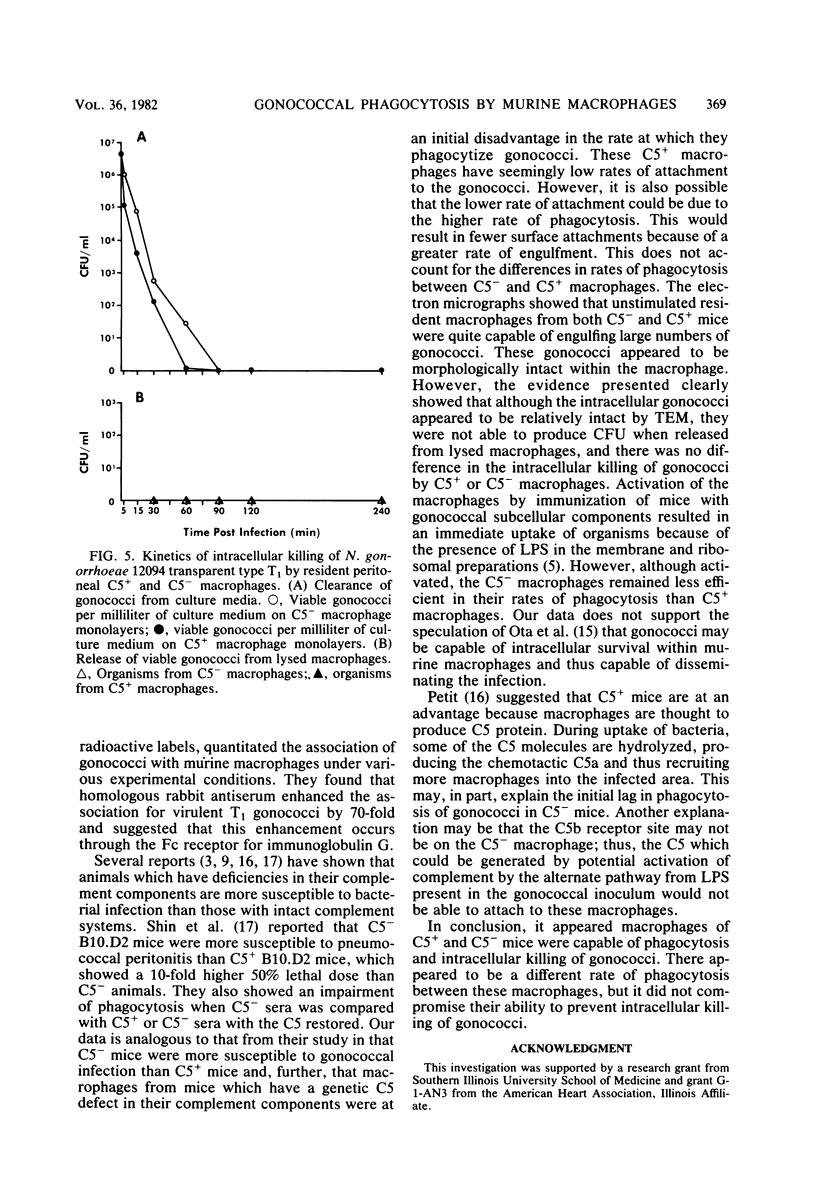
Unstimulated resident peritoneal macrophages were harvested from complement-sufficient (C5+) and complement-deficient (C5-) mice by peritoneal lavage and cultured for 14 h. Adherence to cover slips was determined, and the monolayer was infected with transparent T1 gonococci. At various times after infection, the macrophages were observed for both attachment and phagocytosis of the gonococci by scanning and transmission electron microscopy. this analysis indicated that C5+ macrophages were capable of immediate phagocytosis of gonococci, with maximal phagocytosis occurring by 60 to 90 min. In contrast, C5- macrophages had a greater lag time before initiation of phagocytosis; this event was started by 30 min and completed by 90 min. The intracellular gonococci which were phagocytized by either C5+ or C5- mice were completely killed after 30 min of incubation. It appears that C5- mice are at a disadvantage in the early kinetics of the phagocytosis of gonococci, but that this does not affect the ultimate intracellular destruction of gonococci.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arko R. J., Wong K. H., Steurer F. J., Schalla W. O. Complement-enhanced immunity to infection with Neisseria gonorrhoeae in mice. J Infect Dis. 1979 May;139(5):569–574. doi: 10.1093/infdis/139.5.569. [DOI] [PubMed] [Google Scholar]
- Blake M., Swanson J. Studies on Gonococcus infection. IX. In vitro decreased assocation of pilated gonococci with mouse peritoneal macrophages. Infect Immun. 1975 Jun;11(6):1402–1404. doi: 10.1128/iai.11.6.1402-1404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caren L. D., Rosenberg L. T. The role of complement in resistance to endogenous and exogenous infection with a common mouse pathogen, Corynebacterium kutscheri. J Exp Med. 1966 Oct 1;124(4):689–699. doi: 10.1084/jem.124.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Tewari R. P., Bowser D. V. Immunogenicity of ribosomal preparations from Neisseria gonorrhoeae. Infect Immun. 1980 Apr;28(1):92–100. doi: 10.1128/iai.28.1.92-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Wannemuehler M. J., Miller R. D., Fedyk M. F. Role of outer envelope contamination in protection elicited by ribosomal preparations against Neisseria gonorrhoeae infection. Infect Immun. 1981 Apr;32(1):173–179. doi: 10.1128/iai.32.1.173-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Wunderlich A. C., Corbeil R. R., McCutchan J. A., Ito J. I., Jr, Braude A. I. Disseminated gonococcal infection in mice. Infect Immun. 1979 Dec;26(3):984–990. doi: 10.1128/iai.26.3.984-990.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Comparison of subcutaneous and intraperitoneal staphylococcal infections in normal and complement-deficient mice. Infect Immun. 1976 Feb;13(2):399–406. doi: 10.1128/iai.13.2.399-406.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Buchanan T. M. Quantitative measurement of phagocytosis of Neisseria gonorrhoeae by mouse peritoneal macrophages. Infect Immun. 1978 Jun;20(3):732–738. doi: 10.1128/iai.20.3.732-738.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita E., Matsuura H., Kashiba S. A mouse model for the study of gonococcal genital infection. J Infect Dis. 1981 Jan;143(1):67–70. doi: 10.1093/infdis/143.1.67. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Hughes M., Miler J. J., Syrett C., Turner W. H., Harris J. R., MacLennan I. P. Studies on the mechanism of pathogenicity of Neisseria gonorrhoeae. J Med Microbiol. 1977 Aug;10(3):347–365. doi: 10.1099/00222615-10-3-347. [DOI] [PubMed] [Google Scholar]
- Ota F., Morita J., Yoshida N., Ashton F., Diena B. Studies on gonococcal infection. I. Electron microscopic studies on phagocytosis of Neisseria gonorrhoeae by macrophages. Jpn J Microbiol. 1975 Apr;19(2):149–155. doi: 10.1111/j.1348-0421.1975.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Petit J. C. Resistance to listeriosis in mice that are deficient in the fifth component of complement. Infect Immun. 1980 Jan;27(1):61–67. doi: 10.1128/iai.27.1.61-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Smith M. R., Wood W. B., Jr Heat labile opsonins to pneumococcus. II. Involvement of C3 and C5. J Exp Med. 1969 Dec 1;130(6):1229–1241. doi: 10.1084/jem.130.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]