Abstract
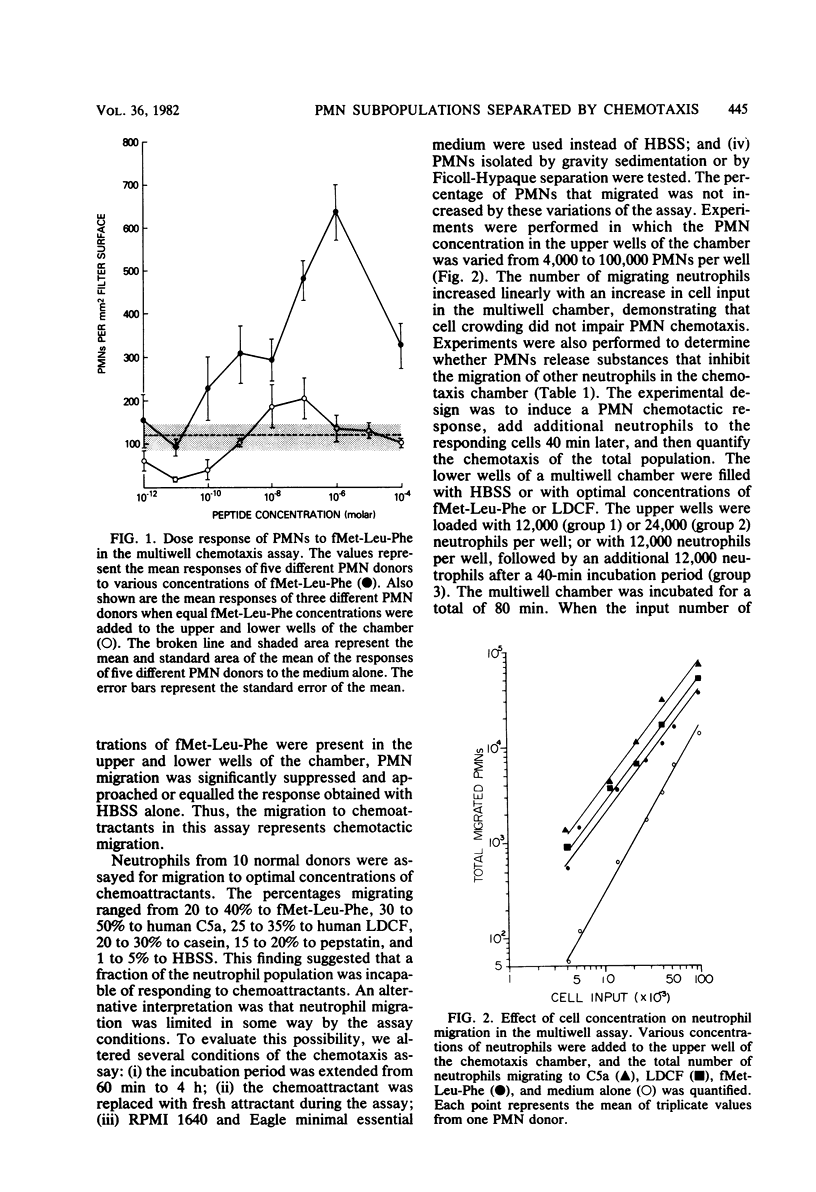
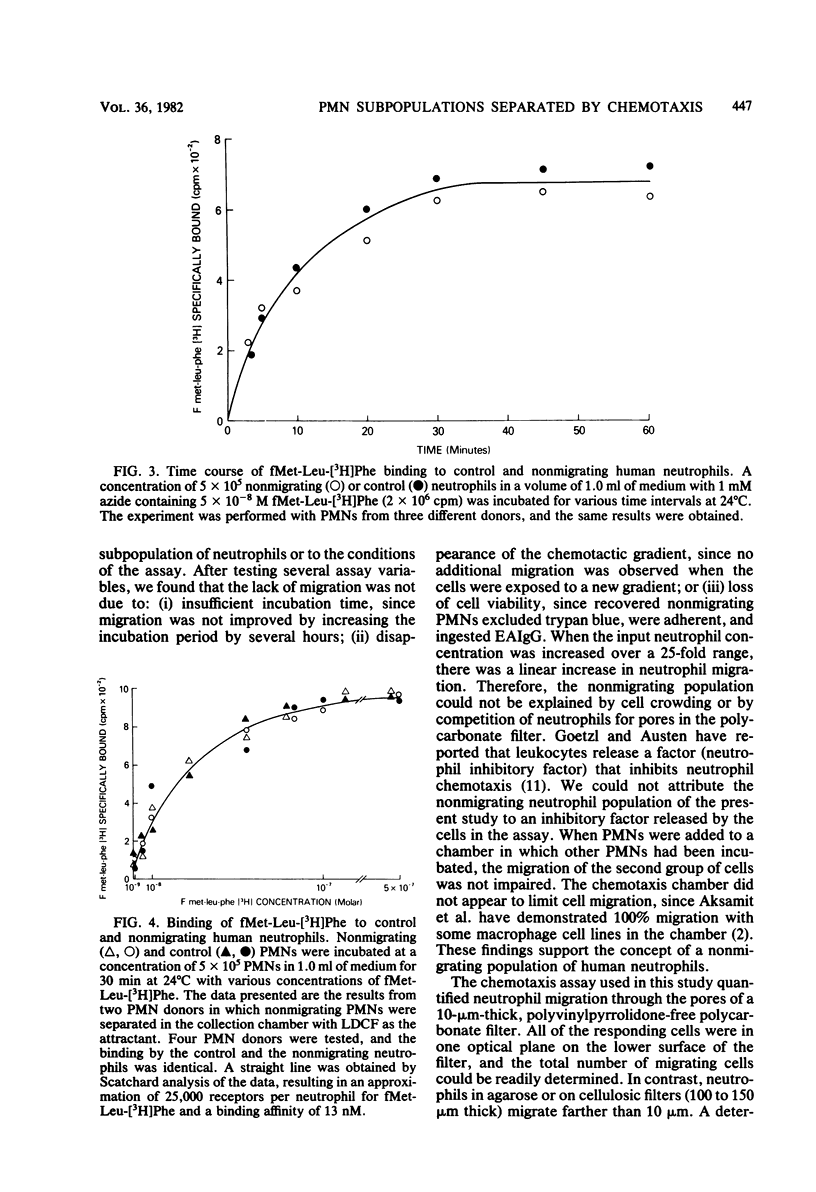
Normal human blood contains a population of neutrophils that migrates to various chemoattractants and a population that fails to migrate. The percentage of neutrophils migrating to optimal concentrations of chemoattractants was quantified: 20 to 40% migrated to N-formylmethionyl-leucyl-phenylalanine, 30 to 50% migrated to human C5a, 25 to 35% migrated to human leukocyte-derived chemotactic factors, 20 to 30% migrated to casein, 15 to 20% migrated to pepstatin, and 1 to 5% migrated to medium alone. Neutrophil migration to the most active chemoattractant was not increased when other chemoattractants were added, indicating that the population of neutrophils migrating to the most active attractant was the same population that was migrating to the other attractants. The percentage of neutrophils migrating to a chemoattractant was not altered by prolonging the assay incubation period or by replacing the attractant with new chemoattractant during the assay, and the percentage was independent of the neutrophil concentration added to the chemotaxis chamber. Nonmigrating neutrophils were isolated with a chemotaxis collection chamber, and they were examined for radiolabeled chemotactic peptide binding. The binding of radiolabeled N-formylmethionyl-leucyl-phenylalanine by nonmigrating and migrating neutrophils was identical.
Full text
PDF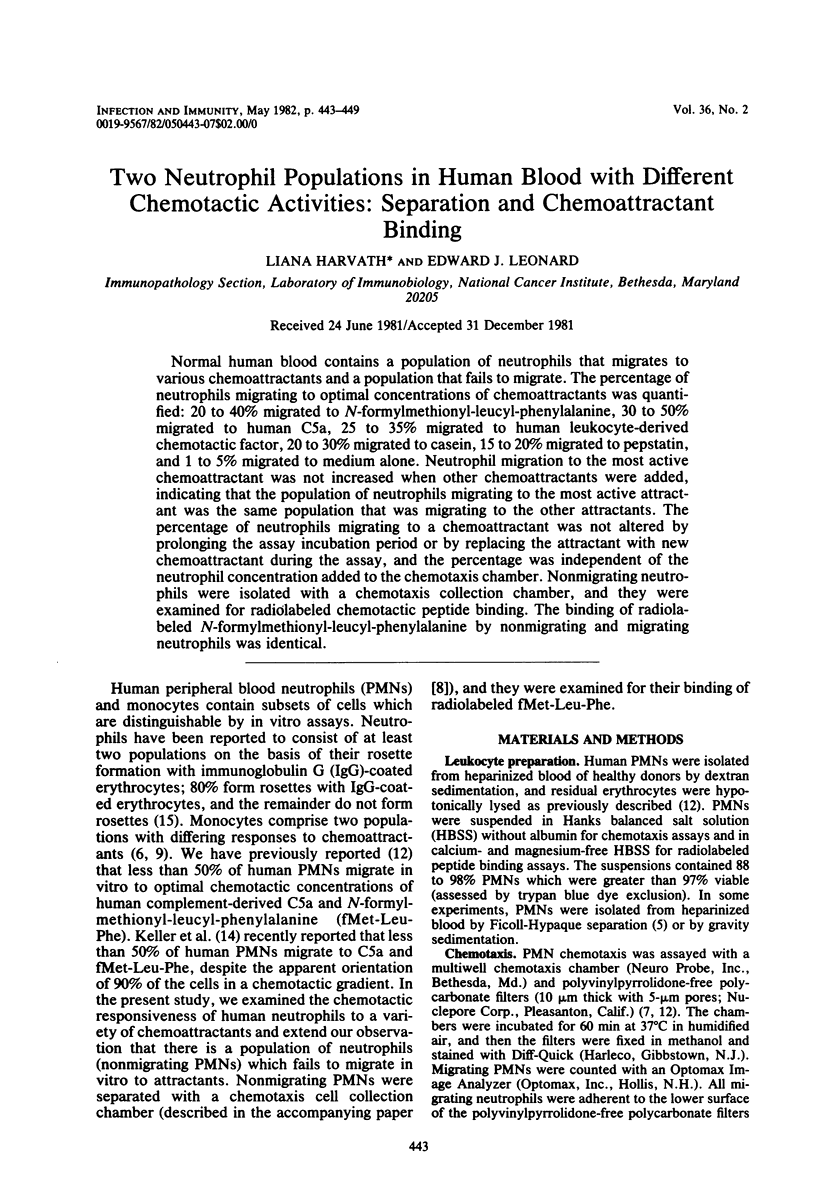




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Pepstatin A--a human leukocyte chemoattractant. Clin Immunol Immunopathol. 1979 Oct;14(2):244–250. doi: 10.1016/0090-1229(79)90146-6. [DOI] [PubMed] [Google Scholar]
- Aksamit R. R., Falk W., Leonard E. J. Chemotaxis by mouse macrophage cell lines. J Immunol. 1981 Jun;126(6):2194–2199. [PubMed] [Google Scholar]
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J. A simple procedure to use whole serum as a source of either IgG- or IgM-specific antibody. J Immunol Methods. 1980;32(1):51–58. doi: 10.1016/0022-1759(80)90116-7. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Falk W., Harvath L., Leonard E. J. Only the chemotactic subpopulation of human blood monocytes expresses receptors for the chemotactic peptide N-formylmethionyl-leucyl-phenylalanine. Infect Immun. 1982 May;36(2):450–454. doi: 10.1128/iai.36.2.450-454.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Borel J. F., Wilkinson P. C., Hess M. W., Cottier H. Re-assessment of Boyden's technique for measuring chemotaxis. J Immunol Methods. 1972 Jan;1(2):165–168. doi: 10.1016/0022-1759(72)90043-9. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Wissler J. H., Damerau B. Diverging effects of chemotactic serum peptides and synthetic f-Met-Leu-Phe on neutrophil locomotion and adhesion. Immunology. 1981 Mar;42(3):379–383. [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Separation and functional characterization of human neutrophil subpopulations. Blood. 1978 Apr;51(4):659–669. [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: dissociation of phagocytic and chemotactic responsiveness. Cell Immunol. 1978 Jan;35(1):99–111. doi: 10.1016/0008-8749(78)90130-2. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Ackerman S. K., Fiegel V. D., Bauman M. P., Douglas S. D. Cytotaxin receptors of neutrophils: evidence that F-methionyl peptides and pepstatin share a common receptor. Infect Immun. 1979 Dec;26(3):996–999. doi: 10.1128/iai.26.3.996-999.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Snyderman R., Fudman E. J. Demonstration of a chemotactic factor receptor on macrophages. J Immunol. 1980 Jun;124(6):2754–2757. [PubMed] [Google Scholar]
- Wilkinson P. C. Synthetic peptide chemotactic factors for neutrophils: the range of active peptides, their efficacy and inhibitory activity, and susceptibility of the cellular response to enzymes and bacterial toxins. Immunology. 1979 Mar;36(3):579–588. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


