Abstract
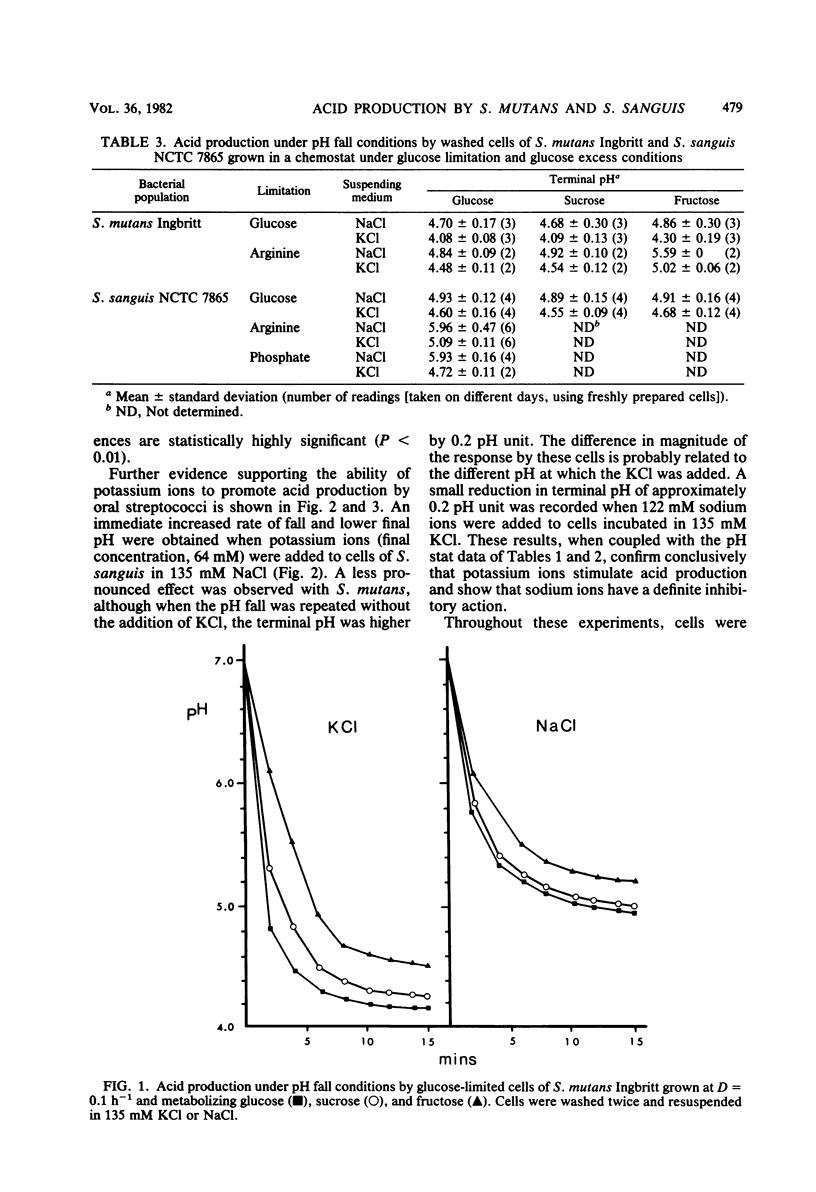
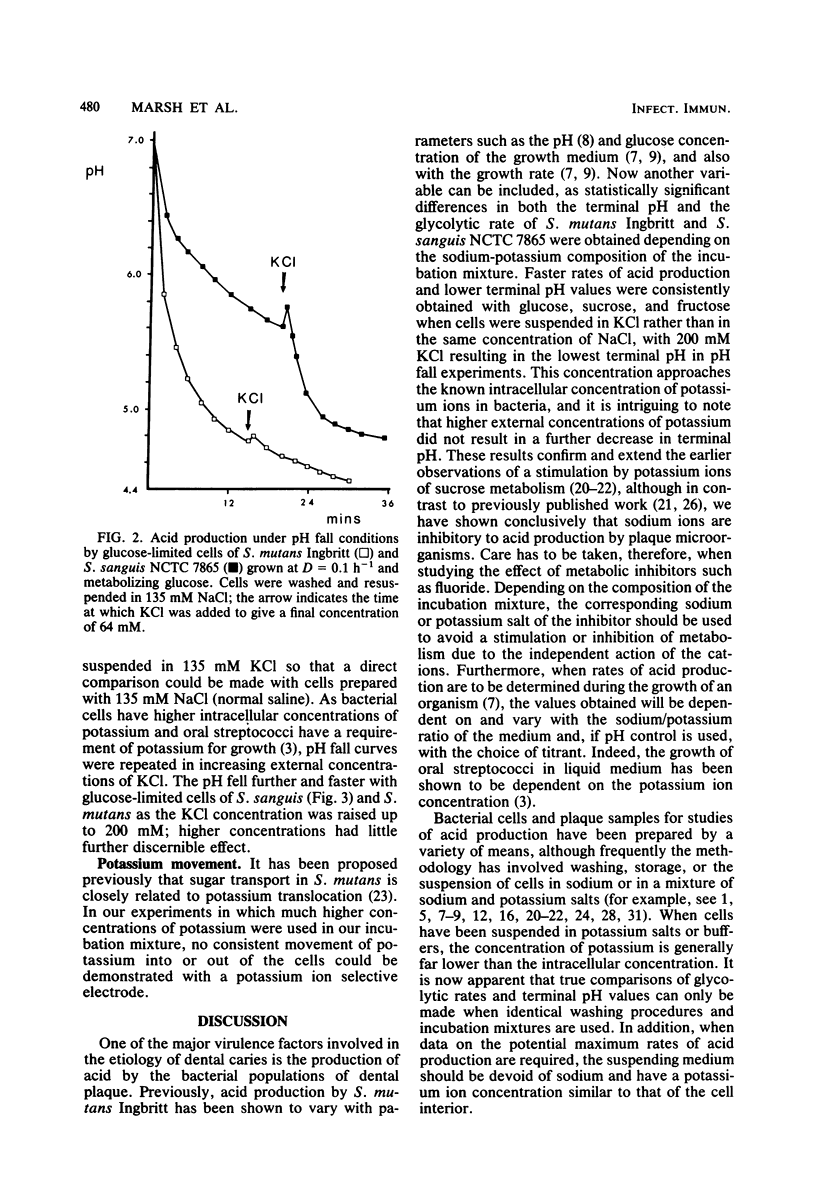
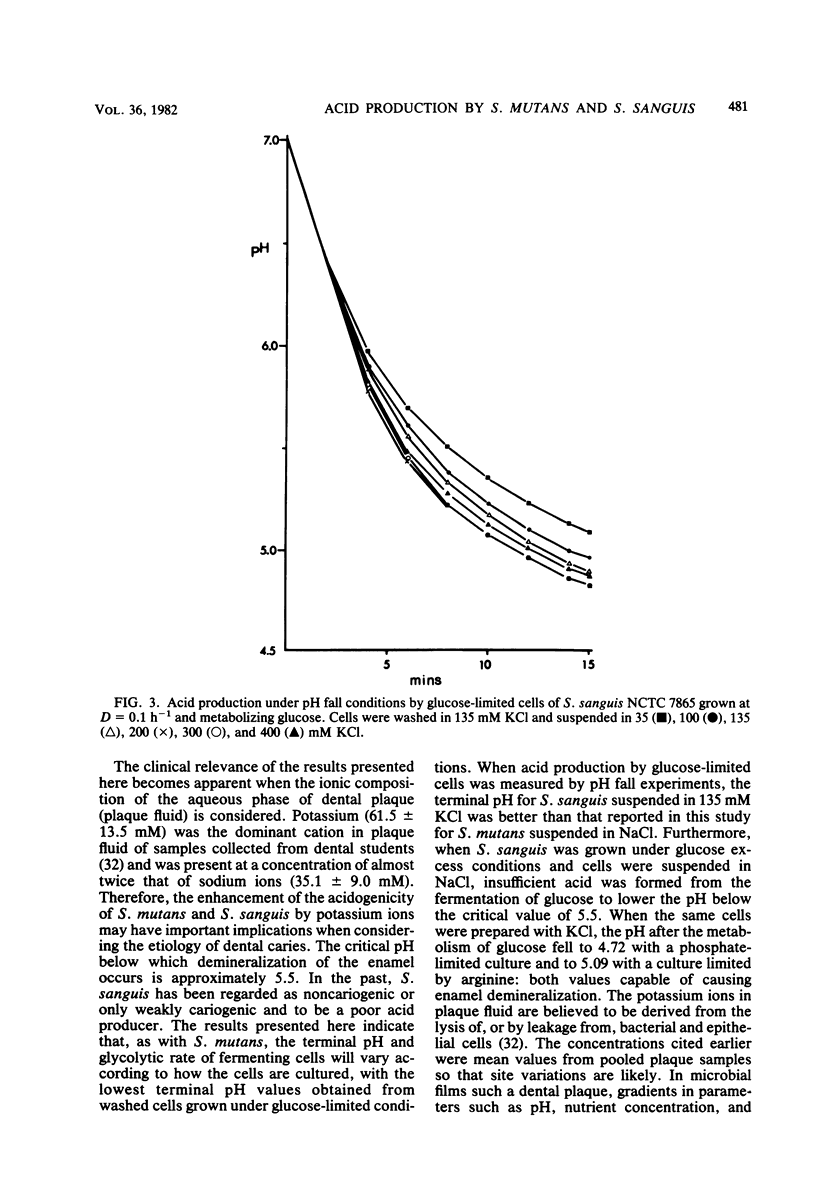
A comparison was made of acid production by cells of Streptococcus mutans Ingbritt and S. sanguis NCTC 7865 that had been washed twice and incubated in different concentrations of sodium and potassium ions. Organisms were grown under defined conditions in a chemostat under both glucose limitation and glucose excess conditions at a dilution rate of 0.1 h−1 (mean generation time, 6.9 h). Acid production after a pulse of glucose, sucrose, and fructose was measured by pH fall experiments and as a rate at pH 7.0. S. mutans produced more acid than S. sanguis as measured by either criterion, although statistically faster rates of acid production and lower terminal pH values were obtained when cells of both species were suspended in KCl rather than in NaCl, with 200 mM KCl resulting in the lowest terminal pH in pH fall experiments. Sodium ions inhibited acid production: 183 mM NaCl reduced the glycolytic rates of S. mutans and S. sanguis metabolizing glucose at pH 7.0 in 135 mM KCl by 39 and 33%, respectively. The most pronounced stimulatory effect of potassium on acid production was by washed cells of S. sanguis that had been grown under arginine and under phosphate limitation. The pH fell by a further 0.86 and 1.21 pH units, respectively, and to below the critical pH for enamel demineralization when these cells were metabolizing glucose in 135 mM KCl compared with the same concentration of NaCl. This enhancement of acid production was not due to potassium translocation, as had been suggested previously, because no movement of potassium ions across the cell membrane could be detected. An alternative explanation is proposed in which sodium ions are excluded from the cell at the expense of membrane energy, i.e., the proton motive force, which could otherwise be used for the transport of sugars.
Full text
PDF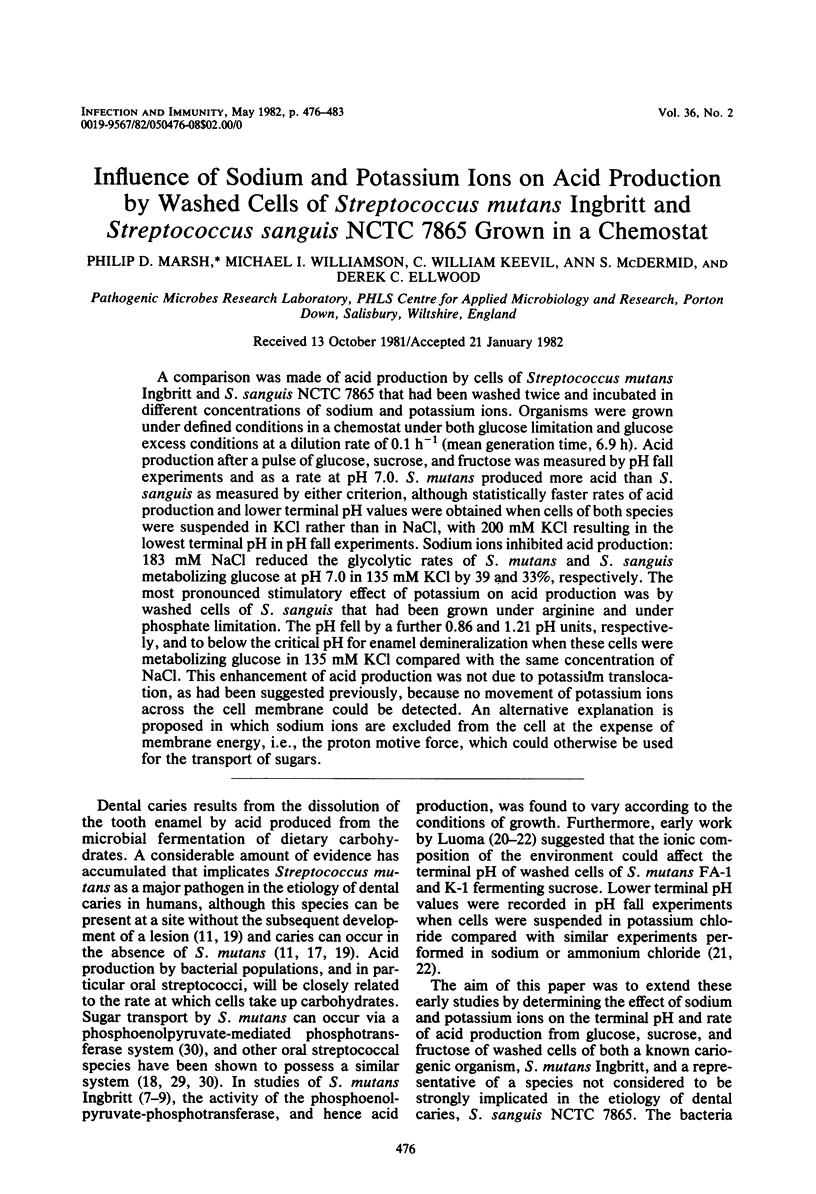




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkhed D., Frostell G. Influence of sucrose concentration on production of organic acids, soluble polysaccharides and reducing sugars in human dental plaque in vitro. Caries Res. 1978;12(4):183–189. doi: 10.1159/000260332. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus mutans. Caries Res. 1970;4(4):305–320. doi: 10.1159/000259653. [DOI] [PubMed] [Google Scholar]
- Cowman R. A., Fitzgerald R. J. Potassium requirement of oral streptococci. J Dent Res. 1976 Jul-Aug;55(4):709–709. doi: 10.1177/00220345760550043601. [DOI] [PubMed] [Google Scholar]
- Edgar W. M. The role of saliva in the control of pH changes in human dental plaque. Caries Res. 1976;10(4):241–254. doi: 10.1159/000260206. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Physiological classification of oral viridans streptococci. J Dent Res. 1976 Jan;55:A166–A176. doi: 10.1177/002203457605500108011. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Thomson P. L., South R. J., Marsh P. D., Bowden G. H., McKee A. S., Fillery E. D., Slack G. L. A longitudinal epidemiological study on dental plaque and the development of dental caries--interim results after two years. J Dent Res. 1977 Oct;56(Spec No):C90–C98. doi: 10.1177/00220345770560032401. [DOI] [PubMed] [Google Scholar]
- Hayes M. L., Roberts K. R. The breakdown of glucose, xylitol and other sugar alcohols by human dental plaque bacteria. Arch Oral Biol. 1978;23(6):445–451. doi: 10.1016/0003-9969(78)90075-4. [DOI] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. I. The sodium circulation. J Biol Chem. 1980 Dec 10;255(23):11396–11402. [PubMed] [Google Scholar]
- Heefner D. L., Kobayashi H., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. II. Energy coupling in everted membrane vesicles. J Biol Chem. 1980 Dec 10;255(23):11403–11407. [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- Huis in 't Veld J. H., Backer Dirks O. Intracellular polysaccharide metabolism in Streptococcus mutans. Caries Res. 1978;12(5):243–249. doi: 10.1159/000260340. [DOI] [PubMed] [Google Scholar]
- Huis in 't Veld J. H., van Palenstein Helderman W. H., Dirks O. B. Streptococcus mutans and dental caries in humans: a bacteriological and immunological study. Antonie Van Leeuwenhoek. 1979;45(1):25–33. doi: 10.1007/BF00400775. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma H. Potassium and sodium content and acid production of nongrowing cariogenic streptococci. Scand J Dent Res. 1971;79(3):202–208. doi: 10.1111/j.1600-0722.1971.tb02010.x. [DOI] [PubMed] [Google Scholar]
- Luoma H. The relationship of potassium, sodium and ammonium ions to sucrose fermentation by a cariogenic streptococcus. Caries Res. 1969;3(3):266–280. doi: 10.1159/000259600. [DOI] [PubMed] [Google Scholar]
- Luoma H., Tuompo H. The relationship between sugar metabolism and potassium translocation by caries-inducing streptococci and the inhibitory role of fluoride. Arch Oral Biol. 1975 Nov;20(11):749–755. doi: 10.1016/0003-9969(75)90047-3. [DOI] [PubMed] [Google Scholar]
- Luoma H. Uptake of phosphate by caries-active and caries-inactive streptococci. Arch Oral Biol. 1968 Nov;13(11):1331–1342. doi: 10.1016/0003-9969(68)90157-x. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K., Virtanen K. K. Effect of 4.5--year use of xylitol and sorbitol on plaque. J Dent Res. 1978 Mar;57(3):441–446. doi: 10.1177/00220345780570030401. [DOI] [PubMed] [Google Scholar]
- Oppermann R. V., Rölla G. Effect of some polyvalent cations on the acidogenicity of dental plaque in vivo. Caries Res. 1980;14(6):422–427. doi: 10.1159/000260485. [DOI] [PubMed] [Google Scholar]
- Otto R., Sonnenberg A. S., Veldkamp H., Konings W. N. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5502–5506. doi: 10.1073/pnas.77.9.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. R., Hayes M. L. Effects of 2-deoxy D-glucose and other sugar analogues on acid production from sugars by human dental plaque bacteria. Scand J Dent Res. 1980 Jun;88(3):201–209. doi: 10.1111/j.1600-0722.1980.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Roberts K. R., Linder L. Phosphoenolpyruvate-dependent glucose phosphotransferase activity in Streptococcus mitis ATCC 903. Scand J Dent Res. 1980 Aug;88(4):316–322. doi: 10.1111/j.1600-0722.1980.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Skinner A., Naylor M. N. Influence of sugar type on the pattern of acid production by Streptococcus mutans. J Dent Res. 1972 Jul-Aug;51(4):1022–1024. doi: 10.1177/00220345720510040501. [DOI] [PubMed] [Google Scholar]
- Tatevossian A., Gould C. T. The composition of the aqueous phase in human dental plaque. Arch Oral Biol. 1976;21(5):319–323. doi: 10.1016/0003-9969(76)90055-8. [DOI] [PubMed] [Google Scholar]


