Abstract
Acidic mining lakes (pH <3) are specific habitats exhibiting particular chemical and biological characteristics. The species richness is low and mixotrophy and omnivory are common features of the plankton food web in such lakes. The plankton community structure of mining lakes of different morphometry and mixing type but similar chemical characteristics (Lake 130, Germany and Lake Langau, Austria) was investigated. The focus was laid on the species composition, the trophic relationship between the phago-mixotrophic flagellate Ochromonas sp. and bacteria and the formation of a deep chlorophyll maximum along a vertical pH-gradient. The shallow wind-exposed Lake 130 exhibited a higher species richness than Lake Langau. This increase in species richness was made up mainly by mero-planktic species, suggesting a strong benthic/littoral – pelagic coupling. Based on the field data from both lakes, a nonlinear, negative relation between bacteria and Ochromonas biomass was found, suggesting that at an Ochromonas biomass below 50 μg C L−1, the grazing pressure on bacteria is low and with increasing Ochromonas biomass bacteria decline. Furthermore, in Lake Langau, a prominent deep chlorophyll maximum was found with chlorophyll concentrations ca. 50 times higher than in the epilimnion which was build up by the euglenophyte Lepocinclis sp. We conclude that lake morphometry, and specific abiotic characteristics such as mixing behaviour influence the community structure in these mining lakes.
Keywords: Acidic lake, Mining lake, Plankton, Mixotrophy, Rotifers, Flagellates, Ochromonas, Lepocinclis, Chlamydomonas, Deep chlorophyll maximum
Introduction
Acidic mining lakes that originate from ceased open-cast lignite mining activities, are very specific habitats which are often characterised by extreme chemical conditions. The key process is the weathering of pyrite and markasite resulting in the generation of high amount of acidity (Friese et al., 1998). Besides the low pH, high concentrations of sulphate, aluminium, iron and other heavy metals which are several orders of magnitude higher than in circum-neutral lakes are typical. In such extreme habitats, only a limited number of species is able to survive and, consequently, a low diversity of plankton organisms is found (Wollmann et al., 2000; Kamjunke et al., 2004). The plankton community consists typically of heterotrophic bacteria, mixotrophic flagellates and a limited number of other algae (Nixdorf et al., 1998; Lessmann et al., 2000; Beulker et al., 2003) and very few heterotrophic protist and rotifer species (Packroff, 2000; Deneke, 2000). Despite the extreme chemical forcing factors, other environmental (e.g. lake morphology, turbulence, etc.) and biological factors (e.g. competition and predation) may shape the plankton community structure in such lakes. For example, bacteria compete with the osmo-mixotrophic Chlamydomonas acidophila (Tittel et al., 2005) for dissolved organic carbon (DOC) (Kamjunke et al., 2008), and bacteria are consumed by the phago-mixotrophic Ochromonas sp. (Schmidtke et al., 2006). In low productive mining lakes (e.g. in Lake 111) consumers such as rotifers are resource limited (Weithoff, 2004). These biotic interactions as well as physical forces such as sediment resuspension and transport of the biota from the benthos/littoral are important processes for structuring the plankton community. The aim of this study was to investigate the effect of lake morphometry and wind exposure (and mixing behaviour) on the plankton communities in two differing mining lakes that are similar in their chemical characteristics. Lake 130 in the Lusatian mining district (Germany) is shallow and wind-exposed with extensive macrophyte beds. The larger part of the lake is polymictic and only at a few deeper areas a thermal stratification establishes in summer. Lake Langau (Lake LG, Austria) is less wind-exposed due to its location and surrounding trees, and stable stratified during summer. Accordingly, the chances of colonizing the pelagial by littoral/benthic (micro-) organisms and by wind-dispersed invaders should be higher in Lake 130 than in Lake LG. Our hypothesis was that higher probability of colonization would result in a more complex food web in Lake 130.
Material and methods
The two mining lakes studied are located in the Lusatian mining area in Eastern Germany (Lake 130, ca. 51°33′N, 13°43′E, ca. 34 ha area, 5 m depth), and in North-East Austria (Lake LG, ca. 48°51′N, 15°44′E, 10 ha area, 10 m depth). Both lakes are ca. 330 km apart; Lake 130 arose ca. 10 years ago, Lake LG is ca. 40 years old.
In 2008, Lake 130 was sampled five times on April 24, May 27, July 3, August 4 and October 17. Samples were taken at 6 depths, at 0, 1, 2, 3, 4 and ca. 5 m using a vertical water sampler. Additionally, physical and chemical parameters (pH, temperature and oxygen) were recorded using a multiparameter probe (Idronaut, Brugheri, Italy). The underwater light intensity as photosynthetic active radiation was recorded with spherical light sensors (Li-Cor 193, Li-Cor, Lincoln, Nebraska, USA).
Subsamples for the analysis of bacteria and phytoplankton were fixed with Lugol’s iodine (1 mL 100 mL−1) and 1 mL 24% sulphuric acid was added to avoid iron precipitation. Phytoplankton was analysed using the Utermöhl procedure (Utermöhl, 1958) using an inverted microscope (Zeiss, Jena, Germany). The cell sizes were measured with video-aided software (Thalheim, Pulsnitz, Germany). From these measurements the cell volume of phytoplankton cells was calculated assuming a regular geometric shape, and converted into carbon content based on previously established conversion factors (Kamjunke et al., 2004).
For the analysis of bacteria, the samples were bleached with sodium-thiosulfate and stained with acridine orange (Hobbie et al., 1977). The samples were then filtered on black membrane filters (0.2 μm, nuclepore) and analysed with a fluorescence microscope (Zeiss, Jena, Germany). Cell sizes were measured with a porton grid and cell volume and carbon content was calculated according to Simon and Azam (1987).
For zooplankton, depth integrated samples (10.5 L) were taken from the epilimnion and hypolimnion based on the temperature measurements. These samples were filtered through 30 μm mesh size and fixed as described above. Samples were analysed with an inverted microscope (Thalheim, Pulsnitz, Germany). The conversion into carbon was according to Weithoff (2005), Gaedke and Kamjunke (2006) and Weithoff (unpublished results).
For the calculation of the ratio of heterotrophy:autotrophy (H:A) we assumed that the osmo-mixotrophic C. acidophila gains 80% of its carbon by photosynthesis and 20% by the uptake of DOC and Ochromonas sp. uses to 43% the autotrophic way for carbon acquisition and to 57% the heterotrophic pathway (Gaedke and Kamjunke, 2006). For Lake LG, the algae in the deep chlorophyll maximum (DCM) were not considered in the calculations, because no information on their nutritional strategy was available.
In the laboratory, untreated samples were filtered through GF/F glass fibre filters for the analysis of the soluble reactive phosphorus (SRP), iron and dissolved organic carbon. SRP was measured photometrically using the molybdene-method, iron using the ferrozine assay (Lovley and Phillips, 1987) and DOC with a carbon analyser (Elementar, Hanau, Germany). From the filters, chlorophyll was extracted overnight with hot ethanol and measured fluorometrically (TD-700, Turner Design, Sunnyvale, California, USA).
The sampling procedure and analyses for Lake LG were similar as for Lake 130 with the following modifications which did not influence our overall results: A total of 9 samples were taken during the growing season in 2005 (September 28 and October 25), 2007 (April 17, June 21, August 29 and November 6) and 2008 (April 1, June 25 and August 1). One or two epilimnetic samples and, if present, one sample from the deep chlorophyll maximum were taken on each occasion. Depth profiles including chlorophyll-fluorescence were measured with a multi parameter probe (YSI Environmental, Yellow Spring, Ohio, USA). Bacterial abundance was determined from epifluorescence cell counts of formalin-fixed (1% final concentration) samples after DAPI staining (Porter and Feig, 1980). The same samples were used to analyse phytoplankton abundance and community composition by flow cytometry (FACS Calibur, Becton Dickinson, San Jose, California, USA) during 2007 and 2008, in addition to cell counts obtained from Lugol fixed samples by the Utermöhl procedure. The agreement between both methods was, on average, within 10% (T. Weisse, unpubl. res.). We report results from the Utermöhl counts to render results from both lakes comparable; however, flow cytometric cell counts were used to assess the phytoplankton abundance close to the lake bottom where high detrital and inorganic particle concentration hindered visual analysis of the samples. Since no size measurements for bacteria are available from Lake LG, constant carbon contents of 15 fg carbon per single cell and 150 fg carbon per filamentous bacteria were assumed.
Results
Physical and chemical characteristics
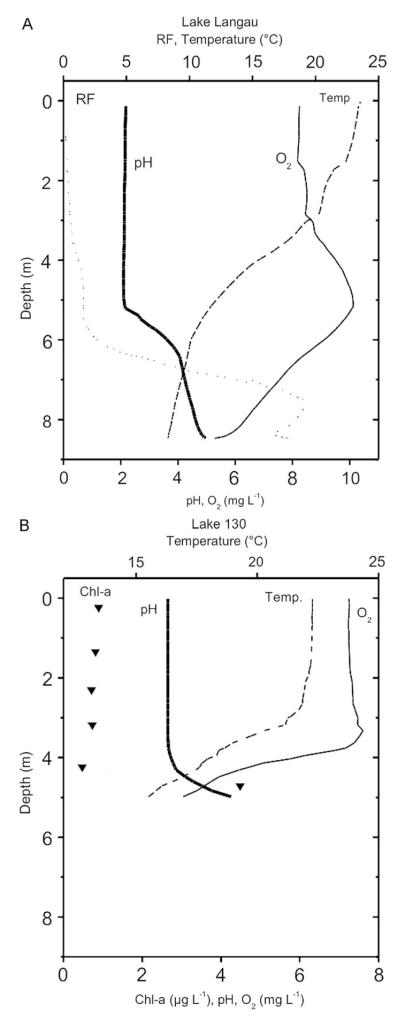
Vertical depth profiles from August 2008 exhibited in both lakes a stable thermal stratification (Fig. 1). The shallow and wind-exposed Lake 130 had a constant temperature up to 3.5 m depth and temperatures decreased downwards to 5 m by ca. 8 °C, whereas in the more sheltered Lake LG, the temperature decreased more gradually over depth with a maximum temperature difference from the surface to the lake bottom of ca. 15 °C; maximum surface temperature was 30.2 °C in June 2007. Due to the high attenuation of the FeS-rich, red-coloured water, light intensity is reduced strongly in both lakes. The 1% surface light intensity was reached at 5–6 m in Lake 130 and at 6–7 m in Lake LG. In the hypolimnion of both lakes, the oxygen concentration decreased towards the lake bottom, but remained above anoxic conditions. A specific feature in the pH profile of Lake LG was that below 5 m, the pH increased to about 5 close to the lake bottom. The stable stratified, low-light, low-oxygen and relatively high pH hypolimnion provided a distinct habitat different from the epilimnion. In Lake 130, the pH was constant over depth except for a slight increase above the sediment.
Fig. 1.
Vertical profiles from Lake Langau (1A, August 1, 2008) and Lake 130 (1B, August 4, 2008). pH, temperature, oxygen concentration and relative chlorophyll fluorescence (Lake LG) or chlorophyll concentration (Lake 130).
Temporal and vertical abundance of plankton organisms
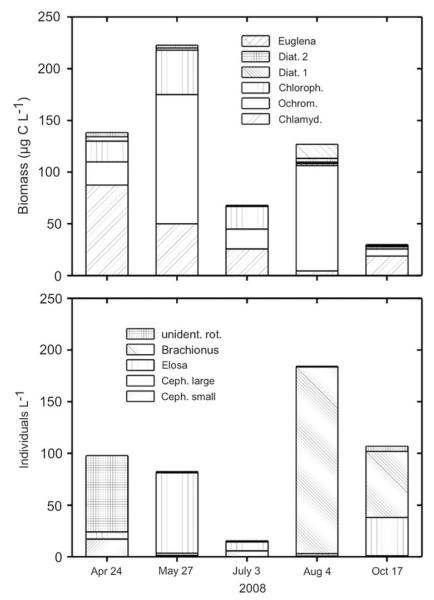
In Lake 130, the bacteria biomass was relatively constant over depth and during the year with a grand mean of 10±3 (SD) μg C L−1. The phytoplankton biomass exhibited a much higher variability both at the total biomass level and also at the species level. The depth integrated biomass ranged from 30 to 220 μg C L−1 (mean 117±74 (SD) μg C L−1), however, the individual species biomass varied more strongly (Fig. 2A) suggesting some compensatory dynamics on the community level. Remarkably, compared to some other mining lakes in the Lusatian region, more species contributed to the total phytoplankton biomass in Lake 130: besides the common mixotrophic flagellates Ochromonas sp. and C. acidophila, two different diatom species (Eunotia spp., typical for benthic habitats), one coccal green alga (not identified) and Euglena sp. occurred at some sampling dates in considerable abundances (Fig. 2A). The chlorophyll-a concentration in the epilimnion was low (mean 1.7 μg chl-a L−1) and increased slightly above the sediment (4.4 μg chl-a L−1, Fig. 1B).
Fig. 2.
Seasonal, depth integrated phytoplankton biomass (2A, Chlamdomonas acidophila, Ochromonas sp., unidentified coccal chlorophyte (Chloroph.), unidentified diatoms 1 and 2 (Eunotia sp.) and Euglena sp.) and zooplankton abundance in Lake 130 (2B, a small Cephalodella sp., a large Cephalodella sp., Elosa worallii, Brachionus sericus and an unidentified rotifer).
In Lake LG, the phytoplankton in the epilimnion was almost exclusively dominated by Ochromonas sp. and C. acidophila with, on average, widely consistently ca. 2.2-fold higher biomasses of Chlamydomonas than Ochromonas (490 and 230 μg C L−1, respectively). Chlorophyll-a concentrations were similarly low (1.7 μg chl-a L−1) as in Lake 130. A specific characteristic of Lake LG in summer was an accumulation of algae at a depth of ca. 7 m resulting in high chlorophyll concentrations (Fig. 1). This DCM was made up exclusively by a euglenophyte, Lepocinclis sp., tentatively identified as Lepocinclis buetschlii. The fluorescence peak at 7.5 m shown in Fig. 1A corresponded to a chlorophyll-a concentration of approximately 90 μg L−1. Cell numbers of Lepocinclis ranged from 7.7–11.0×107 L−1 in this layer. The average cell size of this ellipsoid species was approximately 35×18 μm, yielding a cellular volume of ca. 5900 μm3 (derived from V=π/6×length×width2). Assuming a carbon content of 110 fg μm−3, this results in an exceptionally high carbon biomass of Lepocinclis ranging from 50–70 mg C L−1 in the DCM. Calculating its carbon biomass from conventional chlorophyll to carbon conversion factors, the carbon concentration estimate was one order of magnitude lower. However, flow cytometric analyses revealed that the per cell chlorophyll content of Lepocinclis was lower than that of the smaller Chlamydomonas and Ochromonas (Weisse, 2008 and Weisse, unpubl.). Therefore, irrespective of the uncertainties involved in such calculations, it is clear that Lepocinclis may temporarily dominate the autotrophic biomass averaged over the water column in Lake LG. On an annual average, Lepocinclis is less important, because the DCM is only found in summer under stably stratified conditions.
The species richness of consumers, i.e. rotifers was higher in Lake 130 than in Lake LG (Table 1). In Lake 130, several of these species dominated at different times of the year: in April an unidentified species dominated, in May and July Elosa worallii and in August and October, Brachionus sericus contributed most to the total rotifer biomass (Fig. 2B). In Lake LG in 2007, the only rotifer found was Cephalodella sp. (for 2008, no zooplankton data are available). On average, the abundance of Cephalodella in the DCM was ca. two-fold higher (28 Ind L−1) than in the epilimnion (17 Ind L−1). Heliozoa, the potential planktonic top predators in extremely acidic lakes, occurred only sporadically and in low numbers in both lakes.
Table 1.
Lake morphology and abundance of organisms in Lake 130 and Lake LG.
| Lake 130 | Lake LG | |
|---|---|---|
| Age (years) | 10 | 40 |
| Max. depth (m) | 5 | 10 |
| Mean integrated epilimnetic abundance (0–5 m): | ||
| Bacteria (μg L−1) | 9.6 (7.8–11.5) | 3.7 (0.9–5.7) |
| Ochromonas sp. (μg C L−1) | 55 (7–125) | 230 (47–460) |
| Chlamydomonas acidophila (μg C L−1) | 37 (5–87) | 490 (33–1600) |
| Coccal chlorophyte (μg C L−1) | 18 (2–48) | Not detected |
| Diatom 1 (μg C L−1) | 2 (0.6–4) | Not detected |
| Diatom 2 (μg C L−1) | 2.4 (0.8–4.3) | Not detected |
| Euglena sp. (μg C L−1) | Occurred only once | Not detected |
| Unidentified rotifer (Ind L−1) | 16 (0.2–74) | Not detected |
| Cephalodella sp. small (Ind L−1) | 5 (0–17) | 17 (12–22) |
| Cephalodella sp. large (Ind L−1) | 1 (0–2) | Not detected |
| Elosa worallii (Ind L−1) | 27 (2–77) | Not detected |
| Brachionus sericus (Ind L−1) | 49 (0–180) | Not detected |
| Deep chlorophyll maximum (DCM): | ||
| Lepocinclis buetschlii | No DCM | High, see text |
Data in brackets show the absolute range.
The relation of bacterial to Ochromonas biomass and the ratio of heterotrophy to autotrophy (H:A)
We combined our data from the two studied lakes with data from a previous study on another mining lake (Lake 111) in the Lusatian region (Fig. 3). At low Ochromonas biomass (<50 μg C L−1, ln (50)=3.9) the bacterial biomass was in the range of 10 μg C L−1 (ln (10)=2.3, mainly data from Lake 130) and with further increasing Ochromonas biomass the bacteria declined to lower levels (data from Lakes 111 and LG). The data of the present study broadens the range of the relationship found for Lake 111 providing further evidence that the bacteria are suppressed in these lakes when Ochromonas builds up high biomasses. The average H:A ratio in Lake 130 was 0.9 and thus, considerably higher than in Lake LG with 0.6. The low H:A ratio in Lake LG was mainly attributable to the ca. 2.2-fold higher abundance of Chlamydomonas compared to Ochromonas and the comparably low abundance of bacteria and consumers.
Fig. 3.
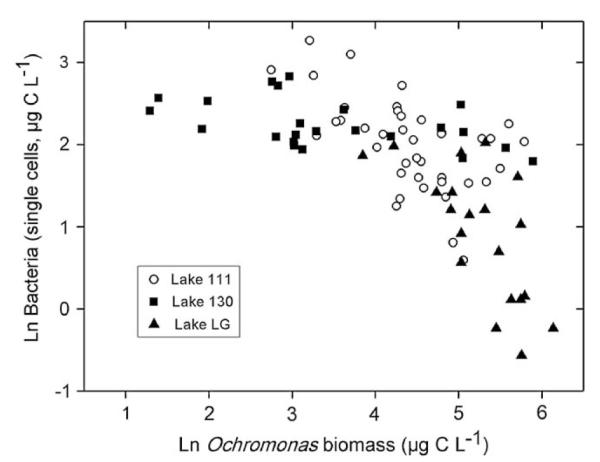
Relationship of ln-transformed bacterial and Ochromonas biomass in the Lakes 111, 130 and LG. Data for Lake 111 from Kamjunke et al. (2004).
Discussion
Plankton composition and vertical structure of the two lakes
The two lakes differed in two prominent aspects: (i) Lake 130 exhibited higher species richness than Lake LG and (ii) in Lake LG a pronounced DCM was build up at a less acidic pH during the summer stratification.
(i) Following our initial hypothesis, the higher species richness in Lake 130 is likely due to the shallow, wind-exposed character of the lake along with the extensive macrophyte beds. The diatoms found are typical benthic forms. Although the contribution of the diatoms to the total phytoplankton biomass is relatively low, their permanent occurrence suggests that they were resuspended and transported from the lake bottom (and littoral) into the well-mixed upper water layers and remained there when turbulence persisted. A similar explanation for the higher rotifer species richness might hold true, because all species found belong to genera that are described as mero-benthic (or mero-planktic) (Koste, 1978) and may have been passively transported into the open water of Lake 130. In laboratory incubation experiments of littoral sediment cores from mining Lake 111, Bell and Weithoff (2003, 2008) showed a marked recruitment of the planktonic population of Cephalodella sp. from the sediment. In contrast to expectations arising from previous measurements at different mining lakes (Bell et al., 2006), heliozoa occurred very rarely in the plankton of Lake 130, although they are also mero-planktic organisms. Since heliozoa have a broad food spectrum ranging from filamentous bacteria over Chlamydomonas to rotifers (Bell et al., 2006), the plankton community in Lake 130 provides adequate resources for them. Nevertheless, it appears that in these extremely acidic mining lakes, the benthic/littoral – pelagic coupling is more important than in circum-neutral lakes because the open water is inhabited by only a few competitors which allow even not purely planktonic organisms to persist. Another remarkable finding was the absence of E. worallii in Lake LG. Although a complete absence cannot be proven, not even a single specimen was encountered in the fixed samples and during regular observations of live zooplankton samples. Compared to Cephalodella, Elosa has lower quantitative food requirements and a higher starvation resistance which enables Elosa to successfully compete with Cephalodella (Weithoff, 2004, 2007). The chemistry of Lake LG does not explain the absence, since Elosa strains from the Lakes 111 and 130 exhibited positive growth in filtered lake water from Lake LG stocked with Chlamydomonas from Lake LG (Weithoff et al., unpublished results).
(ii) The DCM in Lake LG is of particular interest. Chlamydomonas or Lepocinclis potentially dominate DCMs in acidic mining lakes (Steinberg et al., 1999; Tittel et al., 2003 and this study). Species from both genera are common in other acidic habitats as well (Amaral-Zettler et al., 2000, Lessmann et al., 2000; Aguilera et al., 2006). A prerequisite for the formation and persistence of a DCM is reduced wind-induced mixing, which was the case in the sheltered Lake LG.
Several additional factors facilitate the establishment of a DCM in lakes. Among others, a higher grazing pressure in the epilimnion can cause a DCM which has been proposed by Tittel et al. (2003) based on competition theory and predation. Chlamydomonas is small in size (ca. 5–10 μm in length) and can be ingested by rotifers and also by Ochromonas. The trophic link between Chlamydomonas and the rotifers is of further interest. Because the light availability in deep water layers is low and most likely below the compensation point for phototrophic growth, Chlamydomonas most likely subsidises phototrophy by the uptake of DOC. The mode of nutrition of Chlamydomonas, i.e. autotrophic vs. mixo- and heterotrophic, is crucial for their consumers, especially rotifers. It has been found that Chlamydomonas grown autotrophically is a valuable food source for E. woralli and Cephalodella sp. whereas heterotrophically grown Chlamydomonas enable only positive growth in Cephalodella sp. and not in Elosa, potentially because of the different biochemical composition of the flagellates (Weithoff and Wacker, 2007; Wacker and Weithoff, 2009). This might explain the vertical segregation of the rotifer species in Lake 11: Elosa dominated the upper water layers with low food quantities (but of high quality) and Cephalodella dominated the lower water layers at the DCM made up by high Chlamydomonas abundance which acquires its carbon most likely by using the phototrophic and the osmotrophic pathway. It is not yet known whether the nutrition mode of Chlamydomonas also affects losses through Ochromonas.
Reduced grazing losses in deeper water layers probably do not explain the DCM made up by Lepocinclis in Lake LG. Lepocinclis cells were about 35 μm in length which is above the prey size range of Ochromonas (Schmidtke et al., 2006) and most rotifers. Since larger consumers were absent in Lake LG, grazing losses tended to be very low at all depths. These findings suggest that in the case of a DCM by Lepocinclis abiotic factors (e.g. resource availability) are of larger importance. In many circum-neutral lakes with a DCM the reduced grazing losses likely contribute to the formation of a DCM. The algae accumulate at or even below the oxycline where sulphide is present (Adler et al., 2000; Gervais et al., 2003) which is toxic to most zooplankton species reducing the grazing losses.
Ochromonas – bacteria interactions
Although in the three lakes the relationship between bacterial and Ochromonas biomass is not necessarily caused by the same factors, our data suggest that bacteria are strongly suppressed at high Ochromonas densities. Laboratory experiments demonstrated that Ochromonas, a strain isolated from Lake 111, feeds efficiently on bacteria. Calculations from bacteria production and abundance data suggested that in the epilimnion of Lake 111, 88% of the daily bacteria production is grazed by Ochromonas (Kamjunke et al., 2005; Schmidtke et al., 2006). Besides the top-down control, bacteria can also be bottom-up regulated in these mining lakes by the available DOC (Kamjunke et al., 2006). The DOC concentrations in Lake 130 were lower than in Lake LG (2.9 vs. 7.8 mg C L−1) and slightly higher than in Lake 111 (0.7–2.5 mg C L−1, Kamjunke et al., 2004). Since the DOC quality is more important for bacteria than its quantity, these values have limited explanatory power. It has been found that the benthic primary production and the resulting exudates can be important sources for the supply of substrates for bacteria in mining lakes (Koschorreck and Tittel (2002); Kamjunke et al., 2006). This might be an indicator that the DOC in the shallow Lake 130 is of higher quality than the one in Lake LG, where the benthic – pelagic exchange of matter is supposedly less pronounced. This view is reinforced by the high, potentially refractory, DOC concentration in Lake LG which is not taken up by bacteria or Chlamydomonas. In summary, in Lake 130 the DOC pool is small and labile DOC might be produced by benthic and epiphytic algae thereby increasing the planktonic heterotrophy. In Lake LG the DOC pool is large which is obviously not channelled through heterotrophic biomass leading to a larger relative amount of planktonic autotrophy.
Conclusions
Our study revealed that chemically similar acidic mining lakes may harbour a surprisingly different species diversity and plankton community structure. Some species such as the flagellates Chlamydomonas acidophila and Ochromonas spp. are wide-spread in acidic mining lakes, while others such as the flagellate Lepocinclis and the rotifers Elosa worallii and B. sericus are more restricted in their distribution. We identified lake morphometry in combination with wind-induced mixing as a major factor that may indirectly, but strongly influence biological processes in acidic mining lakes. Thus, the acidic lakes tend to behave like neutral ones regardless of the specific chemical properties. The success of bentho-littoral species in colonizing the pelagic may even be greater in acidic lakes, because strong pelagic competitors are absent due to the low pH.
Acknowledgements
We thank Silvia Heim, Christina Schirmer, Peter Stadler and Ulrike Scheffel for technical assistance in the field and laboratory. Part of this work was supported by the Austrian Science Fund (FWF Grant P20118-B17 to T. W.).
Dedicated to Prof. Dr. Walter Geller on the occasion of his 65th birthday anniversary
References
- Adler M, Gervais F, Siedel U. Phytoplankton species composition in the chemocline of mesotrophic lakes. Arch. Hydrobiol. Spec. Issue Adv. Limnol. 2000;55:513–530. [Google Scholar]
- Aguilera A, Manrubia SC, Gomez F, Rodriguez N, Amils R. Eukaryotic community distribution and its relationship to water physicochemical parameters in an extreme acidic environment, Rio Tinto (Southwestern Spain) Appl. Environ. Microbol. 2006;72:5325–5330. doi: 10.1128/AEM.00513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral-Zettler LA, Gomez F, Zettler E, Keenan BG, Amils R, Sogin ML. Microbiology: Eucaryotic diversity in Spain’s River of Fire. Nature. 2000;417:137. doi: 10.1038/417137a. [DOI] [PubMed] [Google Scholar]
- Bell EM, Weithoff G. Benthic recruitment of zooplankton in an acidic lake. J. Exp. Mar. Biol. Ecol. 2003:285–286. 205–219. [Google Scholar]
- Bell EM, Weithoff G. Recruitment of Heliozoa, rhizopods and rotifers from the sediment of an extremely acidic lake during spring and early summer. Lakes Reserv. Res. Manage. 2008;13:105–115. [Google Scholar]
- Bell EM, Weithoff G, Gaedke U. Temporal dynamics and growth of Actinophrys sol (Sarcodina: Heliozoa), the top predator in an extremely acidic lake. Freshwater Biol. 2006;51:1149–1161. [Google Scholar]
- Beulker C, Lessmann D, Nixdorf B. Aspects of phytoplankton succession and spatial distribution in an acidic mining lake (Plessa 117, Germany) Acta Oecol. 2003;24:S25–S31. [Google Scholar]
- Deneke R. Review of rotifers and crustaceans in highly acidic environments of pH values <3. Hydrobiologia. 2000;433:167–172. [Google Scholar]
- Friese K, Hupfer M, Schultze M. Chemical characteristics of water and sediment in acid mining lakes of the Lusatian lignite district. In: Geller W, Klapper H, Salomons A, editors. Acidic Mining Lakes: Acid Mine Drainage, Limnology and Reclamation. Springer; Berlin: 1998. pp. 25–45. [Google Scholar]
- Gaedke U, Kamjunke N. Structural and functional properties of low- and high diversity planktonic food webs. J. Plankton Res. 2006;27:707–718. [Google Scholar]
- Gervais F, Siedel U, Heilmann B, Weithoff G, Heisig-Gunkel G, Nicklisch A. Small-scale vertical distribution of phytoplankton, nutrients and sulphide below the oxicline of a mesotrophic lake. J. Plankton Res. 2003;25:273–278. [Google Scholar]
- Hobbie JE, Daley RJ, Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamjunke N, Gaedke U, Tittel J, Weithoff G, Bell EM. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004;161:289–306. [Google Scholar]
- Kamjunke N, Tittel J, Krumbeck H, Beulker C, Poerschmann J. High heterotrophic bacterial production in acidic, iron-rich mining lakes. Microb. Ecol. 2005;49:425–433. doi: 10.1007/s00248-004-0270-9. [DOI] [PubMed] [Google Scholar]
- Kamjunke N, Bohn C, Grey J. Utilisation of dissolved organic carbon from different sources by pelagic bacteria in an acidic mining lake. Arch. Hydrobiol. 2006;165:355–364. [Google Scholar]
- Kamjunke N, Köhler B, Wannicke N, Tittel J. Algae as competitors of heterotrophic bacteria for glucose. J. Phycol. 2008;44:616–623. doi: 10.1111/j.1529-8817.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- Koschorreck M, Tittel J. Benthic photosynthesis in an acidic mining lake (pH 2.6) Limnol. Oceanogr. 2002;47:1197–1201. [Google Scholar]
- Koste W. Rotatoria. Die Rädertiere Mitteleuropas. Gebrüder Bornträger; Berlin: 1978. [Google Scholar]
- Lessmann D, Fyson A, Nixdorf B. Phytoplankton of the extremely acidic mining lakes of Lusatia (Germany) with pH <3. Hydrobiologia. 2000;433:123–128. [Google Scholar]
- Lovley DR, Phillips EJP. Rapid assay for microbially reduced ferric iron in aquatic sediments. Appl. Environ. Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf B, Mischke U, Lessmann D. Chrysophytes and chlamydomonads: pioneer colonists in extremely acidic mining lakes (pH <3) in Lusatia (Germany) Hydrobiologia. 1998;370:315–327. [Google Scholar]
- Packroff G. Protozooplankton in acidic mining lakes with special respect to ciliates. Hydrobiologia. 2000;433:157–166. [Google Scholar]
- Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–948. [Google Scholar]
- Schmidtke A, Bell EM, Weithoff G. Potential grazing impact of the mixotrophic flagellate Ochromonas sp (Chrysophyceae) on bacteria in an extremely acidic lake. J. Plankton Res. 2006;28:991–1001. [Google Scholar]
- Simon M, Azam F. Protein content and protein synthesis rate of planktonic bacteria. Mar. Ecol. Prog. Ser. 1987;51:201–213. [Google Scholar]
- Steinberg C, Fyson A, Nixdorf B. Extrem saure Seen in Deutschland. B.I.U.Z. 1999;29:98–109. [Google Scholar]
- Tittel J, Bissinger V, Zippel B, Gaedke U, Bell E, Lorke A, Kamjunke N. Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proc. Natl. Acad. Sci. USA. 2003;100:12776–12781. doi: 10.1073/pnas.2130696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel J, Bissinger V, Gaedke U, Kamjunke N. Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist. 2005;156:63–75. doi: 10.1016/j.protis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Utermöhl H. Zur Vervollkommnung der quantitative phytoplankton-methodik. Mitt. Int. Ver. Limnol. 1958;9:1–38. [Google Scholar]
- Wacker A, Weithoff G. Carbon assimilation mode in mixotrophs and the fatty acid composition of their consumers – three rotifers. Freshw. Biol. 2009;54:2189–2199. [Google Scholar]
- Weisse T. The meaning of protist diversity: ecology meets taxonomy. Denisia. 2008;23:297–306. [Google Scholar]
- Weithoff G. Vertical niche separation of two consumers (Rotatoria) in an extreme habitat. Oecologia. 2004;139:594–603. doi: 10.1007/s00442-004-1545-z. [DOI] [PubMed] [Google Scholar]
- Weithoff G. On the ecology of the rotifer Cephalodella hoodi from an extremely acidic lake. Freshwater Biol. 2005;50:1464–1473. [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Weithoff G, Wacker A. The mode of nutrition of mixotrophic flagellates determines the food quality for their consumers. Funct. Ecol. 2007;21:1092–1098. [Google Scholar]
- Wollmann K, Deneke R, Nixdorf B, Packroff G. Dynamics of planktonic food webs in three mining lakes across a pH gradient (pH 2–4) Hydrobiologia. 2000;433:3–14. [Google Scholar]