Abstract
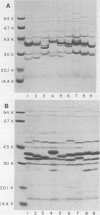
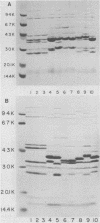
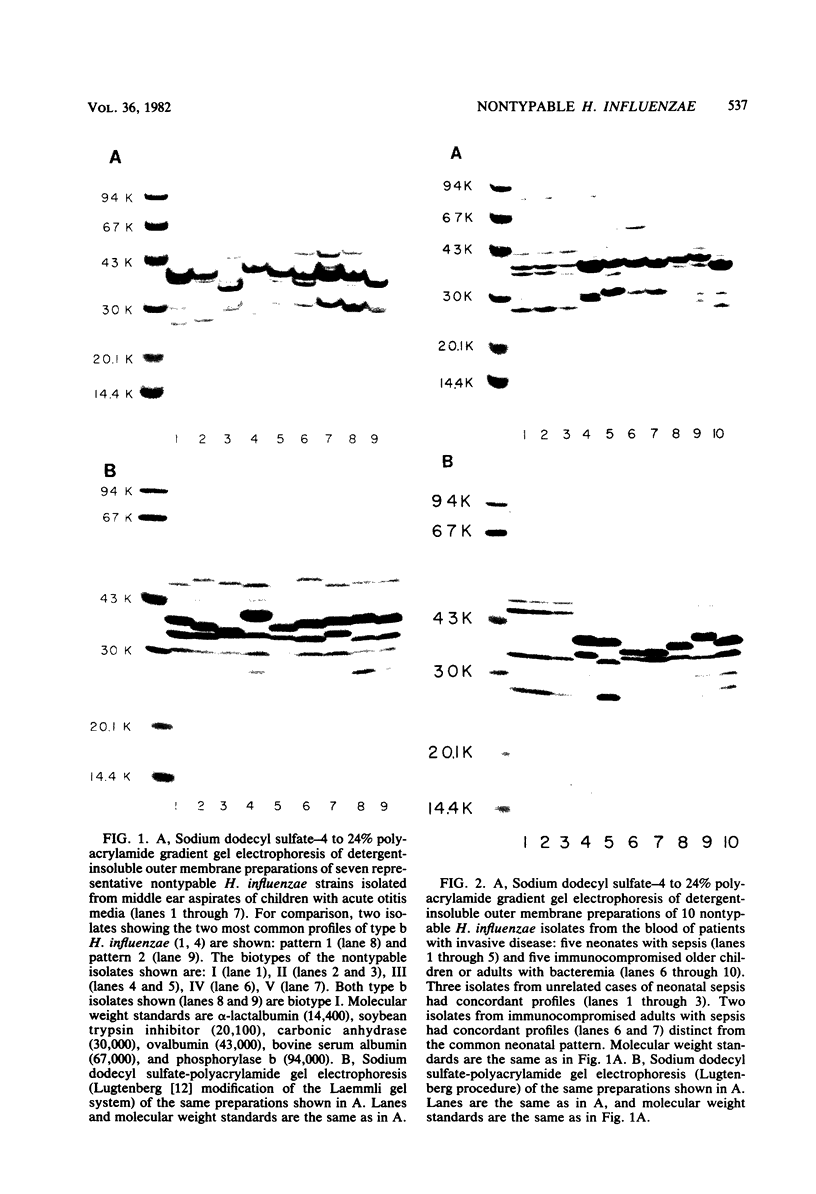
The techniques of biotype determination and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of outer membrane protein preparations were applied to 35 epidemiologically unrelated isolates of pathogenic nontypable Haemophilus influenzae. Three of five isolates obtained from the blood of unrelated newborns with sepsis had concordant major outer membrane from the blood of unrelated older children or adults with bacteremia had concordant major outer membrane protein profiles, distinct from the common profile of neonatal strains, and were biotype II. The outer membrane protein profiles of the remaining 5 isolates from blood, 2 isolated from cerebrospinal fluid, and 23 isolated from middle ear aspirates of children with otitis media were unique, although each isolate had peptides with apparent molecular weights of 16,000 and 31,500. These results suggest that a subset of nontypable isolates associated with bacteremia has distinctive strain markers. Their pathogenicity may relate to a prediction for colonizing the female genital tract in the case of the common neonatal strain or an increased ability to evade host defenses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Comparison of outer-membrane protein subtypes and biotypes of isolates of Haemophilus influenzae type b. J Infect Dis. 1981 Nov;144(5):480–480. doi: 10.1093/infdis/144.5.480. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Nankervis G. A. Infectious arthritis in the neonate caused by Haemophilus influenzae. Am J Dis Child. 1975 Jun;129(6):730–733. doi: 10.1001/archpedi.1975.02120430062017. [DOI] [PubMed] [Google Scholar]
- Howie V. M., Ploussard J. H. Bacterial etiology and antimicrobial treatment of exudative otitis media: relation of antibiotic therapy to relapses. South Med J. 1971 Feb;64(2):233–239. doi: 10.1097/00007611-197102000-00022. [DOI] [PubMed] [Google Scholar]
- Howie V. M., Ploussard J. H., Lester R. L., Jr Otitis media: a clinical and bacteriological correlation. Pediatrics. 1970 Jan;45(1):29–35. [PubMed] [Google Scholar]
- Khuri-Bulos N., McIntosh K. Neonatal Haemophilus influenzae infection. Report of eight cases and review of the literature. Am J Dis Child. 1975 Jan;129(1):57–62. doi: 10.1001/archpedi.1975.02120380037009. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- May J. R., Herrick N. C., Thompson D. Bacterial infection in cystic fibrosis. Arch Dis Child. 1972 Dec;47(256):908–913. doi: 10.1136/adc.47.256.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- OMLAND T. SEROLOGICAL STUDIES ON HAEMOPHILUS INFLUENZAE AND RELATED SPECIES. 8. EXAMINATIONS OF ULTRASONICALLY PREPARED HAEMOPHILUS ANTIGENS BY MEANS OF IMMUNO-ELECTROPHORESIS. Acta Pathol Microbiol Scand. 1964;62:89–106. doi: 10.1111/apm.1964.62.1.89. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]