Abstract
Child maltreatment and biomarkers of allostatic load were investigated in relation to child health problems and psychological symptomatology. Participants attended a summer research day camp and included 137 maltreated and 110 nonmaltreated low-income children, who were aged 8 to 10 years (M = 9.42) and racially and ethnically diverse; 52% were male. Measurements obtained included salivary cortisol and DHEA, body-mass index, waist-hip ratio, and blood pressure; these indicators provided a composite index of allostatic load. Child self-report and camp adult-rater reports of child symptomatology were obtained; mothers provided information on health problems. The results indicated that higher allostatic load and child maltreatment status independently predicted poorer health outcomes and greater behavior problems. Moderation effects indicated that allostatic load was related to somatic complaints, attention problems, and thought problems only among maltreated children. Risks associated with high waist-hip ratio, low morning cortisol, and high morning DHEA also were related to depressive symptoms only for maltreated children. The results support an allostatic load conceptualization of the impact of high environmental stress and child abuse and neglect on child health and behavioral outcomes and have important implications for long-term physical and mental health.
Child maltreatment constitutes a severe, if not the most severe, environmental hazard to children’s adaptive and healthy development. Substantial research has demonstrated the dire effects of child maltreatment on the developmental course. Through a process of probabilistic epigenesis, child abuse and neglect progressively contribute to compromised adaptations on a range of developmental domains central to successful adjustment. These developmental failures pose significant risk for psychopathology across the life course (Cicchetti, 1989; Cicchetti & Valentino, 2006). In addition to socio-emotional and psychological consequences, attention is being directed toward the physiological repercussions of child maltreatment. In this investigation, we consider how the joint impact of chronic stress and consequent high allostatic load serves as a unifying perspective on how diverse biological and psychological systems are affected and thereby influence physical and mental health.
Within this framework, it is important to appreciate that although child maltreatment may occur at all socioeconomic levels, the vast majority of maltreated children are from low-income, highly impoverished populations (NIS-4, Sedlack, Mettenburg, Basena, Petta, McPherson, et al., 2010). Consequently, maltreated children, are exposed to high levels of community stress, including high crime, violence, noise, overcrowding, poor schools, and diminished local resources. These risk factors act within a context of extreme relational stress within the family to confer heightened levels of stress exposure (Cicchetti & Lynch, 1993). Children living within these risky environments regularly endure stressful events and conditions that pose ongoing challenges for their healthy adaptation.
Stressful experiences vary in their degree of challenge to children’s biological and psychological systems. Shonkoff, Boyce, and McEwen (2009) differentiate three types of stress that children may experiences. Normative and routine life challenges, named positive stress, generally promote the learning of problem-solving and coping skills. Time-limited challenges that are embedded within a context of protective factors such as positive, supportive relationships, are considered tolerable stress. Alternatively, toxic stress involves conditions in which the child is exposed to chronic, severe, and prolonged stress, often occurring in the absence of protective factors. Abuse and neglect, parental substance abuse, and family violence all constitute toxic stressors. Therefore, children in low-income families, particularly those in which maltreatment is present, are exposed to greater levels of toxic stress.
Research has demonstrated that children from low socioeconomic status (SES) families exhibit weaknesses across neurocognitive functioning and performance domains that are important for academic achievement (Hackman & Farah, 2009; Hackman, Farah, & Meaney, 2010). Not only are deprivation and lack of cognitive stimulation likely to contribute to these liabilities, but also so are exposure to high levels of environmental stress. Lupien and her colleagues have shown that low SES children have elevated levels of morning cortisol across the elementary school years compared to high SES children (Lupien, King, Meaney, & McEwen, 2001). This finding suggests that increased HPA axis activity may be a response to the chronic and pervasive stress endemic to low-SES environments. In order to investigate relations between environmental risk and physiological biomarkers of stress exposure, Evans (2003) examined cumulative sociodemographic and psychosocial risks with greater prevalence in low-income families and associations with neuroendocrine, cardiovascular, and fat deposition indices in middle childhood. A composite index of allostatic load across the physiological measures was related to higher cumulative SES-related risks. Moreover, in a follow-up investigation in early adolescence, cumulative risk continued to be related to indices of allostatic load among youth in families with low maternal responsiveness (Evans, Kim, Ting, Tesher, & Shannis, 2007). These findings suggest that the impact of poverty on stress-responsive physiological systems may be particularly detrimental when parenting is non-supportive, which has important implications for children in maltreating families.
The concept of allostasis and allostatic load (McEwen, 1998, 2002, 2003; McEwen & Stellar, 1993; McEwen & Wingfield, 2003) offers an integrative model regarding how exposure to chronic stress in development, as is the case in low-income and maltreated children, promotes long-term liabilities for physical and mental health. Allostasis is a process whereby normal physiological adaptations are brought to bear to regulate and protect the body in response to acute stress. In the short term, these adaptations protect physiological systems from stress exposure and adjust homeostasis to address current demands. However, with repeated extreme and chronic stress, the initial normative physiological reactions to stress become progressively inefficient in protecting the individual. Wear and tear on the body ensues, resulting in allostatic overload.
The hypothalamic-pituitary-adrenal (HPA) axis, through the secretion of glucocorticoids, and the sympathetic-adrenal-medullary (SAM) axis, through the release of catecholamines, are primary systems that are activated in response to perceived threat (Sapolsky, Romero, & Munck, 2000). Although acute mobilization of these systems results in an adaptive response to stress, chronic activation results in over- or under-compensation, causing cascading effects within interconnected biological systems (Cicchetti & Tucker, 1994; Masten & Cicchetti, 2010), and increased vulnerability for stress-related diseases (Lupien, Ouellet-Morin, Hupbach, Mai, Buss, et al., 2006). Moreover, changes in the brain may occur, including synaptic and dendritic remodeling, suppressed neurogenesis, and structural atrophy/hypertrophy. Thus, allostatic load and its consequent dysregulation of diverse brain and organ systems can increase the emergence of physical and mental disorders.
Multiple interactive physiological systems are involved in the response to stress, and the linkages among these systems are nonlinear (McEwen, 1998). In addition to the HPA and SAM axes, interconnected networks responsive to allostatic load include the cardiovascular, immune, and metabolic systems. Epinephrine, norepinephrine, cortisol, dehydroepiandosterone (DHEA), and pro- and anti-inflammatory cytokines are regarded as primary mediators of allostatic load, as they operate on the cellular level (McEwen, 2003). Although they are initially protective, prolonged activation leads to damaging effects on the body and brain. Subsequently, due to allostatic overload, associated biological systems overcompensate, resulting in subclinical accommodations of metabolic (e.g., fat deposition), cardiovascular (systolic and diastolic blood pressure), and immune (e.g., c-reactive protein) parameters. Over extended periods of time, the effects of allostatic load on primary mediators and secondary outcomes result in tertiary consequences. Often these include the development of psychological disorders or disease states. This unfolding process of allostatic overload may occur over the life course, with distal outcomes eventuating long after periods of chronic stress. Moreover, it is the collective impact of small alterations across multiple systems that in the aggregate contribute most strongly to morbidity, rather than large changes within any one system. Thus, strategies to investigate the impact of allostatic load have composited evidence for stress-related changes across multiple systems in order to derive allostatic load indexes to evaluate in relation to physical and mental health outcomes (Evans, 2003; Juster, Bizik, Picard, Arsenault-Lapierre, Sindi et al., 2011; Juster, McEwen, & Lupien, 2010; Lupien et al., 2006).
There are different models of how the effects of excesses, deficits, and dysregulation in the primary mediators of allostatic load, particularly cortisol, may affect brain structure and function. Further, developmental considerations are important. In this regard, the timing of periods of severe stress may be critical. For example, variation in the age of maturation of brain structures, notably the hippocampus, prefrontal cortex, and the amygdala, may result in differential effects on functioning and health (Lupien, McEwen, Gunnar, & Heim, 2009; Shonkoff et al., 2009). Sensitive periods in the development of these brain structures may generate heightened vulnerability to the neurotoxic effects of excess glucocorticoids, thereby creating a long-term liability as development proceeds. Alternatively, the cumulative exposure to stressful experiences and concomitant dysregulation of the HPA axis may contribute to ongoing wear and tear on these brain structures. These effects of chronic stress are heightened, given that these areas of the brain have dense concentrations of glucocorticoid receptors, thereby promulgating progressive inefficiency in brain structure and function. For children in low-income environments and those subjected to abuse and neglect, the consequences may be particularly salient for health outcomes across the lifespan.
A valuable series of studies has examined diverse, long-term health outcomes in an extensive sample of HMO members who were exposed to varying adverse childhood experiences (ACE, Felitti, Anda, Nordenberg, Williamson, Spitz, et al., 1998). The ACE factors that were examined included exposure to childhood emotional, physical, and sexual abuse, substance abuse in the family, parental mental illness, domestic violence, and adult criminal behavior. The accumulation of adverse experiences across these risk domains was determined and linked to a range of adult health outcomes. For example, a gradient of exposure to increasing numbers of ACE factors was associated with a cumulative index of health risk behaviors linked with adult disease and mortality, (i.e., smoking, alcoholism, drug abuse, suicide attempts, high number of sex partners, sexually transmitted diseases, inactivity, obesity). Moreover, a dose-response relationship of ACE exposures and leading causes of death, including heart disease, cancer, chronic bronchitis or emphysema, hepatitis, skeletal fractures, and poor self-rated health, also was obtained. Other findings from this body of research have demonstrated that verbal and physical abuse in childhood is related to high Body Mass Index (BMI) and obesity in adulthood (Williamson, Thompson, Anda, Dietz, & Felitti, 2002). Furthermore, ACE factors have been associated with hospitalization for diagnosed auto-immune diseases decades into adulthood (Dube, Fairweather, Pearson, Felitti, Anda, et al., 2009), and graded relationships between ACE factors and prescription rates for psychotropic medications have been found (Anda, Brown, Felitti, Bremner, Dube, et al., 2007). This latter finding further illustrates the high rate of serious adult psychiatric morbidity that ensues among children exposed to high levels of adversity.
Maltreatment also has been associated with adverse health outcomes among children. For example, neglect has been associated with high rates of children being overweight and obese, as indexed by high BMI levels (Knutson, Taber, Murray, Valles, & Koeppl, 2010). Severity of trauma from child abuse also has been linked to higher self-reported poor health symptoms among adolescents, increased BMI, and stress-response immune system indicators (Clark, Thatcher, & Martin, 2010). Moreover, maltreatment in childhood has been associated with higher hospitalization rates for asthma, cardio-respiratory, and infectious diseases in adolescents (Lanier, Jonson-Reid, & Stahlschmidt, 2010). Thus, increasing evidence points to the impact of maltreatment on health during childhood. Furthermore, these health liabilities are likely to extend subsequently across the life course.
Although less attention has been given to physical health outcomes among maltreated children, the detrimental effects of child abuse and neglect on child mental health are well-documented. Child maltreatment is a substantial risk factor for a broad spectrum of child psychopathology. Elevated internalizing and externalizing symptomatology among maltreated children relative to nonmaltreated children has been consistently observed, as well as higher prevalence of clinical-level symptomatology and psychiatric diagnoses, including major depressive disorder, attention-deficit hyperactivity disorder, oppositional defiant disorder, conduct disorder, and post-traumatic stress disorder (Cicchetti & Valentino, 2006; Famularo, Kinscherff, & Fenton, 1992; Putnam, 2003). Furthermore, child maltreatment has long-term negative consequences for adult mental health (Chen, Brown, & Smaile, 2001; Collishaw, Pickles, Messer, Rutter, Shearer, et al., 2007; Edwards, Holden, Felitti, & Anda, 2003; Kendler, Kuhn, & Prescott, 2004; Widom, 1999). Data from the National Comorbidity Study examining 20 DSM-IV diagnoses (Green, McLaughlin, Berglund, Gruber, Sampson, et al., 2010) indicate that child neglect and physical and sexual abuse are associated with higher occurrence of mood, anxiety, substance use, and disruptive disorders in adulthood. Moreover, histories of child abuse and neglect contribute to persistence of psychiatric disorders in different stages of adulthood (McLaughlin, Green, Gruber, Sampson, Zavlasky, et al., 2010). Research prospectively following individuals with documented child abuse and neglect also has demonstrated linkages to increased rates of major depressive disorder and comorbidity with other diagnoses (Widom, Dumont, & Czaja, 2007). Further, the age of onset of abuse and neglect further predicts higher symptomatology and rates of depression, anxiety, antisocial, and alcohol diagnoses (Kaplow & Widom, 2007).
Consistent evidence thus indicates that child abuse and neglect are associated not only with mental health problems in childhood but also with substantial risk for psychopathology across the life course. Increasingly, it is important to investigate the developmental processes and concomitant psychological, neurobiological, and genetic mechanisms that mediate relations between child maltreatment and psychopathology. Moreover, given diverse developmental pathways ensuing from child abuse and neglect experiences, understanding processes that promote resilience also is crucial (Cicchetti, 2010; Cicchetti & Rogosch, 2007).
Not only is the extensive rate of psychopathology among maltreated children a dire consequence in its own right, but also psychopathology in childhood has been linked to negative physical health outcomes many years later in adulthood (von Stumm, Deary, Kivimaki, Jokela, Clark, et al, 2011). Accordingly, the extent to which the experience of child abuse and neglect generates high rates of psychopathology in maltreated children may, in turn, have negative ramifications for physical health. Investigation of the role of allostatic load in both physical and mental health functioning is thus highly warranted.
In our laboratory, we have conducted various investigations of neuroendocrine functioning in maltreated children with a focus on cortisol regulation, a biomarker of allostatic load. Consistent with a tenet of the allostatic load conceptualization specifying that individual components of allostatic load may not reveal large differences in stress-affected individuals independently, we have not found evidence for significant differences between maltreated and nonmaltreated children in global comparisons of cortisol regulation (Cicchetti & Rogosch, 2001a). Rather, greater diversity of neuroendocrine functioning has been observed among maltreated and nonmaltreated children, with greater proportions of maltreated children showing very high or very low levels of cortisol across the day. These diurnal variations were associated with differences in the types of maltreatment children had experienced (Cicchetti & Rogosch, 2001a). Variation in cortisol regulation among maltreated children also has been associated with differences in high levels of internalizing, externalizing, and comorbid symptomatology (Cicchetti & Rogosch, 2001b). In a more recent investigation, we observed that timing of abuse experiences was related to variation in cortisol regulation. Specifically, children who had experienced physical and/or sexual abuse before age 5 and who had elevated internalizing/depressive symptoms uniquely exhibited a pattern of flattened cortisol regulation across the day (Cicchetti, Rogosch, Gunnar, & Toth, 2010). These findings are consistent with an atypical signature of neuroendocrine functioning in adult women with major depressive disorder who had experienced maltreatment during childhood (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008). Moreover, we have discovered that variation in the corticotropin releasing hormone receptor (CRHR1) gene interacts with early abuse, as well as maltreatment experiences more generally, in predicting a flattening of the diurnal cortisol rhythm (Cicchetti, Rogosch, & Oshri, 2011).
Although increasing evidence suggests that maltreatment greatly impacts cortisol regulation and other primary mediators of high allostatic load, no studies to date have examined maltreatment with respect to an overall index of allostatic load and its role in the physical and mental health functioning of low-income maltreated and nonmaltreated children. In the current investigation, we incorporate a multi-domain assessment of systems sensitive to high stress exposure to measure allostatic load and investigate the following hypotheses and research questions:
Children with more indicators of allostatic load will have higher health problems and medical service utilization. Health outcomes also will be more compromised in maltreated children.
Higher allostatic load will relate to higher levels of child psychopathology.
Because all low income children are subjected to high levels of stress, child maltreatment and allostatic load will both contribute to prediction of diverse forms of child psychopathology.
Allostatic load will predict some forms of psychopathology primarily among maltreated children.
Methods
Participants
The participants in this investigation included 247 children (118 female, 129 male) who attended a research summer camp program designed for school-aged low-income maltreated (n =137) and nonmaltreated children (n =110). Children were on average 9.42 years old (SD = 0.88, range = 7.9 to 10.9). The sample was racially (62.3% Black, 21.1% White, 16.6% Biracial or other race) and ethnically (24.3% were Latino) diverse. Informed consent was obtained from parents of maltreated and nonmaltreated children for their child’s participation in the summer camp program and for examination of any Department of Human Services (DHS) records pertaining to the family.
Children in the maltreated group were recruited through a DHS liaison who examined Child Protective Services reports to identify children who had been maltreated and/or were part of a family with a history of maltreatment. Children living in foster care were not recruited for the current investigation. The DHS liaison contacted eligible families and explained the study. Parents who were interested in having their child participate provided signed permission for their contact information to be shared with project staff. These families were representative of those receiving services through DHS. Comprehensive reviews of all DHS records for each family were conducted. Maltreatment information was coded by trained research staff and a clinical psychologist, using the Barnett, Manly, and Cicchetti (1993) nosological system for classifying child maltreatment. Coding is based on all available information and does not rely on DHS determinations.
Because maltreating families primarily have low socioeconomic status (National Incidence Study – NIS-4; Sedlak et al., 2010), nonmaltreating families were recruited from those receiving Temporary Assistance to Needy Families (TANF) in order to ensure socioeconomic comparability between maltreated and nonmaltreated families. A DHS liaison contacted eligible nonmaltreating families and described the project. Parents who were interested in participating signed a release allowing their contact information to be given to project staff for recruitment. The families were recruited as nonmaltreated families after comprehensive DHS record searches confirmed the absence of any documented child maltreatment. Families who received preventative DHS services due to concerns over risk for maltreatment were not included within the nonmaltreated comparison group. In order to further verify a lack of DHS involvement, trained research assistants interviewed the mothers of children recruited for the nonmaltreatment group using the Maternal Child Maltreatment Interview (Cicchetti, Toth & Manly, 2003) and reviewed records in the year following camp participation to assure that all information had been assessed.
Children in the maltreated and nonmaltreated groups were comparable on a number of family characteristics (Table 1). These include maternal education, χ2(1, N =244) = 0.36, p > 0.05, marital status, χ2(3, N =244) = 2.32, p > 0.05, total family income including public assistance t(239) = 1.17, p > 0.05, and family history of receiving public assistance, χ2(1, N =244) = 0.78, p > 0.05.
Table 1.
Family Demographic Characteristics
| Maltreated | Nonmaltreated | |
|---|---|---|
| M(SD) or % | M (SD) or % | |
| Marital Status | ||
| Never married | 34.3% | 37.4% |
| Married | 17.5% | 21.5% |
| Living with partner | 25.5% | 17.8% |
| No longer married | 22.6% | 23.4% |
| Maternal Education | ||
| Did not graduate high school | 45.3 % | 41.4% |
| Total Family Income | ||
| $1,000s including public assistance | 25.88 (14.90) | 28.15 (14.94) |
| Family history of receiving public assistance | 99.3% | 100% |
Note: All group contrasts were nonsignificant.
Procedures
Day Camp Procedures
Maltreated and nonmaltreated children were randomly assigned to groups of ten same-sex and same-age peers. Within these groups five children were maltreated and five were nonmaltreated. Each group was led by three trained camp counselors who were unaware of child maltreatment status and study hypotheses. Children participated in recreational activities throughout the week. After child assent was obtained, children participated in research assessments conducted by trained research assistants and provided saliva samples for subsequent cortisol and DHEA assay (see Cicchetti & Manly, 1990, for detailed descriptions of camp procedures). Additional indicators of allostatic load were collected from children during this time. Trained research assistants also conducted interviews with each child’s caregiver to obtain demographic information and conduct a number of evaluations on the child’s health and psychiatric status. All research assistants were unaware of child maltreatment status and study hypotheses.
Measures
Maltreatment classification system (MCS; Barnett, Manly, & Cicchetti, 1993)
The Maltreatment Classification System (MCS) is designed to assess individual children’s maltreatment experiences. The MCS utilizes DHS records to make independent determinations of maltreatment. The MCS classifies the subtypes that each child experienced, frequency of occurrence, subtype severity, and developmental periods of occurrence in order to designate the recency, onset, and chronicity of maltreatment. Subtypes of maltreatment include neglect, emotional maltreatment, physical abuse, and sexual abuse. Neglect refers to failure to provide for the child’s basic physical needs for adequate food, clothing, shelter, and medical treatment. Neglect also includes lack of supervision, moral-legal neglect, and educational neglect. Emotional maltreatment involves extreme thwarting of children’s basic emotional needs for psychological safety and security. Examples include belittling and ridiculing the child, extreme negativity and hostility, child abandonment, suicidal or homicidal threats, and extreme negativity and hostility. Physical abuse involves nonaccidental physical injury to the child such as bruises, welts, burns, chocking, and broken bones. Sexual abuse involves attempted or actual sexual contact between the child and caregiver for purposes of the caregiver’s sexual satisfaction or financial benefit. Examples of sexual abuse range from exposure to pornography or adult sexual activity to sexual touching and fondling to forced intercourse with the child.
The MCS has demonstrated reliability and validity in classifying maltreatment in a number of studies (Bolger, Patterson, & Kupersmidt, 1988; Dubowitz, Pitts, Lintrownik, Cox, Runyan, et al., 2005, English, Upadhyaya, Litrownik, Marshall, Runyan, et al., 2005, Manly, 2005; Smith & Thornberry, 1995). DHS records were coded using the MCS by trained research staff and a clinical psychologist. All coders achieved adequate reliability before coding records used for the study. Kappas for the presence of each of the maltreatment subtypes ranged from .90 to 1.00; intraclass correlations for severity ratings of individual subtypes of maltreatment ranged from .83 to 1.0.
In the present study, 76.6% of the maltreated children had experienced neglect, 48.9% experienced emotional maltreatment, 23.4% physical abuse, and 8.8% experienced sexual abuse. Therefore, emotional maltreatment and neglect were pervasive throughout the sample while physical and sexual abuse occurred less frequently. Consistent with other samples of maltreatment, the majority of children in this study experienced more than one subtype of maltreatment. More specifically, 51.9% of children had experienced two or more subtypes of maltreatment (M=1.67, SD = 0.73), and 10 out of the 15 possible combinations of the four maltreatment subtypes were present in the sample.
Teacher Report Form (TRF; Achenbach, 1991)
Behavioral symptomatology was assessed at the end of each week by counselors through completion of the TRF. Summer camp counselors evaluated children who were in their group at the end of the week for each cohort of children. Counselors had spent on average 35 hours of observation and interaction with the children at the time that the assessment was completed. The TRF was used in this study because summer camp counselors were able to observe the types of behaviors that are present in classroom-based settings. The counselors were unaware of maltreatment status and research hypotheses. The TRF is a widely used assessment containing 118 items rated for frequency of diverse symptoms and behavioral disturbances. Items load onto eight symptom scales, and three summary scales. The symptom scales include withdrawn, somatic complaints, anxiety/depression, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior. Summary scales include internalizing behavior, externalizing behavior, and total behavior problems. Average intraclass correlations among pairs of raters were .68 for internalizing, .87 for externalizing, and .88 for total behavior problem scores.
Childhood Depression Inventory (CDI, Kovacs, 1982, 2004)
The Child Depression Inventory (CDI) is a self-report questionnaire designed to measure depressive symptomatology in school-age children. The CDI was administered by trained research assistants, who read each question to the child to account for any discrepancies in reading or comprehension level. Children chose from among three option statements for each item, indicating increasing levels of depressive symptoms. Symptoms were rated based upon how the child has been feeling over the past two weeks. The CDI has well-established validity and internal consistency ranges from 0.71 to 0.89 (Kovacs, 2004). Internal consistency in the current study was .87. According to Kovacs (2004), a total score of 19 or greater on the CDI has been an established cutpoint for clinical level depressive symptoms in children. In the present study, 8.1% of children (n=20) met criteria for clinical level depressive symptoms.
Child Health Screen
An assessment of the child’s current physical health status and utilization of community services providers was measured using items taken from the Parent Report Form of the Child Health and Illness Profile – Child Edition (CHIP-CE/PRF; Riley, Forrest, Starfield, Rebok, Robertson, et al., 2004). Parents reported on the health and functional status of their child, addressing the child’s overall health, need for medication and/or additional intervention, impairment, and diagnosis of acute medical and psychiatric illnesses. During the second part of the assessment, parents answered questions relating to their child’s access to and utilization of the health care system. In order to obtain an indication of children who were rated as experiencing the most acute health difficulties, this assessment was scored in a dichotomous manner. Each item was coded dichotomously as zero, if the child did not meet criteria for risk, or one, if the child was classified as at-risk. Children could obtain a total score of 14 based on items chosen for their implication for overall physical and psychiatric health. Items that met the following criteria were scored as a one; These items included (1) physical health rated as “fair” or “poor”, (2) medication taken for more than 12 months due to a behavioral, medical, or other health problem, (3) medical, mental health or educational service utilization for more than 12 months, (4) impairment lasting more than 12 months due to a behavioral, medical, or other health problem, (5) special therapy due to a behavioral, medical, or other health problem lasting more than 12 months, (6) needing treatment for an emotional, developmental, or behavioral problem lasting more than 12 months, (7) diagnosis with a learning disorder, ADHD, depression/anxiety, behavior or conduct disorder, developmental or physical impairment, or Autism, (8) diagnosis with Diabetes, bone or joint problems, asthma, frequent ear infections, or frequent headaches, (9) diagnosis with respiratory, digestive or skin allergies, (10) diagnosis with speech problems, hearing, or visual problems, (11) severity of diagnosed conditions, (12) moderate or severe difficulties in concentration, behavior, emotions, interpersonal relationships resulting in family stress or burden, (13) emergency room visit at least one time in the past year due to accident, injury, or poisoning, and (14) missed 5 or more days of school due to illness or injury. In the present study, scores ranged from zero to eleven for the total number of health risk indicators (M = 2.97, SD = 2.49). Twenty percent of the sample did not have any of these health and psychiatric concerns.
Resting Blood Pressure
Resting systolic and diastolic blood pressure was assessed during summer camp by a trained research assistant using a 760 Series prosphyg aneroid sphygmomanometer and adscope sprague stethoscope. Blood pressure was assessed while the child was seated with his/her arm at heart level. Three readings were taken on separate days during the course of the child’s week at camp. In order to evaluate whether each child was at-risk for hypertension, these values were entered into a computer software system (EZ Blood Pressure Calculator, www.ezbmi.com). This tool utilizes national guidelines based on the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescence (National Institute of Health, 2005) to calculate the expected and actual percentile systolic and diastolic blood pressure levels for each child based on age, gender, height, and weight. Children whose blood pressure levels were below the 90th percentile as calculated by the parameters set forth in the Fourth Report (NIH, 2005) were classified as normal while those who exceeded the 90th percentile children were classified as prehypertensive. Children whose blood pressure values were within the 95th and 99th percentiles were classified as having stage 1 hypertension, and percentile values exceeding the 99th percentile designated stage 2 hypertension. Blood pressure risk was designated if children were classified in the prehypertension, stage 1 hypertension, or stage 2 hypertension category for at least one of the three readings.
Body Mass Index (BMI) & Waist Hip Ratio (WHR)
Weight and height were measured on a single occasion using standard procedures. Weight was measured using a digital scale. Height was measured with the child standing against a wall on which a measuring tape was secured. BMI was calculated by converting height to meters and weight to kilograms. Weight was then divided by height squared. In order to account for differences in BMI based on age and gender, these values were then entered into a computer software system (EZ BMI Calculator, www.ezbmi.com). This tool utilizes national guidelines to calculate expected weight range and actual percentile weight based on gender and age (Kuczmarski, Ogden, Guo, Grummer-Strawn, Hegal, et al., 2002). Children were calculated as underweight (weight <5th percentile), healthy weight (weight 5th to 85th percentile), overweight (weight 5th to 85th percentile), or obese (weight 5th to 85th percentile). BMI risk was assigned based on classification as underweight, overweight, or obese.
Waist circumference was measured at the top of the child’s hipbone, level with the naval. Hip circumference was measured around the fullest part of the child’s hips. Waist-hip ratio (WHR) was then calculated by dividing waist by hip circumference. WHR risk was calculated in two ways. The WHR risk factor included in the AL index was designated as children whose WHR exceeded one standard deviation above the mean of the sample. In other interaction analyses, WHR risk was designated for children with a WHR within the top quartile of the sample.
Cortisol and DHEA
Saliva samples were obtained at standard times each day over the course of the camp week. Samples were collected when the child arrived at camp at 9:00 AM, and before they departed at 4:00 PM. In order to account for the volatility in cortisol levels within the first hour of wakening, all salivary samples were taken after the 45-minute bus ride to camp and initial greeting by staff (Susman, Dockray, Dorn, Schiefelbein, Herwehe, et al., 2007). Cortisol was assayed from saliva for each day and time across the week that it was collected. Dehydroepiandrosterone (DHEA) was assayed from saliva two days, Tuesday and Thursday, because of less variability in DHEA levels. Trained research assistants obtained these saliva samples via a standardized protocol (Granger, Schwartz, Booth, Curran, & Zakaria,, 1999). Children had not consumed food or drink within 30 minutes of sample collection. Children were asked to chew Trident® sugarless original flavor gum to stimulate saliva and then passively drool through a short drinking straw into a 20-mL plastic vial. Saliva samples were immediately frozen and stored at −40°C. Weekly samples were shipped overnight on dry ice for next day delivery to Salimetrics Laboratories (State College, PA) for assay. After thawing, each sample was processed by placing four to five 1-ml aliquots into 1.8-ml cryogenic storage vials and frozen at −80°C. Upon assay, samples were thawed to room temperature and centrifuged at 3000 rpm for 15 minutes. The clear top phase of the sample was pipetted into appropriate test tubes/wells.
Salivary cortisol (in micrograms/deciliter) was assayed using an enzyme immunoassay kit (Salimetrics, State College, PA). This kit is commercially available and uses 25 μl of saliva. Its lower limit of sensitivity is 0.007 μg/dl (range up to 1.8 μg/dl) with average intra- and interassay coefficient of variation of <5.0 and 10.0%, respectively. Salivary DHEA (in picograms/milliliter) was also processed using an enzyme immunoassay kit (Salimetrics, State College, PA.) This kit uses 50 μl of saliva, Its lower limit of sensitivity is 10.0 pg/ml (range up to 1000 pg/ml) with average intra- and interassay coefficient of variation equaling <5.0 and 15.0%, respectively. The antibody used in this assay had no detectible cross-reactivity with DHEA. To adjust values for extreme outliers, assay values greater than +2SD were winsorized to the +2SD level. To account for the significant skew within both the cortisol and DHEA values for each day and time period, all values were log transformed prior to analyses. DHEA log-transformed values for the two assessments were averaged to obtain a mean morning DHEA measurement. DHEA risk was determined if mean values exceeded one standard deviation above the sample mean.
The log-transformed cortisol values for the morning samples were averaged across days to obtain a mean morning assessment of cortisol. An afternoon cortisol measurement was also obtained by averaging the log-transformed afternoon samples across days. Using these log-transformed mean morning and afternoon cortisol measurements, mean diurnal change scores were calculated by subtracting morning from afternoon values. Flattened cortisol risk was designated for children whose cortisol levels changed less than −1 SD relative to the sample from morning to afternoon; these children included all those who did not decrease or who increased in cortisol level across the day. Differences in basal cortisol level were accounted for in these change scores by calculating standard deviations based on the distribution of the quartile representing each child’s mean morning cortisol value. An additional cortisol risk factor was assigned for children whose morning cortisol values were one standard deviation below the sample mean.
Allostatic Load (AL) Composite
The AL composite used in analyses consisted of a total of six risk factors. These biomarkers included blood pressure risk, BMI risk, WHR risk, flattened cortisol risk, low morning cortisol risk, and high morning DHEA risk. Each biomarker was coded dichotomously as zero, if the child did not meet criteria for risk on the biomarker, or one, if the child was classified as at-risk on the respective biomarker. Morning DHEA and WHR risk were assigned if the child had surpassed one standard deviation above the sample. Morning cortisol risk was designated if children were less than one standard deviation below the sample mean. Flattened cortisol risk was designated as less than one standard deviation change from AM to PM, accounting for morning AM values. BMI and blood pressure risk were assigned based on children who exceeded national guidelines based on age, gender, and body size. After these variables were computed, an allostatic load composite was calculated as the sum of these dichotomous risk factors, with a total possible score ranging from zero to six.
Results
Preliminary Analyses
Table 2 presents descriptive information on each of the six allostatic load factors, the AL composite index, and the psychological and health outcome variables. In the present sample, 26.9% of children had zero risk factors, 36.7% had one risk, 21.2% had two risks, 9.4% had three risks, and 2.9% had four risk factors. Table 3 provides the partial intercorrelation coefficients among the AL composite index, independent AL risk indicators, and outcome variables, with age and gender covaried. Although it is difficult to compare raw means for each AL indicator based on the lack of established norms within children, the high prevalence of risk indication for BMI, blood pressure, and WHR underscores the high-risk nature of the present population.
Table 2.
Descriptive Statistics on Allostatic Load Indicators and Psychological/Health Outcome
| Measure | M | SD | Percentage of sample with risk factor |
|---|---|---|---|
| Physiological Risk Factors | |||
| Body Mass Index (BMI) | 19.35 | 4.33 | |
| (Risk: Over/underweight or obese) | 35.1% | ||
| Waist-Hip Ratio (WHR) | 0.944 | 0.045 | |
| (Risk: ≥ 1 SD) | 15.4% | ||
| Blood Pressure (systolic/diastolic) | 61/103 | 5.6/7.3 | |
| (Risk: Any overall risk indication) | 25.5% | ||
| Morning Cortisol (mean log10 values) | −0.985 | 0.21 | |
| (Risk: < 1SD change AM to PM) | 13.8% | ||
| (Risk: ≤ −1 SD) | 12.1% | ||
| Morning DHEA (mean log10 values) | 1.45 | 0.34 | |
| (Risk: ≥ 1 SD) | 13.8% | ||
| Allostatic Load (0–6) | 1.19 | 1.05 | |
|
| |||
| Outcome variables | |||
| Health Screen (CHIP-CE/PRF) | 2.97 | 2.49 | |
| Child Depression Inventory (CDI) | 7.60 | 7.34 | |
| TRF: Externalizing | 51.72 | 8.97 | |
| Internalizing | 46.90 | 7.11 | |
| Total Behavior Problems | 48.24 | 7.74 | |
| Somatic Complaints | 50.76 | 2.57 | |
| Social Problems | 54.90 | 5.43 | |
| Thought Problems | 50.55 | 2.28 | |
| Attention Problems | 51.24 | 2.43 | |
| Delinquent Behavior | 53.88 | 5.39 | |
| Aggessive Behavior | 54.94 | 6.98 | |
Note. TRF = Teacher Report Form.
Table 3.
Partial Correlation Matrix for AL Biomarkers and Outcome Variables (child age and gender as covariates)
| Measures | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. AL | -- | .54** | .51** | .38** | .36** | .46** | .39** | .10 | .16* | .17* | .15* | .11 | .24** | .25** | .14 | .10 | .13 |
| 2. BMI | -- | .15* | −.01 | −.07 | .06 | .11 | .04 | .15* | .19** | .17* | .06 | .11 | .19** | .13 | .05 | .07 | |
| 3. WHR | -- | .01 | .08 | .12 | .09 | .03 | .003 | .10 | .12 | .08 | .17* | .12 | .07 | .05 | .11 | ||
| 4. BP | -- | −.03 | .02 | .11 | −.03 | .11 | .03 | −.03 | .01 | .12 | .09 | .01 | −.01 | −.01 | |||
| 5. Flat cortisol | -- | .22** | −.11 | −.03 | −.01 | −.03 | −.03 | .01 | .05 | −.03 | −.02 | .002 | .04 | ||||
| 6. Low AM cortisol | -- | −.05 | .11 | .05 | .13 | .10 | .13 | −.01 | .11 | −.01 | .13 | .11 | |||||
| 7. High AM DHEA | -- | .17* | .09 | .01 | .01 | .02 | .15* | .10 | .07 | .01 | .01 | ||||||
| 8. CDI | -- | .24** | .26** | .17* | .24** | .13 | .17* | .18* | .22** | .28** | |||||||
| 9. Parent Health Screen | -- | .21** | .22** | .10 | .11 | .16* | .06 | .10 | .09 | ||||||||
| 10. Total Behavior | -- | .57** | .84 | .32** | .76** | .70** | .68** | .80** | |||||||||
| 11. Internalizing | -- | .14 | .34** | .48* | .38** | .12 | .12* | ||||||||||
| 12. Externalizing | -- | .19** | .60** | .55** | .83** | .94** | |||||||||||
| 13. Somatic | -- | .29** | .40** | .13** | .18* | ||||||||||||
| 14. Social | -- | .59** | .46** | .61** | |||||||||||||
| 15. Attention | -- | .48** | .61** | ||||||||||||||
| 16. Delinquent | -- | .83** | |||||||||||||||
| 17. Aggression | -- |
Note. BP = Blood Pressure, CDI = Child Depression Inventory, Parent Health Screen = Parent Report Form of the Child Health and Illness Profile - Child Edition, Total Behavior = TRF Scale: Total Behavior Problems, Somatic = TRF Scale: Somatic Complaints, Social = TRF Scale: Social Problems, Attention = TRF Scale: Attention Problems, Aggression = TRF Scale: Aggressive Behavior
p<0.05,
p<0.01.
Maltreatment, Allostatic Load, and Child Health Problems
In order to determine if maltreated and nonmaltreated children differed in the levels of allostatic load exhibited, an ANCOVA was performed, controlling for child age and gender. The main effect of maltreatment on AL was not significant, F(1,239) = 0.081, p>0.05. This indicated that maltreatment did not independently predict differences in AL levels. Thus, it appears that children within this high-risk poverty sample display biomarkers of AL regardless of whether or not they have been maltreated.
A multiple regression analysis was conducted to evaluate the relationship between maltreatment status, AL, and their interaction in predicting scores on the abridged CHIP-CE/PRF, which was utilized as a health assessment. Age and gender were included as covariates. The main effects of maltreatment (β= 0.147, p=0.031) and AL (β= 0.155, p=0.022), both significantly predicted scores on the health assessment. The interaction between maltreatment and AL was not significant in predicting health assessment scores, (β= 0.07, p>0.05). Therefore, both maltreatment status and AL independently predicted scores on the health screen assessment. As reported by their mothers, maltreated children evinced significantly greater health difficulties and impairments than did nonmaltreated children. Children with increased biomarkers of AL were also rated significantly higher on the health screen than children with fewer physiological risk factors. Thus, among children from low-income families, maltreatment and allostatic load function in an additive fashion to contribute to children’s poorer health outcomes.
Maltreatment, Allostatic Load, and Observed Behavior Problems
Given the deleterious impact of maltreatment on child psychological functioning, AL and maltreatment were examined jointly in relation to mental health outcomes in a series of multiple regression analyses. For each regression analysis, interaction terms were first computed and AL was centered. Analyses were then conducted with age and gender entered in the first step as covariates. In step two, AL and maltreatment were entered as main effects. The interaction between AL and maltreatment was entered in the third step. In addition to these regression analyses, single biomarkers comprising the AL index were examined in relation to child- and counselor-rated psychological outcomes.
Initially, analyses were conducted to examine the three TRF broadband scales as outcome variables. The main effect of maltreatment status was significantly associated with internalizing symptoms, (β= 0.138, p=0.034), externalizing symptoms, (β= 0.238, p<0.0001), and total behavior problems, (β= 0.229, p<0.0001). This indicates that maltreated children were rated significantly higher by counselors on each of these symptom scales compared to nonmaltreated children. The main effect of AL was significant in predicting internalizing symptoms (β= 0.124, p=0.053), externalizing symptoms (β= 0.135, p=0.031), and total behavior problems (β=0.177, p=0.005). Therefore, children with a higher number of AL biomarkers were also rated as displaying significantly greater symptoms. Maltreatment status and allostatic load each independently contributed to differences in the broadband scales. Across all three analyses, the interaction between AL and maltreatment was not significantly related to symptoms (β= 0.013, β= 0.129,β= 0.127, p>0.05 for internalizing, externalizing, and total behavior problems, respectively).
After conducting these analyses with respect to overall symptom ratings, each specific TRF scale was examined as an outcome in a separate multiple regression analysis in order to clarify which areas of functioning were most impaired. These analyses revealed that there were no significant effects of maltreatment status, AL, and their interaction in predicting symptoms of withdrawal (β= 0.801, β= 0.025,β= −0.044, p’s>0.05, respectively), or anxiety/depression (β= 0.091, β= 0.123,β= 0.101, p’s>0.05, respectively). However, there were significant main effects for maltreatment status and AL in predicting social problems, delinquent behavior, and aggressive behavior. For the social problems scale, both maltreatment status (β= 0.196, p=0.002) and allostatic load (β= 0.233, p<0.0001) made significant independent contributions. Similar relations were observed for delinquent and aggressive problems. In predicting delinquent problems, both maltreatment (β= 0.209, p=0.001) and AL (β= 0.131, p=0.037) made independent contributions, whereas the interaction (β= 0.105, p>0.05) did not. Likewise, for aggressive problems, the main effects of both maltreatment (β= 0.208, p=0.001) and AL (β= 0.154, p=0.015) were significant, whereas the interaction effect did not contribute significantly to prediction (β= 0.160, p=0.072). Thus, for these three narrowband subscales, both maltreatment and AL independently predicted higher symptom levels.
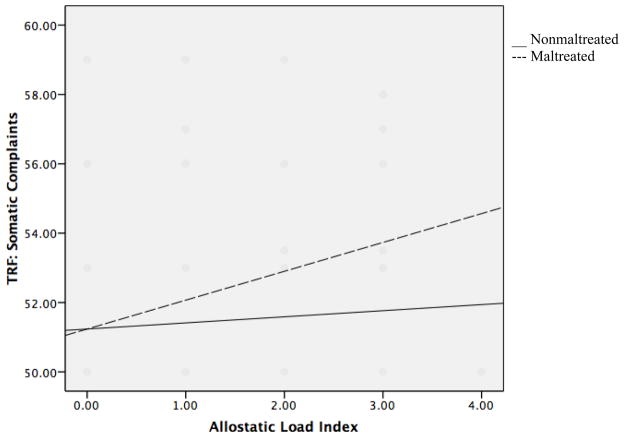
For the remaining TRF narrowband scales, a different pattern of findings emerged involving interaction effects of maltreatment and AL in predicting symptom levels (see Table 4). In the first analysis, maltreatment, AL, and their interaction were entered into a regression predicting child somatic problems. Maltreated children were rated as having significantly greater somatic complaints (β= 0.136, p =0.032), as were children with greater AL (β=0.209, p =0.001). The interaction between maltreatment status and AL was also significant in predicting child somatic complaints (β=0.192, p =0.03). Simple slopes clarified that AL was significantly related to somatic problems for maltreated children (β= 0.263, p =0.002), but not for nonmaltreated children (β= 0.115, p > 0.05, see Figure 1). Maltreatment status contributed to prediction of somatic problems as well as moderated the relationship between AL and psychological outcome.
Table 4.
Regression Results for the main effects of allostatic load, maltreatment, and their interaction in predicting child psychological outcome, controlling for child age and gender
| Outcome | Step | Predictor | Adjusted R2 | ΔR2 | F | df | β | SE |
|---|---|---|---|---|---|---|---|---|
| Somatic Complaints | 1 | Covariates | .023 | .031 | 3.76* | 2,237 | -- | -- |
| Age | .04 | −.01 | .19 | |||||
| Gender | 7.51** | .18 | .17 | |||||
| 2 | Main Effects | .076 | .061 | 5.93 | 4,235 | -- | -- | |
| AL | 11.33 | .21* | .15 | |||||
| Maltx | 4.68 | .14* | .35 | |||||
| 3 | Interaction | .091 | .018 | 5.78 | 5,234 | -- | -- | |
| AL x Maltx | 4.79 | .19* | .30 | |||||
| Attention Problems | 1 | Covariates | .005 | .014 | 1.66 | 2,237 | -- | -- |
| Age | 3.02 | −.11 | .18 | |||||
| Gender | .54 | .05 | .16 | |||||
| 2 | Main Effects | .048 | .05 | 3.99** | 4,235 | -- | -- | |
| AL | 6.35 | .16* | .15 | |||||
| Maltx | 6.38 | .16* | .31 | |||||
| 3 | Interaction | .062 | .018 | 4.17** | 5,234 | -- | -- | |
| AL x Maltx | 4.61 | .19* | .29 | |||||
| Thought Problems | 1 | Covariates | .000 | .008 | .98 | 2,237 | -- | -- |
| Age | 1.94 | .17 | ||||||
| Gender | .06 | .15 | ||||||
| Main Effects | −.007 | .002 | .661 | 4,235 | -- | -- | ||
| AL | .402 | .04 | .14 | |||||
| Maltx | .075 | .02 | .30 | |||||
| Interaction | .028 | .018 | 1.37 | 5,234 | -- | -- | ||
| AL x Maltx | 4.40 | .19* | .28 |
Note. AL = Allostatic Load index, Maltx = Maltreatment status.
p<0.05,
p<0.01.
Figure 1.
Interaction of allostatic load and maltreatment status in predicting somatic complaints on the Teacher Report Form (TRF).
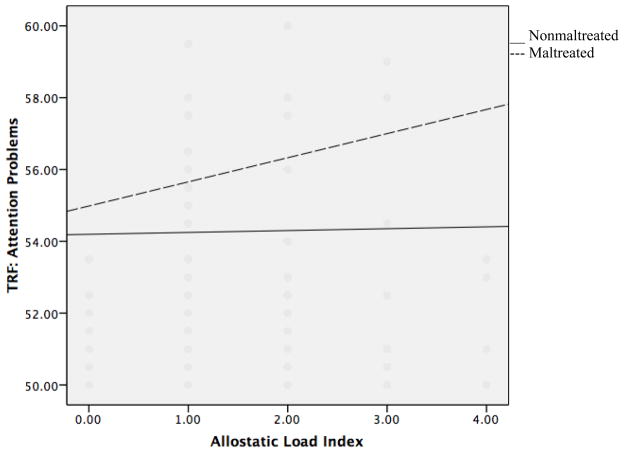
Similar results were found when examining the relationship between AL, maltreatment status, and their interaction, in predicting attention problems. Both maltreatment status (β= 0.161, p=0.012) and AL (β= 0.159, p=0.012) significantly predicted counselor’s ratings of attention problems (β=0.159, p =0.012). However, these effects were clarified by a significant interaction effect (β=0.192, p =0.033). Simple slopes revealed that the relationship between AL and maltreatment was significant for maltreated children (β= 0.244, p =0.005), but was not significant for nonmaltreated children (β= 0.024, p > 0.05, see Figure 2). Consistent with the previous analysis, these results indicate that increased physiological risk is associated with attention problems only for children who have experienced maltreatment. From these two analyses it is evident that only for maltreated children did higher AL predict increased attention difficulties and somatic complaints.
Figure 2.
Interaction of allostatic load and maltreatment status in predicting attention problems on the Teacher Report Form (TRF).
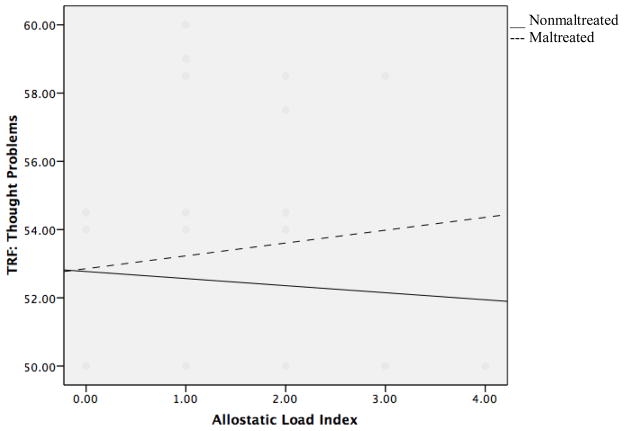
A different pattern of results was found when examining prediction of child thought problems. Neither maltreatment status nor AL independently predicted thought problems, (β=0.018, p>0.05; β=0.041, p>0.05, respectively). However, the interaction between AL and maltreatment status was significantly related to thought problems (β=0.193, p=0.037). Simple slopes revealed that for maltreated children, the relationship between AL and thought problems was marginally significant (β=0.163, p =0.061), whereas for nonmaltreated children the relationship between AL and thought problems was nonsignificant (β= −0.104, p >0.05, see Figure 3). Therefore, higher AL was marginally associated with greater thought problems in maltreated children, whereas no effect of AL was observed for nonmaltreated children.
Figure 3.
Interaction of allostatic load and maltreatment status in predicting thought problems on the Teacher Report Form (TRF).
Maltreatment, Allostatic Load Indicators, and Self-Reported Depressive Symptoms
We also sought to examine the influence of allostatic load and its indicators in predicting child-reported symptoms of depression. First, a regression analysis was performed to determine the relationship between AL and CDI scores. There was not a significant relationship between greater AL and child-rated depressive symptoms (β= 0.108, p=0.087). However, an ANCOVA revealed that there was a significant relationship between maltreatment status and CDI, F(1, 234) = 4.853, p=0.029.
Although the overall influence of AL did not contribute to prediction of depressive symptoms, we considered components of the AL composite to examine potential effects of the individual AL biomarkers. In these analyses to predict CDI scores, individual risk factors, maltreatment status, and their interactions, were examined within additional ANCOVA analyses with child age and gender entered as covariates.
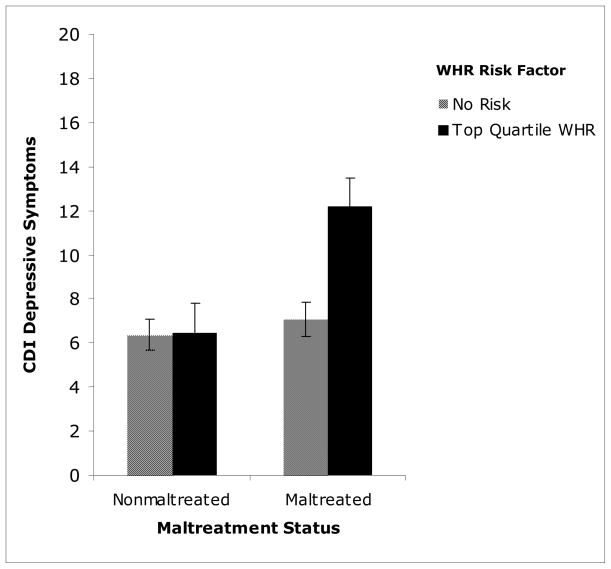
WHR risk and maltreatment status were first examined in relation to CDI scores. In this analysis, children within the top quartile of the sample for WHR were designated as at-risk. Both maltreatment status and WHR risk were significantly related to CDI, though the association between maltreatment and CDI was stronger than that of AL, F(1, 224) = 9.677, p = 0.002; F(1, 224) = 5.415, p=0.021, respectively. Additionally, the interaction between WHR risk and maltreatment status was significantly related to scores on the CDI, F(1, 224) = 6.111, p = 0.014. Follow-up analyses indicated that for nonmaltreated children, the relationship between WHR risk and depressive symptoms was not significant, F(1, 100) = 0.003, p > 0.05. However, for maltreated children, there was a significant relationship between WHR risk and depressive symptoms, F(1, 122) = 11.898, p = 0.001, see Figure 4. Therefore, maltreated children with WHR risk endorsed significantly higher depressive symptoms than maltreated children without this risk factor, as well as both nonmaltreated children with and without this risk factor (p < 0.0001, p<0.0001, respectively). Although maltreatment status itself did not predict significantly greater WHR, these findings suggest that maltreated children who have this biomarker of central adiposity are the most vulnerable to experiencing heightened depressive symptoms.
Figure 4.
Interaction of waist-hip ratio (WHR) and maltreatment status in predicting child-endorsed depressive symptoms on the Child Depressive Inventory (CDI).
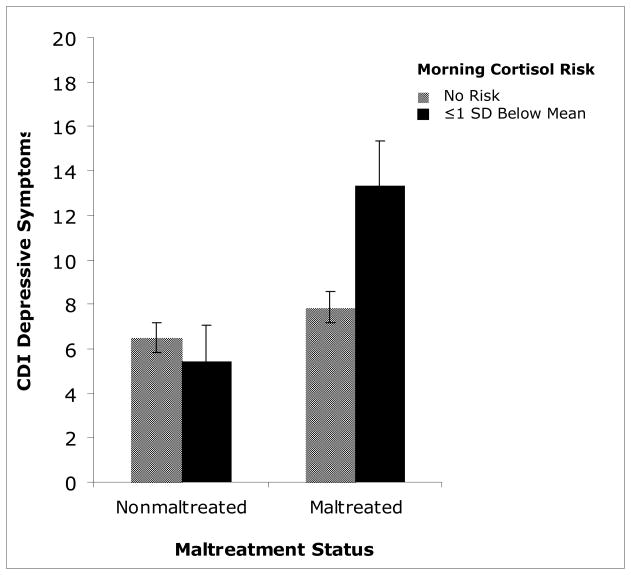
Another allostatic load indicator, low morning cortisol, was examined in an ANCOVA to predict depressive symptoms. The morning cortisol risk variable was scored dichotomously, depending on whether or not cortisol was −1SD below the sample mean. This variable was examined in relation to maltreatment status in predicting scores on the CDI. Maltreatment status was significantly related to child-endorsed depressive symptoms, F(1, 229) = 11.35, p=0.001, whereas low morning cortisol was not significantly associated with depression, F(1, 229) = 2.984, p>0.05. However, the interaction between the cortisol risk factor and maltreatment status was significant, F(1, 229) = 5.391, p=0.021. Examination of the interaction effect revealed that for maltreated children, membership within the low cortisol risk group was significantly related to higher scores on the CDI, F(1, 125) = 6.693, p=0.011. This relationship was not significant for nonmaltreated children, F(1, 102) = 0.368, p>0.05. Therefore, maltreated children with low morning cortisol endorsed significantly greater depressive symptoms compared to maltreated children without this risk factor (p = 0.006), as well as nonmaltreated children with and without this biomarker (p = 0.003, p = 0.001). This analysis indicates that low morning cortisol was associated with increased depressive symptom endorsement only among maltreated children (see figure 5).
Figure 5.
Interaction of low morning cortisol risk and maltreatment status in predicting child-endorsed depressive symptoms on the Child Depressive Inventory (CDI).
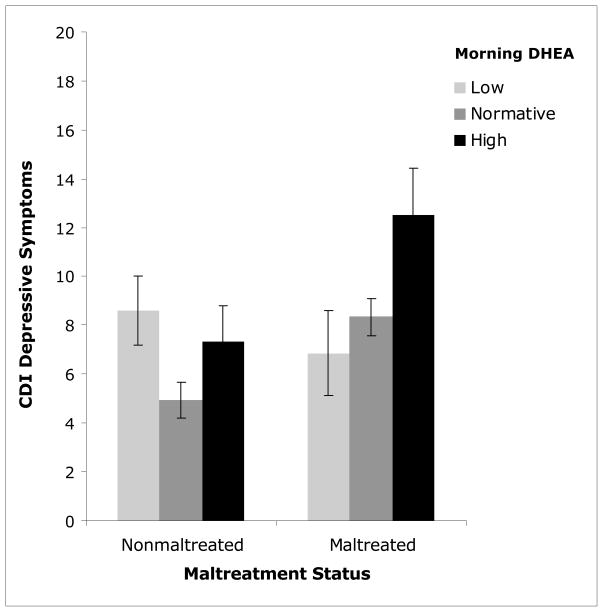
A final AL indicator, variation in morning DHEA, was also examined for its role in influencing depressive symptoms. Morning DHEA scores were differentiated into three categories based on the sample distribution. These categories were coded as “high” scores (≥ +1 SD), “low” scores (≥ −1 SD), and “normative” score (> −1 SD and < +1 SD). Morning DHEA level, maltreatment status, and their interaction, were entered into an ANCOVA predicting CDI scores. Morning DHEA was significantly related to CDI scores, F(1, 224) = 6.072, p=0.003, but maltreatment status was not F(1, 224) = 1.838, p>0.05. However, the interaction between morning DHEA and maltreatment status significantly predicted child depressive symptoms, F(2, 224) = 4.594, p=0.011. These analyses indicated that the relationship between morning DHEA and child depressive symptoms was significant for maltreated, F[2, 123] = 6.598, p=0.002, and nonmaltreated children, F[2, 99] = 3.852, p=0.025, see Figure 6. However, the pattern of relations varied for maltreated and nonmaltreated children. Among nonmaltreated children, those with low morning DHEA and those with high morning DHEA endorsed significantly greater symptoms of depression compared to nonmaltreated children in the normative group (p=0.024; p= 0.041, respectively). In contrast, for maltreated children, those with high morning DHEA endorsed significantly greater depressive symptoms compared to maltreated children with low and normative DHEA (p<0.0001; p=0.004, respectively). It is important to note that the group of maltreated children with high DHEA endorsed significantly greater depressive symptoms than children in all other groups, regardless of maltreatment status or morning DHEA risk (see Figure 6). These analyses implicate high morning DHEA as a significant risk factor for depression in maltreated children, and both high and low morning DHEA as potentially problematic in nonmaltreated children.
Figure 6.
Interaction of morning DHEA and maltreatment status in predicting child- endorsed depressive symptoms on the Child Depressive Inventory (CDI).
Discussion
The current investigation examined the influence of allostatic load on health and psychological functioning among maltreated and nonmaltreated children from low-income families. We sought to determine how early biomarkers of allostatic overload in childhood could presage long-term risk for negative health outcomes. A multidimensional composite of allostatic load was generated. This measure was comprised of primary mediators of the neuroendocrine stress response, including indicators of cortisol and DHEA dysregulation, anthropometric measurements of excess adiposity, i.e., BMI, WHR, and a cardiovascular measure of potential hypertensive risk (cf. Juster et al., 2010). A tenet of allostatic load theory suggests that an accumulation of stress-compromised regulatory processes across multiple systems provides a better global index of the degree of allostatic overload that is operating than any one system measured in isolation.
Children from low-income families experience higher levels of environmental stress than their more advantaged peers and thus are more likely to evince physiological biomarkers of taxed allostasis (Chen, Cohen, & Miller, 2010; Evans et al., 2003, 2007; Lupien et al., 2001). All of the children in the current study were presumed to experience high levels of stress, given the low socioeconomic status neighborhoods in which they were living. Although not all children will respond to stressful environments in the same way, we expected that the likelihood of high allostatic load would be prominent in this low-income sample. Moreover, given the chronic exposure to stress experienced by maltreated children, we anticipated that maltreated children may be at even greater risk for high allostatic load. However, this broad group difference was not borne out; maltreated and nonmaltreated children did not significantly differ on the allostatic load composite. This lack of differences may suggest that our allostatic load composite did not adequately capture the diversity of ways that allostatic processes may be disrupted in maltreated children (cf. Cicchetti & Rogosch, 2001a). Alternatively, it may emphasize the importance of both allostatic load and maltreatment in contributing to adverse health outcomes.
We found that maltreatment and allostatic load jointly predict psychopathology and health difficulties in children. In particular, we we found that both allostatic load and maltreatment contributed independently. Thus, as their levels of allostatic load increased, low-income children, in general, had more health problems, treatment, and interventions. Child maltreatment added to the degree of health problems and treatment usage beyond the level accounted for by the allostatic load composite. Accordingly, the children who demonstrated the highest levels of health problems were those who evinced high levels of allostatic load and who had been maltreated.
This pattern of influences extended to broadband indicators of child psychopathology as reported by camp counselors on the TRF. Higher levels of allostatic load predicted higher levels of total behavior problems, internalizing symptoms, and externalizing symptoms. The presence of child maltreatment added significantly to each of these predictions. Thus, global assessments of the range of child psychopathology, as ascertained by independent adult observers, were predicted by maltreatment and collective aspects of physiological dysregulation. As with our assessment of health functioning, psychological functioning was most compromised for maltreated children with high levels of allostatic load.
Similar additive effects of allostatic load and maltreatment were observed for three narrowband TRF scales, including social, delinquent, and aggressive behavior problems. Interestingly, a common theme of these problem domains likely involves difficulties in social relations. In particular, aspects of bully-victim relations (Shields & Cicchetti, 2001) may be operating. Children with high allostatic load are more likely to be overweight and obese, and such children may experience more teasing and victimization from peers. Coupled with aggressive and rule-violating behavior, very large children may be intimidating and engage in bullying behavior. Dysregulation of neuroendocrine stress systems also may detract from children’s abilities to cope with conflict in social interactions. The effects of child maltreatment on problematic social behavior with peers and conduct disturbances have been extensively documented (Dodge, Pettit, & Bates, 1990; Rogosch & Cicchetti, 1994). Moreover, the relational dysfunction that children experience in abusing and neglecting families contributes to insecure internal working models of self and others, which generate negative relationship expectations and social cognitive biases (Dodge, Pettit, & Bates, 1994; Rogosch, Cicchetti, & Aber, 1995). Thus, allostatic load and child maltreatment, through independent processes, both are contributing in an additive manner to social and externalizing difficulties in low income children.
Moderation effects between allostatic load and child maltreatment also were found, and in these analyses, the impact of allostatic load was apparent only among maltreated children. For example, the one domain of TRF internalizing problems where effects were observed was for somatic complaints. Although both allostatic load and maltreatment made significant contributions to prediction, the interaction effect indicated that high allostatic load was related to somatic complaints only among maltreated children. These results in part corroborate the additive effects of allostatic load and maltreatment on health as reported by mothers. However, with respect to counselor observations of the children, only the maltreated children with high allostatic load exhibited behavior indicative of somatic distress and preoccupation.
Interestingly, the two TRF scales most closely associated with neurocognitive difficulties also showed interaction effects. Allostatic load and maltreatment made independent contributions to attention difficulties; however, a significant interaction effect revealed that high allostatic load was related to attention problems only among maltreated children. While attentional deficits and ADHD are a substantial clinical problem in childhood (Nigg, Hinshaw, & Huang-Pollock (2006), dysregulation of attention also underlies diverse forms of psychopathology (Posner, 2004, 2008). High allostatic load poses risk for the attentional control, and maltreated children appear to be most vulnerable.
Thought problems in an unselected, nonclinical sample are rare. However, given the high risk nature of the children in our sample, mild indications of thought disturbance are important in terms of identifying potential prodromal signs of risk for psychosis (McClellan, 2011). In our findings, an interaction between allostatic load and maltreatment indicated that higher thought problem scores occurred only among maltreated children with high allostatic load. A recent investigation from our laboratory (Toth, Stronach, Rogosch, Caplan, & Cicchetti, 2011) has similarly shown evidence for elevated rates of thought disorder symptoms among maltreated children. It may be that the effects of high stress as indexed by allostatic load may contribute to activation of a diathesis for psychosis among some children who have been subjected to maltreatment. Tracking the developmental progression of these children into late adolescence and young adulthood is crucial in order to determine if the early signs of vulnerability presage emergence of severe disorders.
Our investigation also examined the role of allostatic load and child maltreatment in predicting children’s self-reported depressive symptoms. The findings indicated a significant influence of child maltreatment, but allostatic load did not independently contribute. However, we did observe a number of important interactive effects of components of the allostatic load composite and child maltreatment. In particular, WHR moderated the effect of maltreatment on depressive symptoms, such that higher depressed symptoms were reported only by maltreated children who were in the top quartile of WHR. Thus, children who were maltreated and obese were the most vulnerable to depressive symptoms, whereas nonmaltreated children showed no difference in depressive symptom levels based on WHR risk. This finding bears similarity with the additive effects of allostatic load and maltreatment on observed social problems. Maltreated children who are obese may react more negatively to peer victimization and rejection, and, given more negative representational models of the self, consequently, evince greater tendencies for negative body image, poor self appraisals, low self-esteem, and dysphoric affect. This finding is consistent with other indications that higher BMI in children predicts increased internalizing rather than externalizing symptoms (Bradley, Houts, Nader, O’Brien, Belsky, et al., 2008). We found that both maltreatment and AL independently predicted children’s aggressive symptoms, delinquent behavior, and social problems. In contrast, larger-sized children exposed to the chronic stressor of maltreatment endorse increased depressive symptoms. It is important to better understand the distinction between trajectories toward internalizing and externalizing symptoms in highly stressed, physiologically taxed children, given the known impact of these symptoms on adaptive development.
We also observed interaction effects of stress hormones and maltreatment on children’s depressive symptoms. In particular, child maltreatment was related to high depressive symptoms for children with low morning cortisol. No effect was observed for maltreated children without low cortisol or for nonmaltreated children irrespective of cortisol level. In contrast, when we considered variation in DHEA, a major antagonist of cortisol, we also observed interactional hormonal effects, such that maltreated children with high morning DHEA evinced the highest levels of depressive symptoms, relative to all other children, including maltreated children with lower levels of DHEA and all nonmaltreated children, irrespective of DHEA level. When nonmaltreated alone where considered, we also noted variation in depressive symptoms based on DHEA levels, with children having both very high and very low levels of DHEA evincing relatively higher depressive symptoms.
These neuroendocrine findings suggest that the extremes of neuroendocrine regulation differentiate maltreated children with high levels of depressive symptoms. We focused on low morning cortisol as a biomarker of high allostatic load, given the progressive down-regulation of the HPA axis after chronic overarousal due to stress (Miller, Chen, & Zhou, 2007). In adult women, a history of abuse is associated with a blunted cortisol response to social challenge (Carpenter, Shattuck, Tyrka, Ceracioti, & Price, 2011). Atypical HPA axis functioning has been demonstrated uniquely in women with major depressive disorder and a history of childhood maltreatment (Heim et al., 2008). In our laboratory, we have found a flattening of diurnal cortisol rhythm among children with high internalizing/depressive symptoms and early abuse experiences (Cicchetti, Rogosch, Gunnar, & Toth, 2010). DHEA in relation to depression in children has received little attention. Thus, our finding of high depressive symptoms among maltreated children with very high DHEA is informative. We also have shown previously that high morning DHEA levels are related to low resilience strivings among maltreated children (Cicchetti & Rogosch, 2007). Taken together, evidence for atypical regulation in two primary mediators of allostatic load among maltreated children who are depressed bodes poorly for the long term physical and mental health outcomes of these children.
Overall, our findings provide an important demonstration of how physiological biomarkers indicative of allostatic load collectively contribute to higher levels of health problems as reported by mothers, diverse aspects of child psychological symptomatology as observed by adult raters, and depressive symptoms as reported by children. Within this context, child maltreatment consistently and independently contributed to worse outcomes or the impact of allostatic load and its biomarkers was found to operate only among maltreated children. Thus, both maltreated and nonmaltreated appear to bear the burden of high allostatic load in response to high environmental stress. However, the independence of the allostatic load and maltreatment effects is noteworthy. As a result, at this juncture it cannot be concluded that the impact of maltreatment operates solely through allostatic load mechanisms, given the lack of relations between maltreatment and allostatic load. It may be that the biomarkers that were included in our allostatic load composite did not fully capture all of the ways in which the chronic stress associated with maltreatment may uniquely impact stress-sensitive systems. Alternatively, other processes resulting from maltreatment may operate independently on psychological and physical functioning and cannot be subsumed adequately under an allostatic load framework. For example, one possibility may be the effects of maltreatment on jeopardizing a secure infant attachment organization, and consequent negative representational models that influence subsequent social behavior, interpersonal relationships, and self system processes, and thereby the course of adaptive functioning (Cicchetti, 1989). These processes may provide an overlay on top of the ill effects of high environmental stress resulting in high allostatic load. Our moderation findings also suggest that such psychological effects of maltreatment and trauma may provide a context for greater vulnerability to the negative effects of allostatic load in maltreated children.
Although this is the first study to examine a composite of allostatic load in maltreated children, the research has a number of limitations. The investigation was cross-sectional and thus did not allow for examination of developmental trajectories of allostatic load and emerging associations with health functioning. The study did not include children from families of middle SES backgrounds limiting our ability to demonstrate the unique effects of SES on health outcomes, given the uniformly low SES level of the sample. Demonstrations of the effects of allostatic load may have been more striking with larger variation in the level of general exposure to environmental stress via SES, and the effects of maltreatment may have been further accentuated. Additionally, the strength of the allostatic load index may have benefitted from the addition of other domains, including metabolic indices and immune biomarkers.
Currently, we are striving to address some of these limitations in our laboratory. The children are participating in a longitudinal investigation, which will provide more detail on stability and change in the allostatic load parameters as development proceeds. We also are currently collecting C-reactive protein as a biomarker of immune functioning (i.e., inflammation) (cf. Danese, Moffitt, Pariante, Ambler, Poulton, et al., 2008). Additionally, genetic samples are being obtained in order for us to evaluate genetic influences on neuroendocrine regulation and symptomatology, particularly gene by environment interactions in the context of child maltreatment.
The findings of this investigation have important implications for prevention to promote physical and mental health in the lives of low-income and maltreated children. Heckman (2006) has emphasized the economic and humanitarian value to society of reducing the impact of adverse early family environments on the developing child in order to ameliorate the diminished cognitive capacities ensuing from deprivation and high stress. Allostatic load likely is a major influence on these unrealized potentials, through the negative sequelae of stress occurring in early sensitive periods of brain development and progressive accumulation of liabilities that extend to other stress sensitive systems (Cicchetti, 2002; Shonkoff et al., 2008). While broad-based efforts to reduce the impact of poverty are crucial, targeted interventions to advance cognitive functioning and self-regulatory capacities (Blair & Diamond, 2008; Diamond, 2007) are vital for enhancing self-righting processes and promoting resilience (Cicchetti & Rogosch, 1997, 2007; Luthar & Cicchetti, 2000). For the most vulnerable children in low-SES environments, concerted efforts to prevent the occurrence of child abuse and neglect are of fundamental importance. Early intensive interventions in child maltreatment to establish more sensitive and nurturant parenting, secure attachment, and neurobiological reorganization in maltreated youngsters (Cicchetti, Rogosch, & Toth, 2006; Cicchetti, Rogosch, Toth, & Sturge-Apple, 2011) hold great promise for instilling adaptive developmental trajectories. Consolidated, multi-systemic approaches beginning early in development are necessary to reduce environmental stress exposure, child maltreatment, and allostatic overload in order to improve physical and mental health across the lifespan.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (MH083979) and the Spunk Fund, Inc.
References
- Achenbach T. Manual for the Teacher Report Form and 1991 profile. Burlington: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Anda RF, Brown DW, Felitti VJ, Bremner D, Dube SR, Giles WH. Adverse childhood experiences and prescribed psychotropic medications in adults. American Journal of Preventive Medicine. 2007;32:389–394. doi: 10.1016/j.amepre.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: the interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Abex; 1993. pp. 7–73. [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Development. 1998;69:1171–1197. [PubMed] [Google Scholar]
- Bradley RH, Houts R, Nader PR, O’Brien M, Belsky J, Crosnoe R. The relationship between body mass index and behavior in children. Journal of Pediatrics. 2008;153:629–634. doi: 10.1016/j.jpeds.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effects of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. How research on child maltreatment has informed the study of child development: Perspectives from developmental psychopathology. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. New York: Cambridge University Press; 1989. pp. 377–431. [Google Scholar]
- Cicchetti D. How a child builds a brain: Insights from normality and psychopathology. In: Hartup W, Weinberg R, editors. Minnesota symposia on child psychology: Child psychology in retrospect and prospect. Vol. 32. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 23–71. [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective [Special Article] World Psychiatry. 2010;9:1–10. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children’s development. Psychiatry. 1993;56:96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. Vol. 2. Hillsdale, NJ: Erlbaum; 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Rogosch FA. The role of self-organization in the promotion of resilience in maltreated children. Development and Psychopathology. 1997;9:799–817. doi: 10.1017/s0954579497001442. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001a;13:677–694. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology upon neuroendocrine functioning. Development and Psychopathology. 2001b;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multi-level perspective. Development and Psychopathology. 2007;19(3):787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. Child Development. 2010;25:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of CRHR1, 5-HTTLPR, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011;23 doi: 10.1017/S0954579411000599. [this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and 5-HTT and monoamine oxidase A polymorphisms: Depressive symptomatology among adolescents from low-socioeconomic status backgrounds. Development and Psychopathology. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18:623–650. doi: 10.1017/s0954579406060329. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal maltreatment classification interview. Rochester, NY: Mt. Hope Family Center; 2003. Unpublished measure. [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2. Vol. 3. New York: Wiley; 2006. pp. 129–201. Risk, disorder, and adaptation. [Google Scholar]
- Clark DB, Thatcher DL, Martin CS. Child abuse and other traumatic experiences, alcohol use disorders, and health problems in adolescence and young adulthood. Journal of Pediatric Psychology. 2010;35:499–510. doi: 10.1093/jpepsy/jsp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smaile E. Child abuse and neglect and development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–999. [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of child maltreatment. Archives of General Psychiatry. 2008;65:409–416. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Preschool program improves cognitive control. Science. 2007;318:3187–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Development. 1994;65:649–665. [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz H, Pitts SC, Litrownik AJ, Cox CE, Runyan D, Black MM. Defining child neglect based on child protective services data. Child Abuse & Neglect. 2005;29:493–511. doi: 10.1016/j.chiabu.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Edwards V, Holden G, Felitti V, Anda R. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the Adverse Childhood Experiences study. American Journal of Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- English DJ, Upadhyaya MP, Litrownik AJ, Marshall JM, Runyan DK, Graham JC, et al. Maltreatment’s wake: The relationship of maltreatment dimensions to child outcomes. Child Abuse & Neglect. 2005;29:597–619. doi: 10.1016/j.chiabu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychopathology. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kin P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescence. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: Preliminary findings. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents, and adults. Psychoneuroendocrinology. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Green GG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM-IV disorders. American Journal of Psychiatry. 2010:67. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney M. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews: Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman JJ. Skill formation and economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, et al. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout lifespan development. Development and Psychopathology. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Review. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. Journal of Abnormal Psychology. 2007;116:176–187. doi: 10.1037/0021-843X.116.1.176. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott Childhood sexual abuse, stressful life events, and risk for major depression in women. Psychological Medicine. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory: A self-rated depression scale for school-aged youngsters. Pittsburgh: University of Pittsburgh; 1982. Unpublished manuscript. [Google Scholar]
- Kovacs M. Children’s Depression Inventory (CDI) Toronto: Multi-Health Systems Inc; 2004. [Google Scholar]
- Knutson JF, Taber SM, Murray AJ, Valles NL, Koeppl G. The role of care neglect and supervisory neglect in childhood obesity in a disadvantaged sample. Journal of Pediatric Psychology. 2010;35:523–532. doi: 10.1093/jpepsy/jsp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Hegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistic. Vital Health Statistics. 2002;11(246) [PubMed] [Google Scholar]
- Lanier P, Jonson-Reid M, Stahlschmidt MJ, Drake B, Constantino J. Child maltreatment and pediatric health outcomes: A longitudinal study of low-income children. Journal of Pediatric Psychology. 2010;35:511–522. doi: 10.1093/jpepsy/jsp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nature Reviews: Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, et al. Beyond the stress concept: Allostatic load - a developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. 2. Vol. 2. New York: Wiley; 2006. pp. 578–628. Developmental Neuroscience. [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29:425–43. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D. The construct of resilience: Implications for intervention and social policy. Development and Psychopathology. 2000;12:857–885. doi: 10.1017/s0954579400004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29:425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development and Psychopathology. 1994;6:121–143. [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- McClellan J. Clinically relevant phenomenology: The nature of psychosis. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:642–644. doi: 10.1016/j.jaac.2011.04.010. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsycho-pharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: The good and bad sides of the response to stress. Metabolism. 2002;51(6, Suppl 1):2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedice. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Hinshaw SP, Huang-Pollock C. Disorders of attention and impulse regulation. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2. Vol. 3. New York: Wiley; 2006. pp. 358–403. Risk, disorder, and adaptation. [Google Scholar]
- Posner MI. In: Cognitive neuroscience of attention. Posner MI, editor. New York: Guilford Press; 2004. [Google Scholar]
- Posner MI. The neuropsychology of attention. In: Marien P, Abutalebi J, editors. Neuropsychological research: A review. New York: Psychology Press; 2008. pp. 331–348. [Google Scholar]
- Putnam FW. Ten-year research update review: Child sexual abuse. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Riley AW, Forrest CB, Starfield BS, Rebok GW, Robertson JA, Green BF. The parent report form of the CHIP-Child Edition: Reliability and Validity. Medical Care. 2004;42:210–220. doi: 10.1097/01.mlr.0000114909.33878.ca. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D. Social Development. 1994. Illustrating the interface of family and peer relations through the study of child maltreatment. [Google Scholar]
- Rogosch FA, Cicchetti D, Aber JL. The role of child maltreatment in early deviations in cognitive and affective processing abilities and later peer relationship problems. Development and Psychopathology. 1995;7:591–609. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, et al. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress, Executive Summary. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30:349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Shonkoff JB, Boyce WT, McEwen Neuroscience, molecular biology, and the roots of childrenhood health disparities. Building a new framework for health promotion and disease prevention. Journal of the America Medical Association. 2008;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Smith CA, Thornberry T. The relationship between child maltreatment and adolescent involvement in delinquency. Criminology. 1995;33:451–481. [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortsol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Toth SL, Manly JT, Cicchetti D. Child maltreatment and vulnerability to depression. Development and Psychopathology. 1992;4:97–112. [Google Scholar]
- Toth SL, Pickreign Stronach E, Rogosch FA, Caplan R, Cicchetti D. Illogical thinking and thought disorder in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:659–668. doi: 10.1016/j.jaac.2011.03.002. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, National Institutes of Health. NIH Publication No 05–5267. Bethesda, MD: National Heart, Lung, and Blood Institute; 2005. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Revised May 2005. [Google Scholar]
- von Stumm S, Deary IJ, Kivimaki M, Jokelya M, Clark H, Batty GD. Childhood behavior problems and health at mid-life: 35-year follow-up of a Scottish birth cohort. Journal of Child Psychology, and Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02373.x. pending’. [DOI] [PubMed] [Google Scholar]
- Widom CS. Post-traumatic stress disorder in about and neglected children grown up. American Journal of Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Internal Journal of Obesity. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]