Abstract
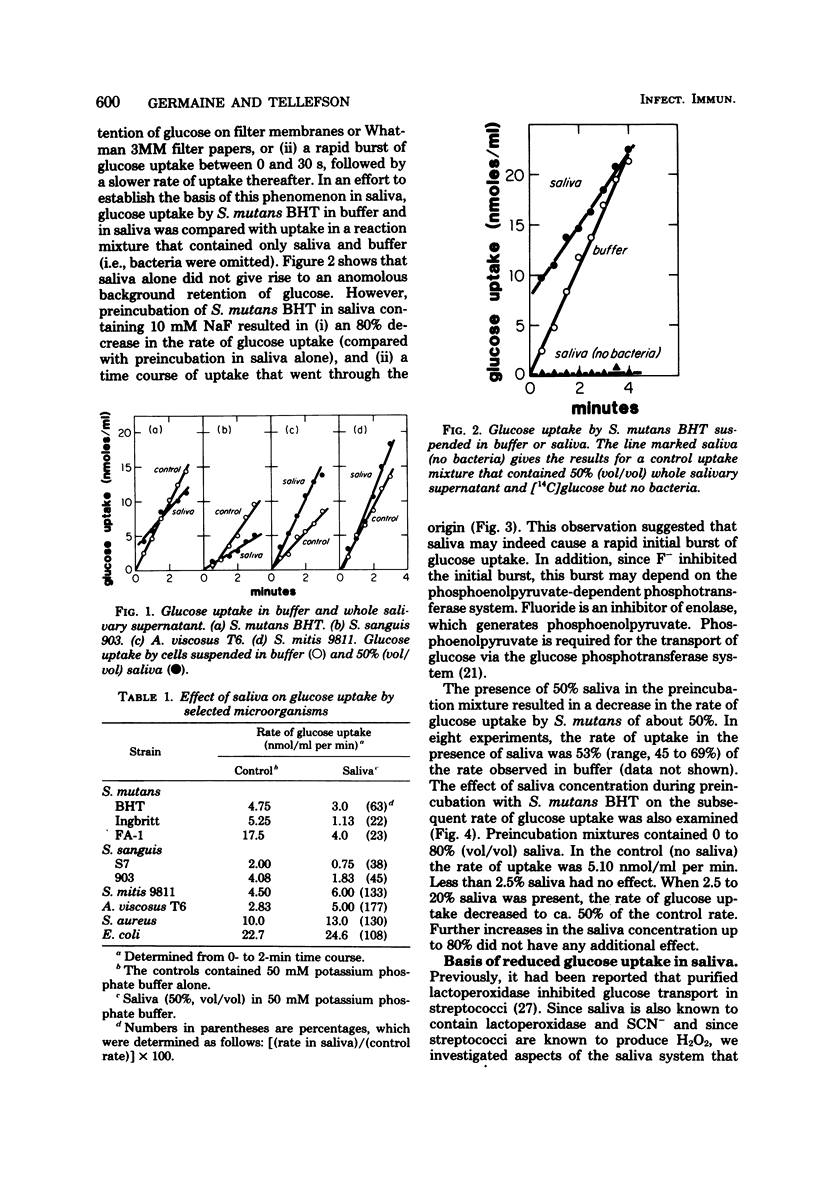
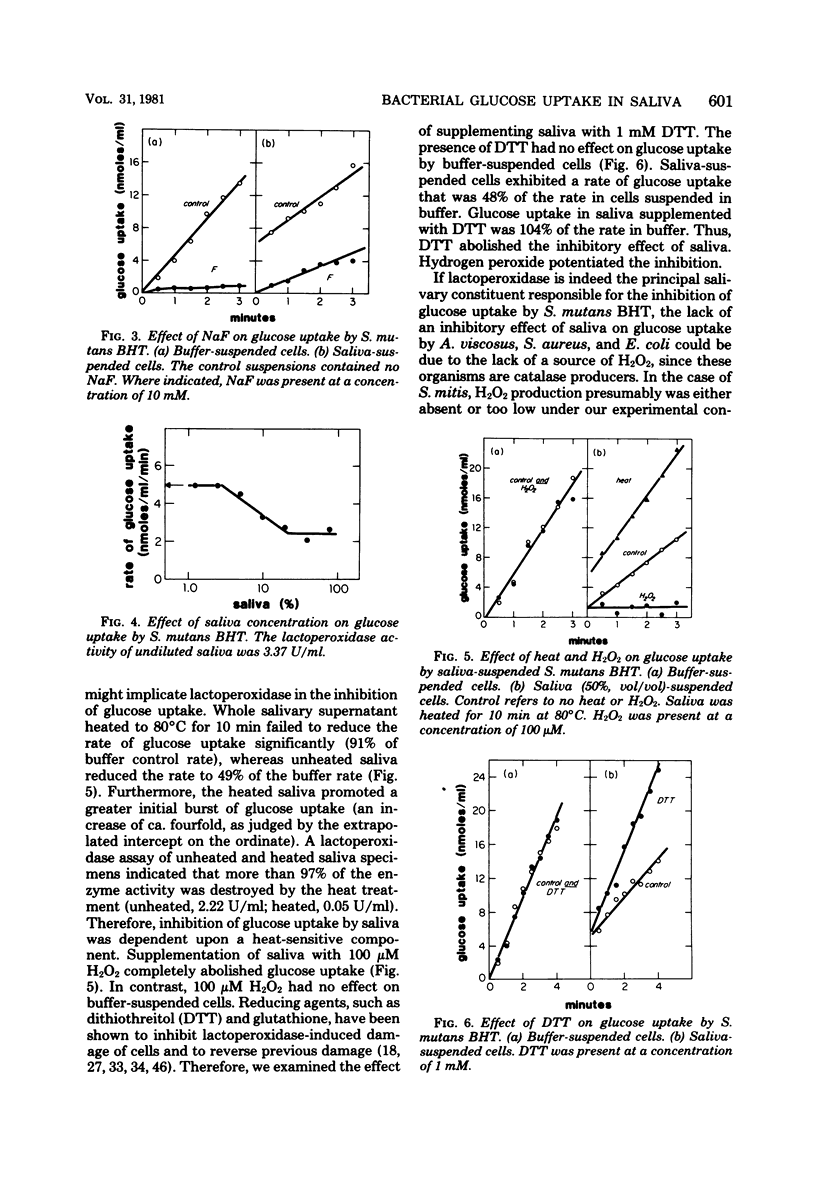
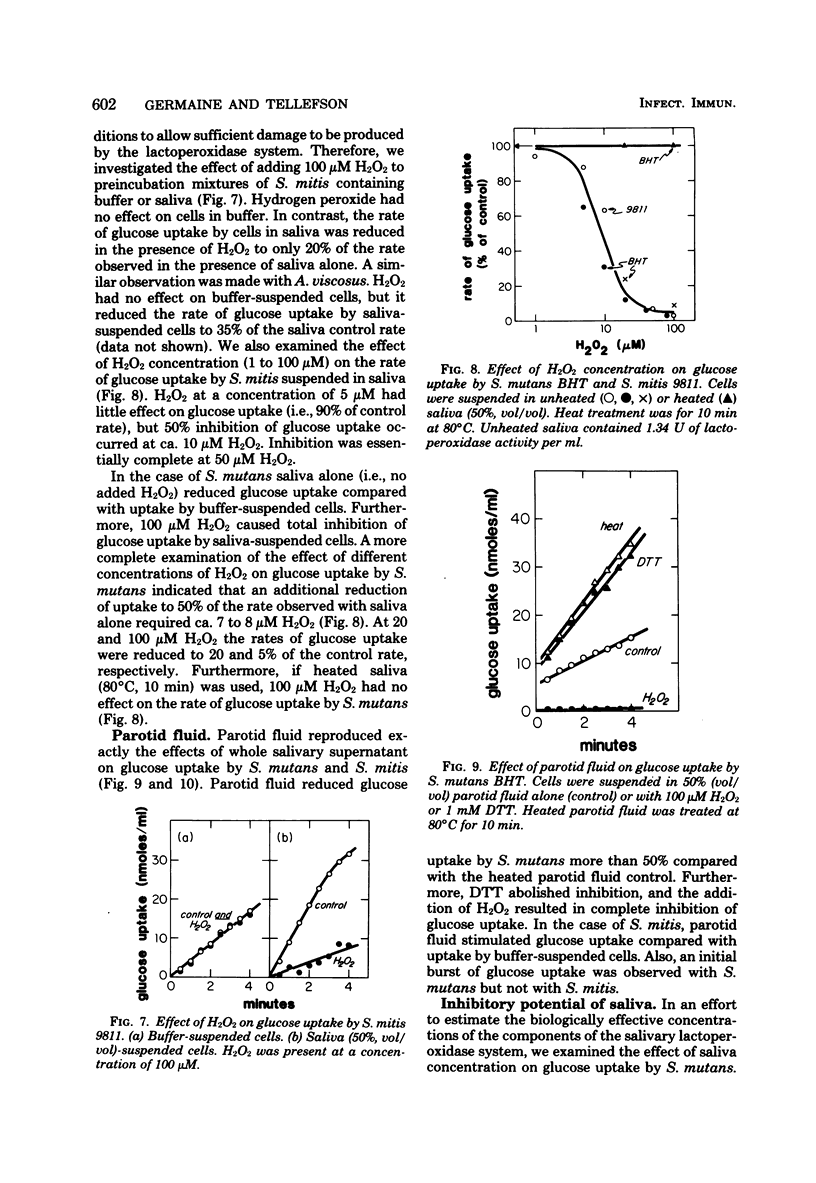
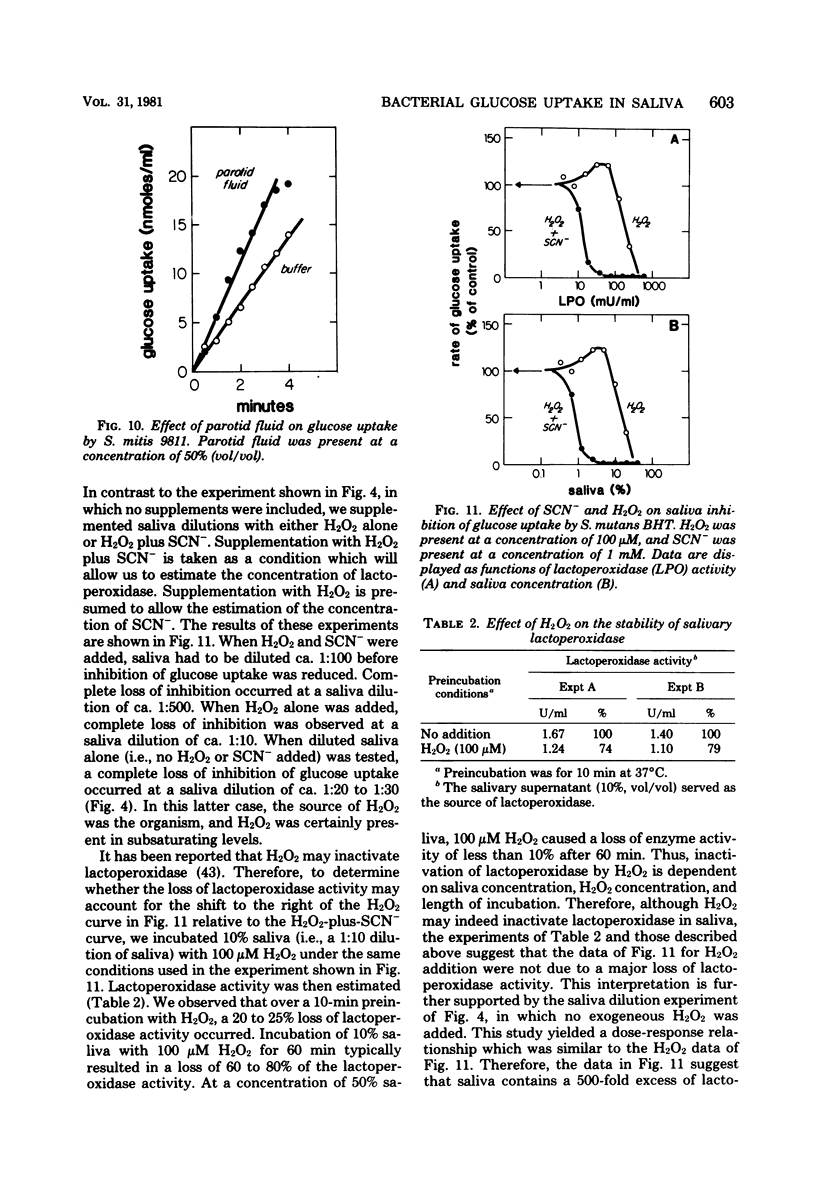
We examined the effects of human whole salivary supernatant and parotid fluid on glucose uptake by Streptococcus mutans, Streptococcus sanguis, Streptococcus mitis, Actinomyces viscosus, Staphylococcus aureus, and Escherichia coli. The following three effects of saliva were observed: (i) inhibition of glucose uptake (S. mutans, S. sanguis), (ii) promotion of a transient, rapid (0 to 30 s) burst of glucose uptake (S. mutans, S. sanguis), and (iii) enhancement of glucose uptake (S. mitis, A. viscosus, S. aureus, E. coli). We observed no differences between the effects of whole salivary supernatant and the effects of parotid fluid. Heat treatment (80°C, 10 min) of saliva or the addition of dithiothreitol abolished inhibition of glucose uptake. Supplementation of saliva with H2O2 potentiated inhibition of glucose uptake. S. mitis and A. viscosus, which were stimulated by saliva alone, were inhibited by H2O2-supplemented saliva; 50% inhibition of glucose uptake by S. mutans and S. mitis required ca. 10 μM H2O2 in 50% (vol/vol) saliva. Loss of the inhibitory action of saliva occurred at about 5% (vol/vol) saliva. Supplementation of saliva dilutions with SCN− and H2O2 extended the inhibitory activity to solutions containing ca. 0.2% (vol/vol) saliva. We suggest that the salivary lactoperoxidase-SCN−-H2O2 system is responsible for the inhibitory activity of saliva reported here. Furthermore, we concluded that lactoperoxidase and SCN− are present in saliva specimens in concentrations that exceed minimal inhibitory levels by factors of ca. 500 and 10 to 20, respectively. The resistance of A. viscosus, S. aureus, and E. coli to the inhibitory potential of saliva alone was probably due to the production of catalase by these organisms. The resistance of S. mitis may have been due to special effects of saliva on H2O2 accumulation by this organism compared with S. mutans and S. sanguis. The basis of saliva-dependent enhancement of glucose uptake and the basis of promotion of a transient, rapid burst of glucose uptake are unknown. The role of the salivary lactoperoxidase-SCN−-H2O2 system in the oral microbial ecosystem is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L., Morrison M. Lactoperoxidase-catalyzed incorporation of thiocyanate ion into a protein substrate. Biochemistry. 1977 Oct 18;16(21):4611–4615. doi: 10.1021/bi00640a013. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Bonilla C. A. Rapid isolation of basic proteins and polypeptides from salivary gland secretions by adsorption chromatography on polyacrylamide gel. Anal Biochem. 1969 Dec;32(3):522–529. doi: 10.1016/s0003-2697(69)80019-9. [DOI] [PubMed] [Google Scholar]
- Bowen W. H. Defense mechanisms in the mouth and their possible role in the prevention of dental caries: a review. J Oral Pathol. 1974;3(6):266–278. doi: 10.1111/j.1600-0714.1974.tb01721.x. [DOI] [PubMed] [Google Scholar]
- DOGON I. L., KERR A. C., AMDUR B. H. Characterization of an antibacterial factor in human parotid secretions, active against Lactobacillus casei. Arch Oral Biol. 1962 Jan-Feb;7:81–90. doi: 10.1016/0003-9969(62)90051-1. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Murrell W. G. Effect of dipicolinic acid on the ultraviolet radiation resistance of Bacillus cereus spores. Photochem Photobiol. 1973 Mar;17(3):145–153. doi: 10.1111/j.1751-1097.1973.tb06344.x. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Simple and rapid procedure for the selective removal of lysozyme from human saliva. Infect Immun. 1979 Dec;26(3):991–995. doi: 10.1128/iai.26.3.991-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gothefors L., Marklund S. Lactoperoxidase activity in human milk and in saliva of newborn infants. Infect Immun. 1975 Jun;11(6):1210–1215. doi: 10.1128/iai.11.6.1210-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon C. B., Klebanoff S. J. A peroxidase-mediated, streptococcus mitis-dependent antimicrobial system in saliva. J Exp Med. 1973 Feb 1;137(2):438–450. doi: 10.1084/jem.137.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973 Mar;18(3):423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., NICKERSON J. F., PERRY W. I., WALKER A. P. Peroxide and peroxidogenic bacteria in human saliva. J Bacteriol. 1957 Jun;73(6):727–735. doi: 10.1128/jb.73.6.727-735.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- MacFarlane T. W., Mason D. K. Changes in the oral flora in Sjögren's syndrome. J Clin Pathol. 1974 May;27(5):416–419. doi: 10.1136/jcp.27.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D., Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;(8):25–47. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M., Arnold R. Lactoperoxidase binding to streptococci. Infect Immun. 1979 Jul;25(1):304–309. doi: 10.1128/iai.25.1.304-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M. Enzyme activity of salivary lactoperoxidase adsorbed to human enamel. Infect Immun. 1977 Jul;17(1):112–116. doi: 10.1128/iai.17.1.112-116.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B., Marshall V. M., BjörckL, Rosén C. G. Nonspecific bactericidal activity of the lactoperoxidases-thiocyanate-hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect Immun. 1976 Mar;13(3):800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONS N. S. Studies on the defense mechanisms of the mucous membranes with particular reference to the oral cavity. Oral Surg Oral Med Oral Pathol. 1952 May;5(5):513–526. doi: 10.1016/0030-4220(52)90231-4. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Harrowe D. J. Bacterial interference: effects of oral antibiotics on the normal throat flora and its ability to interfere with group A streptococci. Infect Immun. 1976 Mar;13(3):808–812. doi: 10.1128/iai.13.3.808-812.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Smith Q. T., Shapiro B. L., Hamilton M. J. Polyacrylamide gel patterns of parotid saliva proteins in Caucasoids and Amerindians. Arch Oral Biol. 1975 May-Jun;20(5-6):369–373. doi: 10.1016/0003-9969(75)90029-1. [DOI] [PubMed] [Google Scholar]
- Sprunt K., Redman W. Evidence suggesting importance of role of interbacterial inhibition in maintaining balance of normal flora. Ann Intern Med. 1968 Mar;68(3):579–590. doi: 10.7326/0003-4819-68-3-579. [DOI] [PubMed] [Google Scholar]
- Steele W. F., Morrison M. Antistreptococcal activity of lactoperoxidase. J Bacteriol. 1969 Feb;97(2):635–639. doi: 10.1128/jb.97.2.635-639.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J. C., Keller P. J. An electrophoretic analysis of the protein components of human parotid saliva. Arch Oral Biol. 1968 Oct;13(10):1213–1222. doi: 10.1016/0003-9969(68)90077-0. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Knuuttila M. L. Antibacterial effect of salivary peroxidases on a cariogenic strain of Streptococcus mutans. J Dent Res. 1977 Dec;56(12):1608–1613. doi: 10.1177/00220345770560123301. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Knuuttila M. L. The antibacterial action of the various components of the lactoperoxidase system on a cariogenic strain of Streptococcus mutans. J Dent Res. 1977 Dec;56(12):1603–1607. doi: 10.1177/00220345770560123201. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Valtakoski J., Knuuttila M. L. Antibacterial activity of lactoperoxidase adsorbed by human salivary sediment and hydroxyapatite. Caries Res. 1977;11(5):257–262. doi: 10.1159/000260277. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella G., Goudswaard J. Measurement of salivary lysozyme. J Dent Res. 1978 Feb;57(2):326–328. doi: 10.1177/00220345780570023001. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


