Abstract
Context:
Adipose tissue (AT) helps to regulate body fat partitioning and systemic lipid/glucose metabolism. We have recently reported lipid/glucose metabolism abnormalities and increased liver triglyceride content in an AT-selective transgenic model overexpressing ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1), the AdiposeENPP1-Tg mouse.
Objective:
The aim of the study was to test the translational hypothesis that AT-ENPP1 overexpression associates with AT dysfunction (changes in AT gene expression, plasma fatty acid, and adipokine levels), increased liver triglyceride deposition, and systemic insulin resistance in humans.
Design/Setting/Participants:
A total of 134 young normoglycemic men and women were subjected to body composition studies, hyperinsulinemic-euglycemic clamps, and AT needle biopsy. Twenty men also had liver/muscle nuclear magnetic resonance spectroscopy.
Main Outcome Measures:
Predetermined measures included AT expression of ENPP1 and other lipid metabolism/inflammation genes, plasma adipokines, and nonesterified fatty acid (NEFA) levels, liver/muscle triglyceride content, and the systemic glucose disposal rate.
Results:
After statistical adjustment for body fat content, increasing AT-ENPP1 was associated with up-regulation of genes involved in NEFA metabolism and inflammation, increased postabsorptive NEFA levels, decreased plasma adiponectin, increased liver triglyceride content, and systemic insulin resistance in men. In women, there were no changes in plasma adiponectin, NEFAs, or glucose disposal rate associated with increasing AT-ENPP1, despite increased expression of lipid metabolism and inflammation genes in AT.
Conclusions:
Increased AT-ENPP1 is associated with AT dysfunction, increased liver triglyceride deposition, and systemic insulin resistance in young normoglycemic men. These findings are concordant with the AdiposeENPP1-Tg phenotype and identify a potential target of therapy for health complications of AT dysfunction, including type 2 diabetes and cardiovascular disease.
Metabolic complications of obesity, including increased liver triglyceride content and insulin resistance, are increasingly prevalent and contribute to a heightened risk for both type 2 diabetes and cardiovascular disease (1–5), two major causes of morbidity and mortality in our population. Weight gain and excessive triglyceride storage in adipocytes are known to induce changes in adipose tissue (AT), such as adipocyte insulin resistance, decreased adiponectin production, and increased TNF-α and IL-6 production. These changes are characteristic of “adipose tissue dysfunction” or “adiposopathy” (6). It is increasingly clear that the weight gain threshold for the development of AT dysfunction and its systemic metabolic consequences varies widely in humans. It can occur with a mild increase in body weight, even within the nonobese range (7, 8); conversely, the threshold may not be reached even in the presence of obesity (9, 10). Therefore, a better understanding of the mechanisms of AT dysfunction would allow better identification of people at risk for systemic metabolic complications and lay the foundation for developing more effective strategies to prevent such complications as ectopic fat deposition and systemic insulin resistance. Along these lines, we have recently shown that ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) can impair adipocyte maturation and triglyceride storage in AT when demand for triglyceride storage is increased by a high-fat diet (11). ENPP1 is a type II transmembrane glycoprotein (targeted to the endoplasmic reticulum lumen with its C-terminal domain) that, when overexpressed in various cell types, is known to interact with the α-subunit of the insulin receptor and decrease activation of the β-subunit and subsequent downstream cellular insulin signaling (12, 13). Although increased ENPP1 expression has previously been linked to insulin resistance (14), we were the first to report that AT-specific ENPP1 overexpression in vivo (in the AdiposeENPP-Tg mouse model) recapitulates abnormalities typically found in the metabolic syndrome, including increased liver triglyceride content and abnormal glucose and fatty acid metabolism (11).
This study was designed to test the overall hypothesis that AT ENPP1 overexpression is associated with AT dysfunction, increased liver triglyceride content and systemic insulin resistance in humans, thus providing translational validity to our recent mechanistic findings in the AdiposeENPP1-Tg model (11). Based on our previous studies in that animal model, we elected to explore specific aspects of AT dysfunction, including the expression of genes involved in lipid metabolism (SREBP1c, ACSL1, CD36, HSL, LPL) and the inflammatory response (macrophage infiltration of AT, shown by CD68 and MAC1 gene expression), plasma concentrations of adipokines (leptin, adiponectin, IL-6, TNF-α) and a marker of systemic inflammation [high sensitivity C-reactive protein (hs-CRP)], and plasma concentrations of nonesterified fatty acid (NEFA).
Subjects and Methods
The Institutional Review Boards at both the University of Texas Southwestern Medical Center at Dallas (UTSW) and the University of Texas Medical Branch at Galveston (UTMB) approved the conduct of this study. A total of 134 subjects were enrolled by public advertisement, and informed consent was obtained from all participants. Subjects with diabetes mellitus or other endocrine disorders, coronary artery disease, or renal insufficiency were excluded. All studies were performed at the Clinical Research Center (CRC), following the protocol shown in schematic Supplemental Fig. 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Each subject was fed an isocaloric diet daily, calculated from his/her height, weight, and age, and containing 30% of calories from fat, 55% from carbohydrate, 15% from protein, and 300 mg cholesterol.
Body composition studies
Participants had anthropometric measurements (8) taken at the beginning of the 4-d CRC-based study, as shown in Supplemental Fig. 1. Body composition was determined using underwater weighing (8), and magnetic resonance imaging was used to measure intraabdominal (visceral) and abdominal sc AT mass, as previously described (15, 16).
Oral glucose tolerance testing (OGTT)
A standard OGTT with 75 g glucose (Tru-Glu100; Fisher Scientific, Pittsburgh, PA) was conducted after 12-h overnight fasting, with glucose and insulin concentrations determined before glucose administration and at 30-min intervals thereafter for 180 min. The main goal of this study was to identify potential glucose intolerance. All study subjects were normoglycemic.
Hyperinsulinemic-euglycemic clamps
On the morning of study d 4, euglycemic-hyperinsulinemic clamp was performed after an overnight fast. In brief, a primed-continuous infusion of regular insulin (Humulin; Squibb-Novo, Princeton, NJ) was started at a rate of 80 mU/m2 (body surface area)/min and was continued for 2 h. Based on our previous studies in young normoglycemic subjects, this infusion protocol induces complete suppression of hepatic glucose output, even in the presence of significant systemic insulin resistance (15). The rate of glucose disposal (Rd) was calculated by subtracting the urinary glucose excretion from the Ra (rate of appearance) during the last 40 min of the study (8).
Nuclear magnetic resonance (NMR) spectroscopy
During d 1 of the study, the first 20 non-Hispanic White males who volunteered from the group of 134 underwent NMR spectroscopy of the soleus muscle and the liver as previously described to measure intracellular triglyceride content (17, 18). Due to a lack of funding, these studies could not be performed for the entire cohort. To minimize the potential confounding effects of gender and ethnicity, we limited our NMR studies to non-Hispanic White males.
AT biopsy
AT was obtained using a 14 G × 9 cm Temno II biopsy needle (Allegiance Healthcare Corp., Mcgaw Park, IL) from the abdominal sc area in the right lower quadrant 2 cm above and medial to the anterior iliac tuberosity. Specimens were frozen in liquid nitrogen immediately after collection.
mRNA quantification
Total RNA was isolated from frozen tissues using RNA STAT-60 (Tel-Test, Friendswood, TX). Genomic DNA was removed from the total RNA preparations using Dnase 1 (DNA Free, Ambion; Invitrogen, San Diego, CA). RNA from each sample was diluted to 5 ng/μl and 100 ng reverse-transcribed in a 100-μl reaction using random hexamer priming (TaqMan Reverse Transcription kit; Applied Biosystems, Foster City, CA). The primers were designed using Primer Express version 2.0 (Applied Biosystems) and synthesized by Integrated DNA Technologies for the following genes: SREBP1c, ACLS1, CD36, HSL, LPL, CD68, and MAC1. Each PCR contained 2 μl cDNA, 150 nm each of forward and reverse primers, and 5 μl SYBR Green Universal PCR Master Mix (Bio-Rad Laboratories Inc., Hercules, CA). Thermal cycling and data collection were performed using the ABI Prism 7900HT instrument (Applied Biosystems). The data were analyzed using SDS version 2.2 software (Applied Biosystems). Relative quantification of gene expression was by the comparative cycle threshold method (User Bulletin no. 2, Applied Biosystems) with cyclophilin mRNA as the endogenous control for total RNA content. The amplification efficiency with each primer set was determined by analyzing the slope of the standard curve (User Bulletin no. 2).
Biochemical measurements
Cholesterol and triglycerides were measured by enzymatic methods (8). High-density lipoprotein (HDL)-cholesterol was determined in the supernatant after precipitating apolipoprotein B-containing lipoproteins using heparin-manganese chloride. Low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald equation. Adiponectin, leptin, IL-6, and TNF-α were measured using multiplex immunoassays (Millipore, Billerica, MA). hs-CRP was measured by a highly sensitive nephelometric assay using a monoclonal antibody to CRP coated on polystyrene beads (Dade Behring, Newark, DE). For plasma free fatty acid determination, plasma lipids were extracted with methanol:chloroform [1:2 (vol/vol)] and heptanes. Heptadecanoic acid (Sigma Aldrich, St. Louis, MO) was added to the plasma samples as an internal standard. The extracted lipids were dried under nitrogen flow and plated on thin-layer chromatography plates. The bands corresponding to fatty acids were extracted and analyzed using a gas chromatography-flame ionization detector.
Statistical analysis and calculations
After conversion to a SAS (SAS version 9.1; SAS Institute, Cary, NC) database, all variables were examined using plots, summary statistics, and tests of normality for continuous variables. All variables except gender demonstrated varying amounts of asymmetry.
Because a significant interaction was found between gender and the studied associations, two groups were identified based on gender and compared for general characteristics using Student's t test for independent variables. We examined the association between ENPP1 gene expression and liver/skeletal muscle intracellular triglyceride content among the 20 normoglycemic men who had NMR spectroscopy. To account for asymmetry in the small number of samples, Spearman correlations were estimated. Subjects within each gender group were compared across tertiles (thirds) of sc abdominal AT-ENPP1 gene expression using standard summary statistics. Groups were compared statistically using ANOVA, with Tukey-Kramer multiple comparisons of the natural logarithms of the dependent variable. The multiple endpoint variables we tested were identified a priori, and the P values are presented without further adjustment for multiplicity. The variables were back-transformed to their natural units for presentation. Multiple regression analysis for Rd value on tertiles of sc abdominal AT ENPP1 gene expression was computed in a model that included body fat content and plasma adiponectin (see Table 3).
Table 3.
Multiple regression of Rd values on tertiles of sc abdominal adipose ENPP1 mRNA, adjusted for body fat and plasma adiponectin (type III sums of squares)
| Degrees of freedom | F value | P > F | |
|---|---|---|---|
| Adjusted for log body fat | |||
| Men | |||
| Model | 3 | 26.11 | <0.0001 |
| AT-ENPP1 tertiles | 2 | 5.08 | 0.0087 |
| ln body fat | 1 | 70.56 | <0.0001 |
| Women | |||
| Model | 3 | 7.04 | 0.0004 |
| AT-ENPP1 tertiles | 2 | 0.74 | 0.4837 |
| ln body fat | 1 | 21.1 | <0.0001 |
| Adjusted for log body fat and log plasma adiponectin | |||
| Men | |||
| Model | 4 | 21.45 | <0.0001 |
| AT-ENPP1 tertiles | 2 | 1.7 | 0.1903 |
| ln body fat | 1 | 52.41 | <0.0001 |
| ln adiponectin | 1 | 4.73 | 0.0334 |
| Women | |||
| Model | 4 | 4.24 | 0.0077 |
| AT-ENPP1 tertiles | 2 | 0.31 | 0.7353 |
| ln body fat | 1 | 10.7 | 0.0027 |
| ln adiponectin | 1 | 1.05 | 0.3129 |
Results
Table 1 summarizes the general characteristics of the study subjects by gender. Gender differences were noted for body composition, fat distribution, plasma adipokines, and Rd values. No gender difference was found for AT expression of genes involved in adipocyte lipid metabolism (SREBP1c, ACSL1, CD36, HSL, LPL) or markers of AT macrophage infiltration (CD68 and MAC1 gene expression; data not shown).
Table 1.
General characteristics of study participants
| Men | Women | P value | |
|---|---|---|---|
| n | 75 | 59 | |
| Ethnicity (% total population) | 0.2 | ||
| Non-Hispanic White | 76 | 79 | |
| Hispanic White | 4 | 5 | |
| Black | 3 | 4 | |
| Asian | 16 | 12 | |
| Other | 1 | 0 | |
| Age (yr) | 27 ± 4 | 29 ± 7 | 0.05 |
| Body mass index (kg/m2) | 25 ± 4 | 28 ± 9 | 0.04 |
| Systolic blood pressure (mm Hg) | 119 ± 9 | 116 ± 13 | 0.14 |
| Diastolic blood pressure (mm Hg) | 71 ± 9 | 72 ± 8 | 0.4 |
| Waist circumference (cm) | 84 ± 15 | 67 ± 19 | <0.0001 |
| Body fat (% of total body weight) | 21 ± 7 | 29 ± 9 | <0.0001 |
| Subcutaneous fat (% of total body weight) | 3.2 ± 1.5 | 4.3 ± 2.4 | 0.01 |
| Intraperitoneal fat (% of total body weight) | 1.2 ± 0.5 | 0.8 ± 0.4 | <0.0001 |
| Plasma total cholesterol (mg/dl) | 169 ± 31 | 169 ± 33 | 0.09 |
| Plasma LDL-cholesterol (mg/dl) | 111 ± 28 | 100 ± 30 | 0.004 |
| Plasma HDL-cholesterol (mg/dl) | 41 ± 11 | 52 ± 15 | <0.0001 |
| Plasma triglyceride (mg/dl) | 94 ± 54 | 90 ± 42 | 0.6 |
| Plasma leptin (ng/ml) | 6 ± 8 | 22 ± 21 | <0.0001 |
| Plasma adiponectin (μg/ml) | 16 ± 11 | 25 ± 12 | 0.0003 |
| hs-CRP (mg/liter) | 0.8 ± 0.9 (0.45; 0.1–4.75) | 1.4 ± 2.0 (0.53; 0.1–7.47) | 0.08 |
| IL-6 (pg/ml) | 1.2 ± 1.1 | 0.9 ± 0.7 | 0.2 |
| Plasma TNF-α (pg/ml) | 1.3 ± 1.1 | 1.0 ± 0.6 | 0.04 |
| Plasma glucose at baseline OGTT (mg/dl) | 92 ± 5 | 87 ± 6 | 0.0009 |
| Plasma glucose at 2-h OGTT (mg/dl) | 108 ± 19 | 108 ± 14 | 0.9 |
| Fasting insulin (μU/ml) | 18 ± 22 | 11 ± 7 | 0.02 |
| Rd (mg/min · kg of body weight) | 7.4 ± 2.8 | 6.3 ± 2.4 | 0.02 |
The data are shown as mean ± sd [skewed data are expressed as (median; minimum-maximum)] unless otherwise indicated. All women were premenopausal. The P value for the difference between men and women for each parameter was calculated using the Mantel-Haenszel χ2 test for ethnicity and the Student's t test procedure for comparison of means.
To explore which systemic metabolic changes and changes in AT function were associated with increased AT-ENPP1 expression, we examined the association between AT-ENPP1 mRNA levels and: 1) AT expression of genes involved in lipid metabolism; 2) AT expression of genes involved in inflammation; 3) plasma concentrations of fatty acids and adipokines; and 4) systemic insulin resistance to peripheral glucose disposal. To this end, we identified three study groups, based on tertiles of AT-ENPP1 gene expression. Because of a significant interaction among gender, AT-ENPP1 expression, and the studied outcome variables, we conducted separate analyses for men and women. As shown in Table 2, age and adiponectin differed across tertiles of AT-ENPP1 gene expression in men. For women, we found no differences across tertiles.
Table 2.
General characteristics of the three study groups identified by sc abdominal AT ENPP1 mRNA tertiles
| AT-ENPP1 ≤ 0.75 | AT-ENPP1 > 0.75 ≤ 1.5 | AT-ENPP1 >1.5 | ANOVA P valuea | |
|---|---|---|---|---|
| Men | ||||
| n | 23 | 20 | 32 | |
| Age (yr) | 26 ± 5 | 25 ± 3 | 28 ± 4 | 0.027 |
| Body mass index (kg/m2) | 25.1 ± 3.3 | 25.4 ± 4.7 | 24.8 ± 3.9 | 0.874 |
| Waist circumference (cm) | 83 ± 18 | 80 ± 19 | 87 ± 8 | 0.176 |
| Body fat (% of total body weight) | 20.8 ± 7.0 | 22.8 ± 8.9 | 20.5 ± 6.6 | 0.661 |
| Subcutaneous fat (% of total body weight) | 2.8 ± 1.5 | 3.3 ± 1.6 | 3.3 ± 1.5 | 0.488 |
| Intraperitoneal fat (% of total body weight) | 1.0 ± 0.5 | 1.1 ± 0.5 | 1.3 ± 0.5 | 0.271 |
| Plasma total cholesterol (mg/dl) | 164 ± 24 | 173 ± 36 | 170 ± 31 | 0.734 |
| Plasma HDL cholesterol (mg/dl) | 41 ± 11 | 41 ± 11 | 41 ± 10 | 0.976 |
| Plasma triglyceride (mg/dl) | 89 ± 50 | 94 ± 57 | 99 ± 57 | 0.773 |
| Plasma adiponectin (mg/ml) | 18.7 ± 9.3 | 20 ± 12 | 13 ± 11 | 0.004 |
| hs-CRP (mg/liter) | 0.65 ± 0.65 | 0.61 ± 1.1 | 0.90 ± 0.89 | 0.076 |
| IL-6 (pg/ml) | 1.38 ± 1.76 | 1.07 ± 0.95 | 1.07 ± 0.59 | 0.682 |
| Plasma TNF-α (pg/ml) | 1.195 ± 0.735 | 1.686 ± 1.575 | 1.176 ± 0.930 | 0.569 |
| Rd (mg/min · kg of body weight) | 8.1 ± 2.4 | 6.4 ± 2.1 | 6.8 ± 3.0 | 0.146 |
| Women | ||||
| n | 22 | 24 | 13 | |
| Age (yr) | 28.6 ± 5.8 | 27.8 ± 7.8 | 29.8 ± 7.2 | 0.562 |
| BMI (kg/m2) | 27.1 ± 11.8 | 26.8 ± 5.9 | 27.6 ± 7.6 | 0.822 |
| Waist circumference (cm) | 67 ± 16 | 70 ± 23 | 60 ± 20 | 0.401 |
| Body fat (% of total body weight) | 24.8 ± 8.8 | 31.1 ± 8.6 | 29.4 ± 10.4 | 0.077 |
| Subcutaneous fat (% of total body weight) | 3.4 ± 1.7 | 5.0 ± 2.8 | 4.1 ± 2.4 | 0.279 |
| Intraperitoneal fat (% of total body weight) | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.930 |
| Plasma total cholesterol (mg/dl) | 167 ± 35 | 174 ± 35 | 162 ± 28 | 0.576 |
| Plasma HDL cholesterol (mg/dl) | 51 ± 14 | 55 ± 15 | 49 ± 15 | 0.487 |
| Plasma triglyceride (mg/dl) | 98 ± 46 | 81 ± 34 | 89 ± 50 | 0.487 |
| Plasma adiponectin (mg/ml) | 28.2 ± 9.6 | 26.4 ± 13.7 | 17 ± 9 | 0.150 |
| hs-CRP (mg/liter) | 1.5 ± 2.1 | 0.9 ± 0.9 | 24 ± 31 | 0.467 |
| IL-6 (pg/ml) | 0.660 ± 0.308 | 1.180 ± 0.879 | 1.006 ± 0.685 | 0.338 |
| Plasma TNF-α (pg/ml) | 0.943 ± 0.551 | 0.908 ± 0.727 | 1.159 ± 0.499 | 0.284 |
| Rd (mg/min · kg of body weight) | 6.7 ± 2.6 | 6.4 ± 2.1 | 6.6 ± 2.4 | 0.988 |
The data are shown as means ± sd.
P values are based on one-way ANOVA of the natural logarithm of the variable.
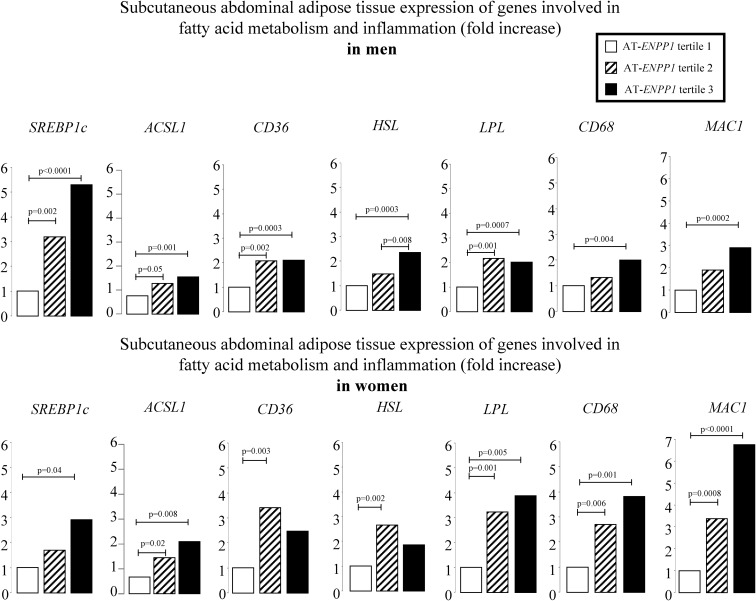
We next applied appropriate regression models for sc abdominal ENPP1 expression to predict the natural log of the relative expression of genes involved in fatty acid metabolism and inflammation. Figure 1 depicts the least-squares means of predicted values after adjusting for total body fat content, a significant correlate of each of the genes evaluated. Up-regulation of genes involved in fatty acid synthesis (SREBP1c, ACSL1), transport (CD36), and lipolysis (HSL, LPL) was found in the highest AT-ENPP1 tertile. The same tertile had higher expression of markers of AT macrophage infiltration (CD68 and MAC1).
Fig. 1.
The least-squares means of predicted values after adjusting for total body fat content are reported for sc abdominal AT expression of genes involved in fatty acid metabolism and inflammation. Values were analyzed after log-transformation and then back-transformed to their natural units for presentation. The groups identify tertiles of AT-ENPP1 gene expression; P values are based on one-way ANOVA of the natural logarithms with Tukey-Kramer adjustment for multiple comparisons. The model included body fat content and ENPP1 gene expression. SREBP1c encodes sterol regulatory element-binding protein, responsible for up-regulating the genes required for de novo lipogenesis. ACSL1 encodes long-chain-fatty-acid-CoA ligase 1, which converts free long-chain fatty acids into fatty acyl-CoA esters, thereby playing a key role in lipid biosynthesis and fatty acid degradation. CD36 protein binds many ligands, including long-chain fatty acids. HSL encodes hormone-sensitive lipase, which regulates triglyceride mobilization in AT. LPL encodes lipoprotein lipase, which has dual functions as a triglyceride hydrolase and ligand/bridging factor for receptor-mediated lipoprotein uptake. CD68 is a marker for the various cells of the macrophage lineage. MAC1 encodes macrophage-1 antigen, a macrophage marker.
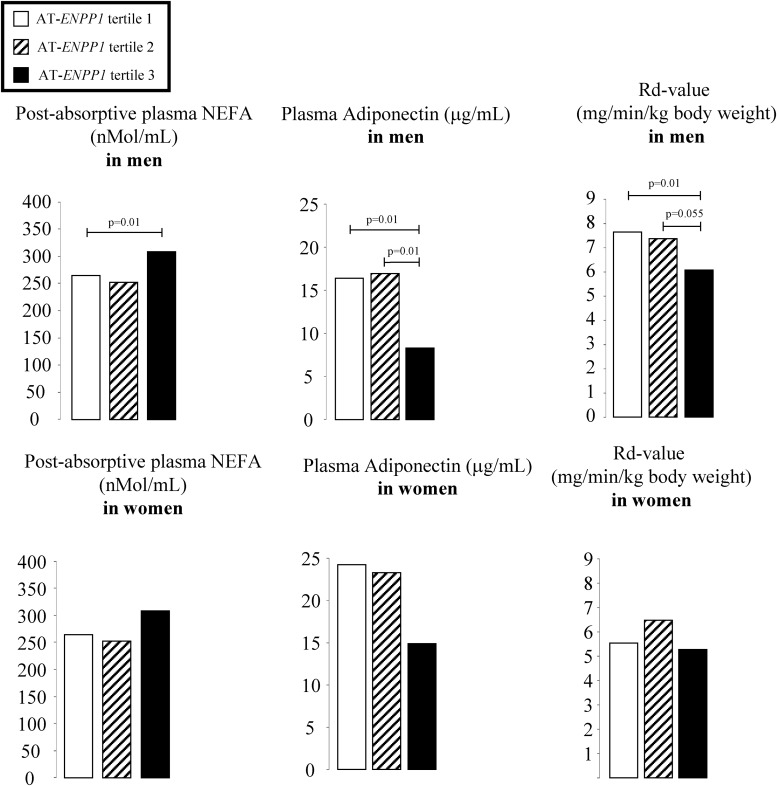
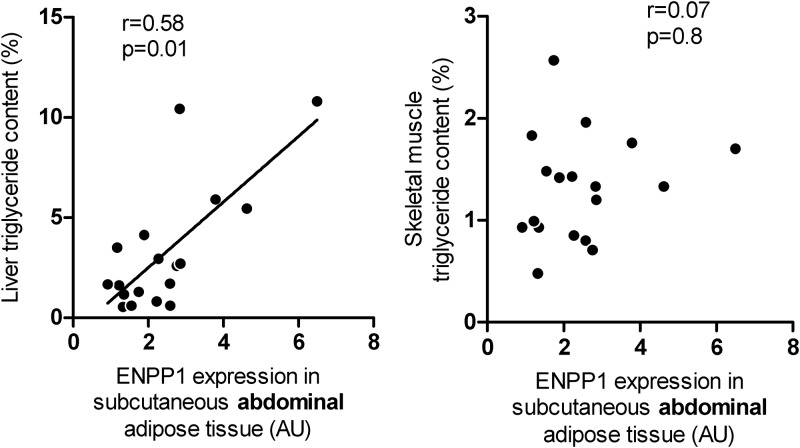
We also applied the regression models for sc abdominal ENPP1 expression to predict the natural log of NEFA, adiponectin, hs-CRP, and Rd values. There was no association between AT-ENPP1 expression and plasma hs-CRP in either men or women. Figure 2 depicts least-squares means of the predicted values after adjusting for the natural logarithm of total body fat content, also a significant correlate of the Rd value. In the men but not the women, postabsorptive plasma free fatty acid concentrations were higher and plasma adiponectin concentrations were lower in the highest tertile of sc AT-ENPP1 gene expression. Plasma NEFA levels were suppressed similarly in the three tertiles during the last 40 min of the hyperinsulinemic-euglycemic clamp. Although AT-ENPP1 gene expression was associated with a lower Rd value, independent of total body fat content, statistical significance was lost after adjusting for both total body fat and plasma adiponectin (Table 3). Another goal of this study was to estimate the correlation between AT-ENPP1 expression and fat content in liver and muscle. To minimize possible gender and ethnic effects, we enrolled only non-Hispanic White males for this part of the study and obtained NMR quantitation of intracellular triglyceride content in both liver and skeletal muscle. These volunteers were enrolled from the 134 subjects who underwent complete body composition and metabolic studies. In this subgroup, the average age was 27 ± 3 yr, and body fat content was 18 ± 6% of total body weight. As shown in Fig. 3, ENPP1 gene expression in sc abdominal AT was highly correlated with liver triglyceride content (r = 0.58; P = 0.01). This correlation remained significant even after statistical adjustment for ip fat mass and plasma adiponectin concentrations (r = 0.58; P = 0.018). Abdominal sc AT-ENPP1 expression was not correlated with skeletal muscle intracellular triglyceride content.
Fig. 2.
The least-squares means of predicted values (after adjusting for total body fat) of plasma NEFA, adiponectin, and Rd values are reported for males and females by tertiles of AT-ENPP1 expression. The values were analyzed after natural log-transformation and then back-transformed to their natural units for presentation. The groups identify tertiles of AT-ENPP1 gene expression, and P values are based on one-way ANOVA of the natural logarithms of variables with Tukey-Kramer adjustment for multiple comparisons. The model included body fat content and ENPP1 gene expression.
Fig. 3.
The relationship between sc adipose expression of ENPP1 [in arbitrary units (AU)] and liver/skeletal muscle intracellular triglyceride content, determined by NMR spectroscopy in normoglycemic male volunteers. Correlation coefficients were derived using Spearman rank correlation analysis.
Discussion
This study shows that, after statistical adjustment for body fat content, increased ENPP1 expression in sc abdominal AT of young normoglycemic men is associated with several parameters of AT dysfunction, including up-regulation of genes involved in fatty acid transport, fatty acid synthesis, lipolysis and inflammation; increased postabsorptive plasma NEFA, and decreased plasma adiponectin concentrations, and with increased systemic insulin resistance to glucose disposal. Our study also shows that the reported association between AT-ENPP1 and systemic insulin resistance in men is eliminated by statistical adjustment for plasma adiponectin concentrations. Finally, our study shows a marked gender effect, with no relationship being detected between AT-ENPP1 and plasma adiponectin, NEFA, or systemic insulin resistance in premenopausal women. Taken together, our results demonstrate the translational value of previous mechanistic studies performed in AdiposeENPP1-Tg male mice (11) and lend support to the view that AT ENPP1 may affect systemic lipid and glucose metabolism via modulation of AT function in young normoglycemic men.
Although the high-ENPP1 group had a higher plasma free fatty acid concentration than the low-ENPP1 men (Fig. 2), they also had higher AT expression of fatty acid transporter and lipolysis genes, as well as elevated fatty acid/triglyceride synthesis genes (Fig. 1). In the presence of stable body weight, this is compatible with both increased adipocyte triglyceride synthesis and increased lipolysis. Our study did not specifically address lipid dynamics in AT. However, an increasing body of evidence supports the idea that fatty acids not used for oxidative metabolism recycle back to triglycerides within the AT (19, 20). Because glycerol-3-phosphate availability, a key regulator of this reesterification process (21), could be insufficient in insulin-resistant adipocytes, we speculate that the high-ENPP1 subgroup could have increased lipolysis (increased lipolysis gene expression) incompletely matched by increased reesterification (increased fatty acid transporter and fatty acid synthesis gene expression). In these circumstances, triglycerides reaching the AT via plasma lipoproteins will not be stored efficiently, with a net result of fatty acid “spillover” and increased plasma NEFA concentrations, as observed (Fig. 2). Although we cannot exclude a role of other covariates, such as parallel changes in ENPP1 expression in hepatocytes, increased fatty acid spillover from the AT would increase substrate availability for hepatocytes and help to explain the correlation between AT-ENPP1 levels and liver triglyceride content in men (Fig. 3). Interestingly, this correlation was not affected by statistical adjustment for visceral fat content and plasma adiponectin, known covariates for hepatic fat content.
Another important known link between AT dysfunction and liver triglyceride content is increased production of inflammatory cytokines driven by increased macrophage infiltration. AT macrophage infiltration supporting low-grade systemic inflammation has been reported to play a mechanistic role in the increased liver triglyceride content, increased liver triglyceride deposition, and insulin resistance associated with obesity (22–25). We did not find a correlation between markers of macrophage infiltration (CD68 and MAC1 gene expression) and liver triglyceride content in the 20 men studied via liver NMR. However, as shown in Fig. 1, CD68 and MAC1 were significantly increased in the larger cohort of men with high AT-ENPP1, thus suggesting AT macrophage recruiting and activation of inflammatory pathways when AT-ENPP1 expression is increased. Notably, whereas AT inflammation is known to be increased in obesity, the association between AT-ENPP1 and inflammatory gene expression in our study was found to be significant after statistical adjustment for body fat content. We did not find parallel changes in circulating inflammatory cytokines, except for lower adiponectin (Fig. 2). Interestingly, plasma adiponectin was also found to be lower in the AdiposeENPP1-Tg mouse (11). In this model, as well as in stably transfected 3T3-L1 cells overexpressing ENPP1, high ENPP1 protein was shown to block adipocyte maturation (26). Therefore, although our study in humans does not prove causality, it is tempting to speculate that lower plasma adiponectin could be a manifestation of defective adipocyte maturation associated with increased AT-ENPP1. Previous studies have shown that decreased plasma adiponectin concentrations could have a role in promoting hepatic triglyceride accumulation (27). This effect could be related to AMP kinase-mediated suppression of fatty acid synthesis and stimulation of fatty acid oxidation promoted by adiponectin (28–31). Mice overexpressing AT adiponectin were recently shown to have lower plasma fatty acid concentrations and decreased hepatic lipogenesis (32). However, our observation that the correlation between AT-ENPP1 expression and liver triglyceride content is not affected by statistical adjustment for plasma adiponectin would suggest that other mechanisms may play a more significant role in explaining this association. One such mechanism could be increased substrate availability to the liver due to higher plasma NEFA concentrations (33). Our study protocol for hyperinsulinemic-euglycemic clamps did not include a low insulin infusion step because our main goal was to evaluate systemic insulin sensitivity to glucose disposal. Likely as a result, uniform suppression of plasma NEFA was observed during hyperinsulinemia (data not shown). However, physiological plasma insulin concentrations during postabsorptive conditions did not suppress plasma NEFA in the highest AT-ENPP1 tertile as compared to that in the lowest tertile (Fig. 2). These results suggest the need for further studies to better define the role of NEFA metabolism in the association between AT-ENPP1 and liver triglyceride content we observed in young normoglycemic men. Future studies will also have to include both genders and specifically measure AT metabolism of fatty acids in relation to ENPP1.
Elevated plasma free fatty acid concentrations are also known to influence systemic glucose metabolism via decreasing insulin-induced glucose disposal in skeletal muscle (33, 34). As shown in Fig. 2, progressive worsening of systemic insulin resistance to peripheral glucose disposal with increasing AT-ENPP1 expression was observed in men. These differences were significant after statistical adjustment for total body fat content, which is a common correlate of insulin resistance. However, statistical significance was lost after adjusting for both body fat content and plasma adiponectin concentrations (Table 3). Clearly, our study design does not allow conclusions on causality. However, as mentioned above, the ENPP1-induced defect in adipocyte maturation shown in 3T3L1 (26) and in our AdiposeENPP1-Tg mouse (11) model could account for the low plasma adiponectin found in men in our study, and could mechanistically explain the systemic insulin resistance associated with high AT-ENPP1 expression in men. Although not specifically addressed in this study, our associative findings clearly support the need to explore a mechanistic hypothesis of adiponectin-mediated systemic insulin resistance when AT-ENPP1 is increased.
Interestingly, we did not find a significant effect of AT-ENPP1 on postabsorptive NEFA and plasma adiponectin levels or systemic insulin resistance to glucose disposal in premenopausal normoglycemic women. The women in our cohort had 40% lower AT-ENPP1 expression than men (P = 0.02, after adjustment for body fat content). Consequently, fewer women were in the highest AT-ENPP1 tertile, which could account for the lack of statistical significance for differences in postabsorptive NEFA and adiponectin levels among the tertile groups (Fig. 2). The same considerations apply to the lack of consistency in the gene expression changes with AT-ENPP1 tertiles in women (Fig. 1). However, not even a trend was observed for a correlation between AT-ENPP1 and insulin resistance in young women, an observation that ought to be addressed in future studies in postmenopausal women. Future investigations will also provide more detailed insights into AT insulin signaling and gene expression changes induced by ENPP1 in adipocytes by assessing the fat distribution, body composition, AT gene expression/protein, and insulin resistance in a large cohort of participants. We also recognize that this study included multiple variables, raising the possibility of type 1 error. However, our design aimed at providing a comprehensive evaluation of predefined outcome variables that are biologically related. We believe this approach and the sequential work done in animals first and in humans second should reduce the probability of spurious findings related to multiplicity.
In conclusion, our findings in humans provide translational value to previous mechanistic studies in the AdiposeENPP1-Tg mice and identify AT-ENPP1 as a potential contributor to AT dysfunction, increased liver triglyceride content, and systemic insulin resistance to glucose disposal in young normoglycemic men. Additional studies are needed to elucidate the observed gender differences and to outline the specific mechanistic pathways involved in this potential target of therapy for prevention of chronic diseases related to AT dysfunction and insulin resistance, such as type 2 diabetes and cardiovascular disease.
Supplementary Material
Acknowledgments
We acknowledge the Institute for Translational Sciences, Key Resources Clinical Research Center, Biostatistics, Epidemiology and Research Design at the University of Texas Medical Branch at Galveston for support in conducting this study. We thank Dr. David Konkel of the University of Texas Medical Branch at Galveston (UTMB) Institute of Translational Sciences Editorial Office for critically editing the manuscript. We thank Beverley Adams-Huet of the Department of Clinical Sciences at the University of Texas Southwestern Medical Center at Dallas (UTSW) for statistical analysis consultation. For technical support, we acknowledge Geetika Saraf at UTMB and Thanalakshmi Seenivasan and Chandna Vasandani at the UTSW. We thank Dr. Scott M. Grundy from the Center for Human Nutrition at the UTSW for his continuous advice during the conduct of the study.
M.C. performed experiments and wrote the first draft of the manuscript. H.D., W.P., M.S., D.T., and E.H.L. performed experiments. N.A. oversaw the entire study, performed experiments, reviewed/edited, and is the guarantor of the manuscript.
This work was supported by National Institutes of Health Grant R01 DK072158 from the National Institute of Diabetes and Digestive and Kidney Diseases and in part by Grant 1UL1RR029876 from the National Center for Research Resources.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AT
- Adipose tissue
- ENPP1
- ectonucleotide pyrophosphatase/phosphodiesterase-1
- HDL
- high-density lipoprotein
- hs-CRP
- high sensitivity C-reactive protein
- LDL
- low-density lipoprotein
- NEFA
- nonesterified fatty acid
- NMR
- nuclear magnetic resonance
- OGTT
- oral glucose tolerance testing
- Rd
- rate of glucose disposal.
References
- 1. Bao W, Srinivasan SR, Berenson GS. 1996. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa Heart Study. Circulation 93:54–59 [DOI] [PubMed] [Google Scholar]
- 2. DeFronzo RA, Ferrannini E. 1991. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14:173–194 [DOI] [PubMed] [Google Scholar]
- 3. Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. 2002. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 40:937–943 [DOI] [PubMed] [Google Scholar]
- 4. Reaven GM. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 5. Zavaroni I, Bonora E, Pagliara M, Dall'Aglio E, Luchetti L, Buonanno G, Bonati PA, Bergonzani M, Gnudi L, Passeri M, Reaven GM. 1989. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med 320:702–706 [DOI] [PubMed] [Google Scholar]
- 6. Bays H, Abate N, Chandalia M. 2005. Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future Cardiol 1:39–59 [DOI] [PubMed] [Google Scholar]
- 7. Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. 1998. The metabolically obese, normal-weight individual revisited. Diabetes 47:699–713 [DOI] [PubMed] [Google Scholar]
- 8. Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. 1999. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab 84:2329–2335 [DOI] [PubMed] [Google Scholar]
- 9. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. 2008. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 10. Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. 2008. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 11. Pan W, Ciociola E, Saraf M, Tumurbaatar B, Tuvdendorj D, Prasad S, Chandalia M, Abate N. 2011. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am J Physiol Endocrinol Metab 301:E901–E911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldfine ID, Maddux BA, Youngren JF, Reaven G, Accili D, Trischitta V, Vigneri R, Frittitta L. 2008. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr Rev 29:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maddux BA, Goldfine ID. 2000. Membrane glycoprotein PC-1 inhibition of insulin receptor function occurs via direct interaction with the receptor α-subunit. Diabetes 49:13–19 [DOI] [PubMed] [Google Scholar]
- 14. Frittitta L, Youngren JF, Sbraccia P, D'Adamo M, Buongiorno A, Vigneri R, Goldfine ID, Trischitta V. 1997. Increased adipose tissue PC-1 protein content, but not tumour necrosis factor-α gene expression, is associated with a reduction of both whole body insulin sensitivity and insulin receptor tyrosine-kinase activity. Diabetologia 40:282–289 [DOI] [PubMed] [Google Scholar]
- 15. Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. 1995. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 96:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abate N, Burns D, Peshock RM, Garg A, Grundy SM. 1994. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35:1490–1496 [PubMed] [Google Scholar]
- 17. Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. 2008. Effects of N-3 fatty acids on hepatic triglyceride content in humans. J Investig Med 56:780–785 [DOI] [PubMed] [Google Scholar]
- 18. Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. 2007. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology 46:716–722 [DOI] [PubMed] [Google Scholar]
- 19. Boden G, Chen X, Desantis RA, Kendrick Z. 1993. Effects of insulin on fatty acid reesterification in healthy subjects. Diabetes 42:1588–1593 [DOI] [PubMed] [Google Scholar]
- 20. Jensen MD, Ekberg K, Landau BR. 2001. Lipid metabolism during fasting. Am J Physiol Endocrinol Metab 281:E789–E793 [DOI] [PubMed] [Google Scholar]
- 21. Nye C, Kim J, Kalhan SC, Hanson RW. 2008. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab 19:356–361 [DOI] [PubMed] [Google Scholar]
- 22. Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. 2009. Prevention of steatosis by hepatic JNK1. Cell Metab 10:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. 2008. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. 2012. The inflammasome puts obesity in the danger zone. Cell Metab 15:10–18 [DOI] [PubMed] [Google Scholar]
- 25. Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. 2011. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA 108:15324–15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang J, Fu M, Ciociola E, Chandalia M, Abate N. 2007. Role of ENPP1 on adipocyte maturation. PLoS One 2:e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pisto P, Ukkola O, Santaniemi M, Kesäniemi YA. 2011. Plasma adiponectin—an independent indicator of liver fat accumulation. Metabolism 60:1515–1520 [DOI] [PubMed] [Google Scholar]
- 28. Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. 2009. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 382:51–56 [DOI] [PubMed] [Google Scholar]
- 29. Kadowaki T, Yamauchi T. 2005. Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- 30. Kadowaki T, Yamauchi T, Kubota N. 2008. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582:74–80 [DOI] [PubMed] [Google Scholar]
- 31. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 32. Shetty S, Ramos-Roman MA, Cho YR, Brown J, Plutzky J, Muise ES, Horton JD, Scherer PE, Parks EJ. 2012. Enhanced fatty acid flux triggered by adiponectin overexpression. Endocrinology 153:113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boden G, Chen X, Ruiz J, White JV, Rossetti L. 1994. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homko CJ, Cheung P, Boden G. 2003. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes 52:487–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.