Abstract
Carotid body chemoreceptors increase their action potential (AP) activity in response to a decrease in arterial oxygen tension and this response increases in the post-natal period. The initial transduction site is likely the glomus cell which responds to hypoxia with an increase in intracellular calcium and secretion of multiple neurotransmitters. Translation of this secretion to AP spiking levels is determined by the excitability of the afferent nerve terminals that is largely determined by the voltage-dependence of activation of Na+ channels. In this review, we examine the biophysical characteristics of Na+ channels present at the soma of chemoreceptor afferent neurons with the assumption that similar channels are present at nerve terminals. The voltage dependence of this current is consistent with a single Na+ channel isoform with activation around the resting potential and with about 60-70% of channels in the inactive state around the resting potential. Channel openings, due to transitions from inactive/open or closed/open states, may serve to amplify external depolarizing events or generate, by themselves, APs. Over the first two post-natal weeks, the Na+ channel activation voltage shifts to more negative potentials, thus enhancing the amplifying action of Na+ channels on depolarization events and increasing membrane noise generated by channel transitions. This may be a significant contributor to maturation of chemoreceptor activity in the post-natal period.
Keywords: Carotid body, Na+ channel, post-natal maturation, action potential
1. Introduction
Carotid body chemoreceptors transduce a decrease in arterial oxygen tension or decrease in pHa into increased action potential (AP) activity on the sinus nerve. This increased activity is essential in mediating the increased drive to breathe during hypoxia and also plays important roles in arousal from sleep and increasing sympathetic activity. The sensitivity to hypoxia undergoes a significant developmental change being relatively insensitive at birth and increasing to adult levels over the first weeks to month of life (Kholwadwala and Donnelly, 1992; Marchal et al., 1992; Mulligan, 1991; Pepper et al., 1995). This is likely due to the changing definition of ‘hypoxia’ at birth since the normal fetal PaO2 (i.e., ‘normoxia’) is about 30 torr and rises to 90 after birth. This developmental increase is also dependent on the oxygen level in the post-natal period. Birth into an hypoxia environment appears to ablate the signal to evoke resetting of chemoreceptor sensitivity and results in a greatly reduced ventilatory response to acute hypoxia (Eden and Hanson, 1987; Hanson et al., 1989). Perhaps surprisingly, birth into an enriched oxygen atmosphere also results in a reduction in the hypoxic ventilatory response (Eden and Hanson, 1986).
An understanding of these developmental factors is dependent on understanding the mechanism by which hypoxia results in increased nerve activity. Here, the glomus cell, a secretory cell within the carotid body and associated with chemoreceptor nerve endings, is believed to play a central role. At least some glomus cells respond to acute hypoxia with a depolarization, thereby activating voltage-dependent calcium channels and allowing an influx of calcium (Buckler and Vaughan-Jones, 1994; Wasicko et al., 2006; Wasicko et al., 1999). The rise of intracellular calcium concentration triggers secretion of dense-cored vesicles and, perhaps, cleared cored vesicles, which are often present in glomus cells (McDonald and Mitchell, 1975). The vesicular release likely contains one or more excitatory neurotransmitters that initiate action potentials in the afferent nerve endings, but we have not yet achieved a clear identification of the excitatory agents (Reyes et al., 2007b).
This last step – the initiation of action potentials - is the primary focus of this brief review, and, in particular, the potential role or roles for voltage-activated Na+ channels in this process. The normal assigned role of Na+ channels is supporting action potential propagation in which a depolarized portion of an axon will provide a current sink that depolarizes a neighboring portion of the nerve fiber. However, Na+ channels may also play an important role in amplifying depolarizing events secondary to the release of neuromodulators or neurotransmitters, and thus dictate the relationship between transmitter release and initiation of APs.
2. Sodium channels in synaptic transmission
2.1 Importance of sodium channels in post-synaptic AP initiation
Transmission at many synapses is relatively independent of the type or number of Na+ channels. For instance, the neuromuscular junction transmits APs over the synapse in a 1:1 fashion with a safety factor estimated at 5-7 times (Wood and Slater, 2001). That is, the magnitude of the post-synaptic depolarization caused by a presynaptic AP is 5-7 times greater than required to transmit across the junction (Wood and Slater, 2001). Thus, a loss of Na+ channels or Na+ channel current due to application of drugs or a reduction in extracellular Na+ concentration has little effect on neuromuscular transmission at low blocking levels.
Most synapses, however, do not demonstrate a 1:1 coupling but rely on the summation of multiple excitatory post-synaptic potentials (EPSPs) from one or more sources to generate an AP. In this case, factors that stabilize or hyperpolarize the postsynaptic membrane would serve to inhibit AP initiation and factors which enhance EPSP magnitude would facilitate AP generation. One such factor is synaptic strengthening by modification of pre-synaptic and post-synaptic sites (Davis and Goodman, 1998). In addition, EPSPs can also be enhanced by voltage-dependent activation of Na+ channels which have a threshold for activation around the resting potential (Crill, 1996). This has been shown to boost the magnitude of the EPSP and result in enhanced AP generation in multiple systems (Crill, 1996; Schwindt and Crill, 1995; Stuart and Sakmann, 1995).
2.2 Na+ channel isoforms
Based on the pioneering work of Hodgkin and Huxley using squid giant axon, the activation and inactivation of Na+ channels can be modeled using an activation scheme of the binding of three activation particles and the binding of a single inactivation particle (Fig 1) (Hodgkin and Huxley, 1952). In the original work, Na+ currents of clamped squid axons were elicited using voltage protocols similar to that used in figure 2. An ensemble average of Na+ current was measured as a function of voltage and time and fit to power function of exponentials. In the model, the gates for activation (m) and inactivation (h) are controlled by a membrane-bound charged particle whose position (bound or unbound) is controlled by an energy barrier. As the membrane is depolarized, the energy barrier is lower and the probability for binding to the gate is higher.
Fig 1.
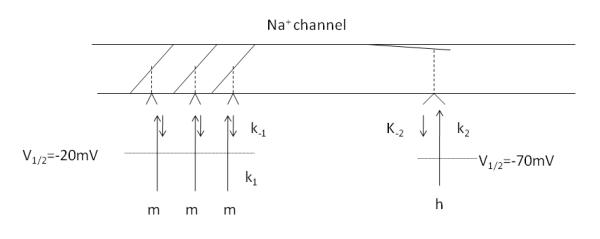
Schematic diagram of a Na+ channel based on the model of Hodgkin and Huxley (Hodgkin and Huxley, 1952). Activation (m) and inactivation (h) particles move to a binding site with a forward rate (k1 and k2) as the membrane potential is depolarized. Upon binding the gate swings open (m gate) or closed (h gate). Both sites have a finite probability of dissociating (k−1 and k−2), particularly at potentials around resting potential, giving rise to a stochastic membrane noise source. V1/2 is the potential at which half the particles are bound.
Fig 2.
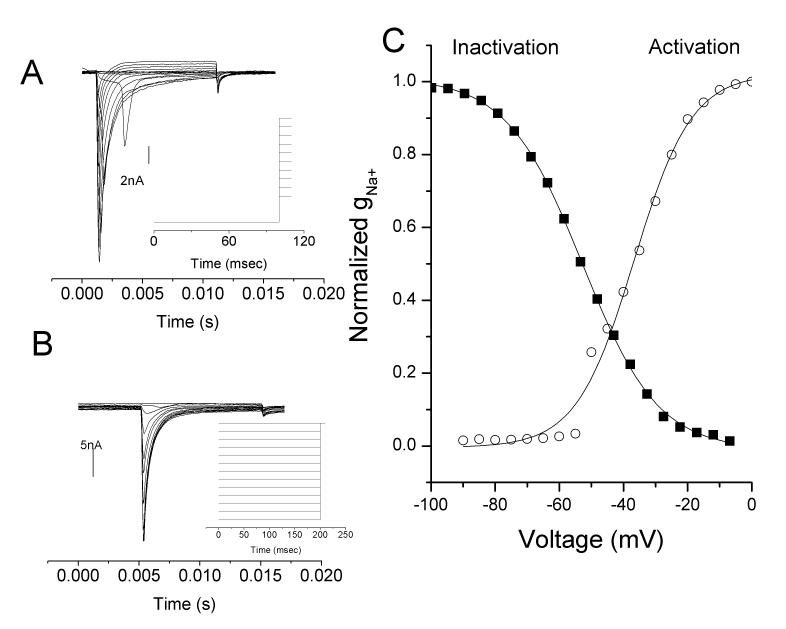
Steady-state activation and inactivation Na+ current profiles for a chemoreceptor neuron recorded in an intact ganglion of a P15 rat carotid body/petrosal preparation. (A) Steady state activation to different command potentials over the range of −90 to 0mV following a conditioning potential of −120mV to place all channels in the closed state (ie, no h particle binding of figure 1). (B) Steady state inactivation in the same neurons showing evoked currents during a step to −10mV following a conditioning step over the range of −100 to −10mV (ie, various amounts of h particle binding of figure 1). (C) Boltzman fit to the currents shown in A and B for activation and inactivation curves. Note region of overlap suggesting a persistent Na+ current in that voltage range. From (Donnelly, 2011).
This model has proven accurate in characterizing the activation and inactivation characteristics of nine isoforms of voltage gated Na+ channels found in humans, termed Nav1.1-Nav1.9. These channels differ in their voltage of activation and voltage of inactivation as well as tissue localization. For instance, Nav1.2 is the primarily isoform in the central nervous system and in unmyelinated axons (Boiko et al., 2003; Yao et al., 2002); Nav1.8 and Nav1.9 are predominantly found in small (pain) neurons of the peripheral nervous system (Akopian et al., 1999; Djouhri et al., 2003); Nav1.7 significantly mediates pain sensing (Chahine et al., 2005; Nassar et al., 2004). A more complete description of tissue localization is given elsewhere (Chahine et al., 2005). In broad terms, isoforms can be grouped into tetrodotoxin sensitive (TTX-S) subtypes (Nav1.1, 1.2, 1.3, 1.4, 1.6, 1.7) and (relatively) TTX-resistant (TTX-R)(Nav1.5, 1.8, 1.9) (Chahine et al., 2005) based on the ability of the toxin to block the channel. The voltages of activation and inactivation for TTX-R isoforms are about 10mV more positive than TTX-S isoforms and, thus, are less likely to participate in the initiation of AP initiation, since the activation voltage is farther removed from the resting potential.
2.3 Kinetics of activation and inactivation of Na+ channels
Besides increasing the magnitude of depolarization events caused by external events (eg EPSPs), the voltage-dependent activation of an inward (i.e., depolarizing) current around the resting potential can lead to instability of the membrane potential, characterized as spontaneous oscillations. Nature utilizes these oscillations in a number of neuronal networks. For instance, rhythmic oscillations due to sodium currents generated around the resting potential underlies the rhythmic discharge behavior of interneurons in the spinal cord which produce a rhythmic motor discharge in the absence of descending and peripheral synaptic inputs (Ziskind-Conhaim et al., 2008) and a similar spontaneous firing occurs in tuberomammillary neurons (Taddese and Bean, 2002); fast rhythmic bursting bursting in layer 2/3 cortical neurons is enhanced by persistent inward currents caused by Na+ channels (Traub et al., 2003); oscillations in entorhinal cortex layer V neurons are caused by spontaneous Na+ channel activity around the resting potential (Agrawal et al., 2001).
An intriguing aspect of voltage-gated Na+ channels is the relationship between their inactivation characteristics and the normal resting potential in cells. Resting potential in chemoreceptor afferent neurons, for instance, is about −58mV (Belmonte and Gallego, 1983; Donnelly, 1999; Iturriaga et al., 2007) but the half inactivation potential (i.e., the voltage where half the inactivation particles, h, are bound) for TTX-S currents is about −70mV, meaning more than half of the Na+ channels are not available to open and support AP generation (Rush et al., 2005). This suggests that either Nature is a poor designer of channels and should have moved the inactivation potential in the positive direction or the inactivation characteristic is used to support a role different than providing the ionic basis for the rise of the AP. One possible role is due to the finite probability for the inactivation particle, h, to dissociate from its binding site, thus allowing current to flow and generating a depolarization. When averaged over a large number of channels in a relatively large structure (e.g. in a soma) this would be manifest as a net inward current whose magnitude increases with slight depolarizations, termed a persistent Na+ current. When present in a small structure with a comparatively small number of channels (e.g. nerve terminals) the small, episodic currents contribute to membrane noise and even form the basis for AP generation in small nerve fibers (Chow and White, 1996).
2.4 Characteristics of Na+ currents in mature chemoreceptor afferent neurons
The first intracellular recordings of chemoreceptor afferent neurons were undertaken in cat ganglia by Belmonte and Gallego using sharp electrodes (Belmonte and Gallego, 1983). Carotid bodies were harvested, intact, with the petrosal ganglia, allowing an unequivocal identification of chemoreceptor modality based on spontaneous AP activity and response to excitatory agents (Belmonte and Gallego, 1983; Belmonte et al., 1988). The high electrical resistance of sharp electrodes precluded the ability to perform voltage-clamp measurements on Na+ channels, but some channel characteristics could be inferred from the AP waveform. All petrosal chemoreceptor neurons with a conduction velocity faster than 2m/s demonstrated a “hump” on the falling phase of the action potential which was sensitive to the removal of extracellular Na+ ion (Belmonte and Gallego, 1983; Gallego, 1983; Varas et al., 2003). The biphasic waveform suggests the presence of two types of Na+ channels with differing rates of inactivation, one being particularly slow. Only one Na+ channel isoform, Nav1.8, has the characteristics to account for the hump and, hence, its presence in the soma of cat myelinated chemoreceptor cells seems likely.
We had performed similar studies on rat petrosal chemoreceptor neurons recorded with sharp electrodes (Donnelly, 1999). Some characteristics were similar to that reported in cat, such as resting membrane potential. But some characteristics were starkly different: i) all rat chemoreceptor neurons had conduction velocities under 1m/s (Donnelly, 1999) while the preponderance of cat afferents were myelinated (Belmonte and Gallego, 1983; Iturriaga et al., 2007); ii) a prolonged depolarization produced a train of APs in the rat (Donnelly, 1999) but only one to few in the cat (Belmonte and Gallego, 1983); no evidence of “hump” was present in tracings from rat chemoreceptor neurons (Donnelly, 1999).
In order to better resolve the basis for these differences, patch clamp recordings were undertaken on neurons dissociated from petrosal ganglia, which had been pre-labeled from the carotid body (Cummins et al., 2002). Measurements of Na+ currents in these neurons showed a uniform population with half-activation potential of −23mV and half inactivation potential of −70mV. Later, we recorded Na+ current in intact ganglia in which the chemoreceptor modality could be definitely identified based on spontaneous activity and response to excitatory agents (Fig 2)(Donnelly, 2011). In both cases the inactivation/membrane potential relationship was smoothly fit by a single Boltzman’s function, suggesting the presence of only a TTX-S type current and a lack of TTX-R current. Since TTX-R current inactivates atmore positive voltages than TTX-S currents, its presence would have yielded a poor fit to a single Boltzman’s function. In contrast to chemoreceptor neurons, a study of all cells in petrosal ganglia determined that >50% of cells only express TTX-R current and another 10% express a mixture of TTX-R and TTX-S (Stea and Nurse, 1992). If carotid body innervation was based on a non-biased representation of petrosal neurons, then the chance of only observing TTX-S currents was estimated at 5.7E-48 (Donnelly, 2011), suggesting that chemoreception modality was closely associated with a TTX-S Na+ channel isoform.
What, then, to make of the TTX-R current in cat chemoreceptor neurons? Firstly, this was primarily observed in myelinated chemoreceptor neurons, which appear to be absent in the rat. The very small sample of non-myelinated chemoreceptor neurons in the cat showed little in the way of an AP “hump” and also generated multiple APs during a sustained depolarization, unlike myelinated fibers (Belmonte and Gallego, 1983). In another study of cat, all chemoreceptors were myelinated and only neurons without the hump (i.e., only expressing TTX-S) demonstrated multiple APs during a prolonged depolarization (Varas et al., 2003). The species reconciliation, as best we can do it, is that rat chemoreceptor neurons are unmyelinated and only express TTX-S while cat chemoreceptor neurons are primarily myelinated and express a significant amount of TTX-R. Since the activation voltage for TTX-R is positive to TTX-S the lack of multiple AP generation in the cat during sustained depolarization is due to the greater degree of depolarization required to reach threshold and, thus, greater inactivation of the TTX-S component following the AP as well as greater activation of voltage-gated K+ currents.
This leaves unresolved the specific rolls of TTX-S and TTX-R in determining chemoreceptor modality, but part of the solution may lie in the cellular distribution of ion channels. For unmyelinated, non-chemoreceptive fibers, Nav1.2 isoform is homogenously distributed in the axon and nerve terminals (Gong et al., 1999; Westenbroek et al., 1989). Nav1.2 also appears to be the primary isoform in chemoreceptor neurons (of rat) based on the kinetics of recovery from inactivation (Cummins et al., 2002). On the other hand, myelinated fibers appear more complicated in their isoform distribution. Immature nodes of Ranvier of non-chemoreceptive neurons initially express Nav1.2 but this is replaced by Nav1.6 during myelination (Boiko et al., 2001). Nav1.6, is also found in the initial segment and nodes of Ranvier without detectable levels in the axon terminals or soma suggesting a subcellular segregration of channel isoforms (Boiko et al., 2001; Van Wart et al., 2007). Similarly, the TTX-R isoform, Nav1.8, appears to be primarily located in the soma but is able to translocate following nerve injury so its distribution is dependent on experience (Novakovic et al., 1998). Thus, the presence of a TTX-R current in the soma of cat chemoreceptor neurons may not be indicative of the type of Na+ current present in the cat axons. AP propagation in cat chemoreceptor fibers is blocked by TTX suggesting the conducting portion of the axon is primarily supported by TTX-S type channels (Gallego, 1983). However, this does not preclude a role for TTX-R current at nerve terminals of myelinated axons. In a non-chemoreceptor fiber – pain fibers of the guinea pig cornea – sodium currents at the nerve terminals appear to continue in the presence of TTX, suggesting a TTX-R type channel at the terminal (Brock et al., 1998). In summary, a TTX-S channel, likely Nav1.2, plays a major role in AP initiation and conduction in unmyelinated chemoreceptive fibers of rat. In myelinated fibers, which are prevalent in the cat, Na+ channel distribution and functional roles in spike initiation and conduction have not been resolved.
2.5 Developmental changes in characteristics of Na+ currents in chemoreceptor afferent neurons
Two studies have examined this question based on the hypothesis that changes in excitability caused by changes in Na+ current characteristics may contribute to chemoreceptor maturation. In the first, using dissociated neurons pre-labeled from the carotid body, the magnitude of the Na+ current increased in the post-natal period, but this was associated with an increase in cell size. When normalized to cell size, the density of Na+ current was unchanged (Cummins et al., 2002). In addition, neither voltage-dependence of activation or voltage-dependence of inactivation changed in the post-natal period (Cummins et al., 2002). However, there were some concerns that the act of dissociation or low recording temperature may have influenced the results since dissociation enhances excitability (Zhang et al., 1997). Recordings in intact petrosal ganglia yielded different results. Although channel current density was again unchanged in the post-natal period, the voltage dependence was changed. The voltage for activation was significantly left-shifted in mature chemoreceptor neurons but no change occurred in the voltage for inactivation (Fig 2)(Donnelly, 2011). The net effect of the voltage shift is a greater overlap between the activation and inactivation curves and this coincides with the resting potential of these neurons.
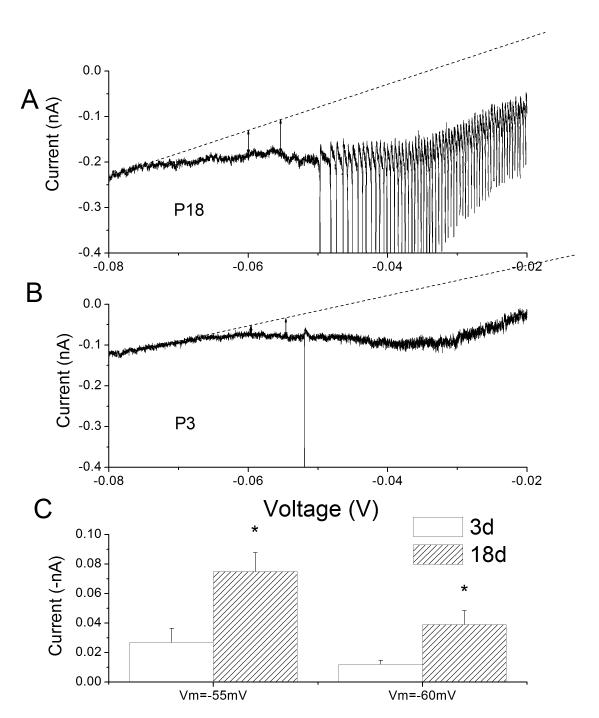
The overlap region is an area of incomplete inactivation and partial activation, producing a non-inactivating or persistent Na+ current (INaP). This is more clearly recorded by applying a ramp depolarization which is applied slow enough to allow a steady-state to form in the activation and inactivation processes (Fig 3). As predicted from the activation/inactivation relationships, a slow ramp depolarization produced a downward deflection of the current trace suggesting an enhancement of excitability (Fig 3). Furthermore, the ramp depolarizations produced greater inward (depolarizing) currents in the mature compared to the immature. This developmental increase has been previously observed in pyramidal neurons from rat sensorimotor cortex (Alzheimer et al., 1993).
Fig 3.
Voltage-dependent inward currents during ramp depolarizations increase with age. (A) Representative somal currents as a function of voltage during a ramp depolarization from −80mV to +20mV (20mV/sec) in an intact petrosal chemoreceptor neurons from a P18 rat (A) and P3 rat (B). Sharp downward deflections are ectopic-generated APs. Dotted line is the leak subtracted, zero current level. (C) Net inward current was measured at −60, and −55 mV which covers the range of estimates of chemoreceptor resting potentials. Net inward current was significantly larger in mature (P18) than immature (P2-P3) cells. Adapted from (Donnelly, 2011).
3 Potential roles of Na+ currents in the AP generation process
As mentioned above, Na+ channel activation around resting potential may serve to boost the magnitude of a depolarizing event like an EPSP. Could the Na+ channels themselves be responsible for AP generation? The question is difficult to directly investigate since high fidelity (ie, patch clamp) recording of chemoreceptor nerve terminals has not yet been undertaken. However, in silico modeling studies may give some clue as a potential role.
Using the activation/inactivation characteristics of Na+ currents as developed above, we modeled the effect of placement of these channel in small nerve fibers. Resting potential, leak current and voltage-activated outward (K+) current parameters were based on somal measurements and extrapolated to the nerve ending structure using the same current density. Leak and voltage-activated K+ currents were modeled as an ensemble process (that is, not as individual channels and produced no voltage noise). As the nerve structure became smaller, the effect of channel noise produced by the opening of individual Na+ channels (due to the occasional dissociation of the h particle of figure 1) became greater, occasionally producing APs. A similar modeling result using different Na+ channel characteristics had been previously described (Chow and White, 1996). In both cases, as the nerve structure became smaller, the noise produced by channel opening increased, eventually leading to spontaneous AP generation.
The theoretical spike train shared many characteristics previously recorded on real single-unit chemoreceptor spike trains. The interspike-interval distribution of both trains is random (Poisson) at both low and high discharge rates (neglecting the effects of the refractory period on interval distribution) (Biscoe and Taylor, 1963; Eyzaguirre and Koyano, 1965). Since prolonged somal depolarization of rat chemoreceptor neurons produces a repetitive discharge pattern and assuming an extrapolation of somal electrical characteristics to the nerve ending, it suggests that a prolonged (> a few milliseconds) depolarization does not occur at the nerve terminal. If it did then the pattern would diverge from random, particularly at higher (stimulated) discharge rates. The theoretical model also predicted the AP generation process would be highly sensitive to levels of extracellular Na+, a result which had been described previously (Donnelly et al., 1998).
This type of spike generation process may offer an explanation to an apparent paradox. Single-unit chemoreceptor discharge activities have been recorded in a number of species (cat, rabbit, dog, goat, mouse) and all have a low rate under normoxia or hyperoxia (<1Hz) and a discharge rate over 10-20Hz under severe hypoxia, that is, a 10-20 fold increase in discharge rate (Eyzaguirre and Koyano, 1965). However, measurements of transmitter release suggest a much smaller increase in magnitude of change. ATP acting through P2X receptors is currently a strong candidate for mediating much of the nerve excitation during hypoxia. However, the increase in ATP release in going from hyperoxia (or normoxia) to strong hypoxia is much lower: 1-2X in whole rat carotid body (Buttigieg and Nurse, 2004)and 2X in whole cat carotid body (Fitzgerald et al., 2009). How can a 1-2 fold increase in excitatory transmitter release produce a 10-fold increase in nerve activity? If a quantal release of transmitter produced an EPSP then, at best, there should be a 1:1 relationship. (A two-fold increase in transmitter release produces a two-fold increase in nerve activity.)
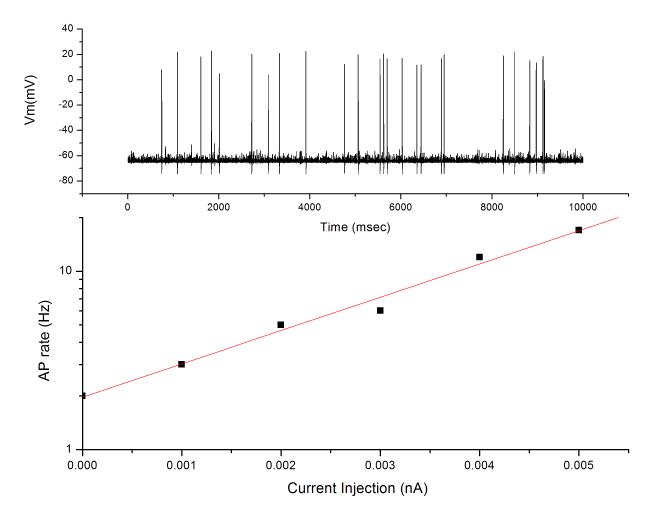
Using the same simulated nerve-ending model as described above, a series of small depolarizing currents was applied to the nerve ending model and the effect on (theoretical) AP generation rates observed. The relationship was not linear, but rather exponential, such that a two-fold increase in depolarizing current produced a 10-fold increase in AP generation (Fig 4). Although currently lacking experimental confirmation, this model of AP generation presents a possible explanation for the relatively small increase in excitatory transmitter release observed in several studies.
Fig 4.
Simulated APs generated in a model axon of 0.5μM diameter with a Na+ channel density of 30/μm2. (upper) Representative 10s tracing of spontaneous AP generation due to the membrane noise generated by the stochastic openings of Na+ channels. (lower) Application of a continuous depolarizing current in the model increased AP generation rate in an exponential fashion. Note log scale on ordinate.
3.1 Role of INaP in chemoreceptor function
In order to test the potential role of INaP in chemoreceptor AP generation we undertook experiments utilizing drugs that preferentially reduce INaP compared to the transient, voltage-activated Na+ current. The most commonly used drug for this type of study is riluzole – a drug used clinically to reduce the spasticity of Amyotrophic Lateral Sclerosis and that appears to stabilize the inactivated state of the Na+ channel. Although high concentrations of this drug reduces the transient current, at low concentrations – ca 5-10μM – INaP is preferentially reduced (Spadoni et al., 2002; Urbani and Belluzzi, 2000). In isolated chemoreceptors, riluzole reduced AP activity in normoxia and during hypoxia without changing the AP conduction time, suggesting an effect of riluzole on Na+ channels which is independent on their role in AP conduction (Faustino and Donnelly, 2006a). Finally, in unanesthetized rats, riluzole greatly reduced the ventilatory response to acute hypoxia but had a much smaller effect on the ventilatory response to acute hypercapnia (Faustino and Donnelly, 2006a).
Other drugs that are used clinically may also reduce INaP. Two widely used anti-epileptic drugs are phenytoin and lamotrigine, both of which target Na+ channels. Similar to riluzole, administration, in vivo, significantly reduces the ventilatory response to acute hypoxia and, in isolated chemoreceptors, reduces the AP activity during both normoxia and acute hypoxia (Faustino and Donnelly, 2006b).
Taken together, these results suggest that the persistent current generated by Na+ channels around the resting potential play an important role in determining the level of chemoreceptor afferent activity. Secondly, it suggests that clinically useful drugs that target Na+ channels (e.g. drugs for ALS, epilepsy and pain) may have the unintended consequence of reducing the ventilatory response to hypoxia and, hence, raise the possibility of ventilatory instabilities produced by these drugs.
4 Summary and future directions
Most work directed to understanding the mechanism of chemo-transduction has focused on the glomus cell – a secretory cell associated with chemoreceptor nerve terminals. Several oxygen-sensitive channels have been identified in these cells and they initiate membrane depolarization, a rise in intracellular calcium and release of secretory granules. With this schema in place, it seems logical that this secretion is stimulatory to the afferent nerve fibers by producing EPSP like depolarizations. However, there are a number of observations suggesting that the workings are more complicated, including a greater role for the afferent nerve endings. (These aspects are not presented in their entirety.)
 Applications of drugs which block receptors for our best-candidate excitatory transmitters sometimes fail to ablate or even reduce chemoreceptor AP generation. For instance, simultaneous application of suramin (to block purinergic) and mecamylamine (to block nicotinic receptors) in cat failed to produce a large reduction in chemoreceptor activity or the ventilatory response to hypoxia (Reyes et al., 2007a, b).
Applications of drugs which block receptors for our best-candidate excitatory transmitters sometimes fail to ablate or even reduce chemoreceptor AP generation. For instance, simultaneous application of suramin (to block purinergic) and mecamylamine (to block nicotinic receptors) in cat failed to produce a large reduction in chemoreceptor activity or the ventilatory response to hypoxia (Reyes et al., 2007a, b). The histologic appearance of glomus cell/afferent nerve contacts demonstrates some synaptic characteristics but lack clustering of synaptic vesicles. This led Verna to write, “I wonder if it is possible to say that the existence of a chemical synapse between glomus cells and sensory nerve endings is really demonstrated!” (Verna, 1997). Similarly, Gronblad examined vesicle fusion sites and failed to observe any fusion events in the area between glomus cells and nerve endings ‘despite careful scrutiny’ (Gronblad, 1983). Without a defined synaptic structure, it seems unlikely that EPSP like events can be generated from relatively distant transmitter release sites.
The histologic appearance of glomus cell/afferent nerve contacts demonstrates some synaptic characteristics but lack clustering of synaptic vesicles. This led Verna to write, “I wonder if it is possible to say that the existence of a chemical synapse between glomus cells and sensory nerve endings is really demonstrated!” (Verna, 1997). Similarly, Gronblad examined vesicle fusion sites and failed to observe any fusion events in the area between glomus cells and nerve endings ‘despite careful scrutiny’ (Gronblad, 1983). Without a defined synaptic structure, it seems unlikely that EPSP like events can be generated from relatively distant transmitter release sites. Hypoxia-induced release of catecholamine, which can be detected in real time using voltammetry and considered as a proxy for release of co-stored transmitters, may poorly encode the magnitude of nerve response (Donnelly, 1996; Iturriaga et al., 1996). The most dramatic example of this dissociation is produced by high levels of carbon monoxide which cause both catecholamine release and an increase in afferent nerve activity. Exposure to intense white light completely reverses the nerve excitation with little effect on catecholamine release leaving the nerve response dissociated from glomus cell secretion(Buerk et al., 1997).
Hypoxia-induced release of catecholamine, which can be detected in real time using voltammetry and considered as a proxy for release of co-stored transmitters, may poorly encode the magnitude of nerve response (Donnelly, 1996; Iturriaga et al., 1996). The most dramatic example of this dissociation is produced by high levels of carbon monoxide which cause both catecholamine release and an increase in afferent nerve activity. Exposure to intense white light completely reverses the nerve excitation with little effect on catecholamine release leaving the nerve response dissociated from glomus cell secretion(Buerk et al., 1997). Simultaneous intracellular (sharp electrode) recordings of glomus cells and afferent nerve endings in a slice preparation of rodent carotid body failed to detect any excitatory coupling between the glomus cell and nerve ending – a result that Eyzaguirre characterized as “puzzling” (Eyzaguirre, 2005; Jiang and Eyzaguirre, 2006).
Simultaneous intracellular (sharp electrode) recordings of glomus cells and afferent nerve endings in a slice preparation of rodent carotid body failed to detect any excitatory coupling between the glomus cell and nerve ending – a result that Eyzaguirre characterized as “puzzling” (Eyzaguirre, 2005; Jiang and Eyzaguirre, 2006). In at least two instance, sinus nerve neuromas established hypoxia responsiveness despite the apparent absence of glomus cells (Kienecker and Knoche, 1977; Kienecker et al., 1978; Mitchell et al., 1972). This suggests that chemoreceptor nerve fibers may possess an innate hypoxia-sensing mechanism.
In at least two instance, sinus nerve neuromas established hypoxia responsiveness despite the apparent absence of glomus cells (Kienecker and Knoche, 1977; Kienecker et al., 1978; Mitchell et al., 1972). This suggests that chemoreceptor nerve fibers may possess an innate hypoxia-sensing mechanism.
These experiments strongly suggest that there are other important elements contributing to chemoreceptor transduction. Here, we have focused on the potential role of Na+ channels in the afferent nerve fibers. These channels, as reflected by the characteristics of Na+ currents at the soma, seem wedded to the chemoreceptor modality. That is, most C-fibers express TTX-R currents, sometimes solely TTX-R current, yet all rat chemoreceptor cells that we have recorded only express TTX-S currents. The chance probability of this happening is very low. TTX-S currents, which activate around the resting potential, are known to generate persistent inward currents that can boost depolarization events and generate channel noise that can, by itself, generate APs. A role for these channels is suggested by experimental results demonstrating that drugs which reduce INaP cause a large decrease in chemoreceptor activity. Furthermore, the magnitude of INaP increases in the post-natal period at the same time chemoreceptor activity becomes more sensitive to acute hypoxia. While causality has not been established, the correlation is intriguing.
In addition to contributing to AP generation, INaP may be a direct contributor to hypoxia transduction. Hypoxia and mitochondrial blockers increase INaP in caudal hypothalamic neurons which sense hypoxia (Horn and Waldrop, 2000). In rat hippocampus, both hypoxia and inhibition of oxidative metabolism enhance INaP (Hammarstrom and Gage, 1998, 2000). Hypoxia also enhances INaP in cardiac myocytes (Ju et al., 1996), an effect which can be reproduced in HEK293 cells expressing the cardiac Na+ channel isoform (Fearon and Brown, 2004).
The question of how APs are generated in chemoreceptor nerve terminals would be best resolved with high resolution (i.e., patch clamp) recordings of nerve terminal activity. At present, the technical challenge appears to be beyond our abilities. An alternate might be the examination of post-synaptic activity in the co-culture model of Nurse and colleagues where glomus cells and petrosal neurons are allowed to reestablish synaptic connections in culture (Zhong et al., 1997). Post-synaptic activity from the petrosal neurons is recorded from the soma and, if the physical separation is small then post-synaptic activity is recorded through the cable properties of the connection. While this has proven quite useful in addressing the roles of several neurotransmitters, it is likely not useful for determining the role of INaP. The ability of a few Na+ channels to generate a significant depolarization is dependent on the low capacitance and high axial resistance of small nerve fibers. The somal coupling through a short cable, essential for the benefits of co-culture recording, would obviate depolarizing events caused by Na+ channels due to the high shunt capacitance imposed by the soma.
In conclusion, chemoreceptor afferent nerve fibers should be considered as equal partners to the glomus cell in establishing the chemoreceptor modality. In this regard, an identification of the types and placement of Na+ channels in the chemoreceptor nerve terminals may better establish their role in the process. In addition, an understanding of how development or modulatory factors such as hypoxia and reactive oxygen species alter the biophysical characteristics of chemoreceptor Na+ channels may shed new light on how chemoreceptor sensitivity is altered by age and environment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal N, Hamam BN, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in rat entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. doi: 10.1016/s0306-4522(00)00455-3. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslava V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nature Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Postnatal development of a persistent Na+ current in pyramidal neurons from rat sensorimotor cortex. J Neurophysiol. 1993;69:290–292. doi: 10.1152/jn.1993.69.1.290. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J. Physiol. (London) 1983;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Gallego R, Morales A. Membrane properties of primary sensory neurones of the cat after peripheral reinnervation. J. Physiol. (London) 1988;405:219–232. doi: 10.1113/jphysiol.1988.sp017330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Taylor A. The discharge pattern recorded in chemoreceptor afferent fibres from the cat carotid body with normal circulation and during perfusion. J. Physiol. (London) 1963;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol. 1998;512(Pt 1):211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. (London) 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerk DG, Chugh DK, Osanai S, Mokashi A, Lahiri S. Dopamine increases in cat carotid body during excitation by carbon monoxide: implications for a chromophore theory of chemoreception. J. Autonomic Nervous System. 1997;67:130–136. doi: 10.1016/s0165-1838(97)00098-2. [DOI] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Chahine M, Ziane R, Vijayaragavan K, Okamura Y. Regulation of Na v channels in sensory neurons. Trends Pharmacol Sci. 2005;26:496–502. doi: 10.1016/j.tips.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Chow CC, White JA. Spontaneous action potentials due to channel fluctuations. Biophysical J. 1996;71:3013–3021. doi: 10.1016/S0006-3495(96)79494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Ann. Rev. Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Waxman SG, Donnelly DF. Characterization and developmental changes of Na+ currents of petrosal neurons with projections to the carotid body. J. Neurophysiol. 2002;88:2993–3002. doi: 10.1152/jn.00350.2002. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Chemoreceptor nerve excitation may not be proportional to catecholamine secretion. J. Appl. Physiol. 1996;81:657–664. doi: 10.1152/jappl.1996.81.2.657. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Developmental changes in membrane properties of chemoreceptor afferent neurons of the rat petrosal ganglia. J. Neurophysiol. 1999;82:209–215. doi: 10.1152/jn.1999.82.1.209. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Developmental changes in the magnitude and activation characteristics of Na(+) currents of petrosal neurons projecting to the carotid body. Respir Physiol Neurobiol. 2011;177:284–293. doi: 10.1016/j.resp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Panisello JM, Boggs D. Effect of sodium perturbations on rat chemoreceptor spike generation: implications for a Poisson model. J. Physiol. (London) 1998;511:301–311. doi: 10.1111/j.1469-7793.1998.301bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effect of hyperoxia from birth on the carotid chemoreceptor and ventilatory responses of rats to acute hypoxia. J. Physiol. (London) 1986;374:24P. [Google Scholar]
- Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J. Physiol. (London) 1987;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol Res. 2005;38:341–345. doi: 10.4067/s0716-97602005000400005. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Koyano H. Effects of hypoxia, hypercapnia and pH on the chemoreceptor activity of the carotid body in vitro. J. Physiol. (London) 1965;178:385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino EV, Donnelly DF. An important functional role of persistent Na+ current in carotid body hypoxia transduction. J Appl Physiol. 2006a;101:1076–1084. doi: 10.1152/japplphysiol.00090.2006. [DOI] [PubMed] [Google Scholar]
- Faustino EV, Donnelly DF. Lamotrigine and phenytoin, but not amiodarone, impair peripheral chemoreceptor responses to hypoxia. J Appl Physiol. 2006b;101:1633–1640. doi: 10.1152/japplphysiol.00633.2006. [DOI] [PubMed] [Google Scholar]
- Fearon IM, Brown ST. Acute and chronic hypoxic regulation of recombinant hNa(v)1.5 alpha subunits. Biochem Biophys Res Commun. 2004;324:1289–1295. doi: 10.1016/j.bbrc.2004.09.188. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Chang I, Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res. 2009;1270:39–44. doi: 10.1016/j.brainres.2009.02.078. [DOI] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J. Physiol. (London) 1983;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- Gronblad M. Improved demonstration of exocytotic profiles in glomus cells of rat carotid body after perfusion with glutaraldehyde fixative containing a high concentration of potassium. Cell Tissue Res. 1983;229:627–637. doi: 10.1007/BF00207702. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AKM, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J. Physiol. (London) 1998;510:735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AKM, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J. Physiol. (London) 2000;529:107–118. doi: 10.1111/j.1469-7793.2000.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Kumar P, Williams BA. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J. Physiol. (London) 1989;411:563–574. doi: 10.1113/jphysiol.1989.sp017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (London) 1952;10:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn EM, Waldrop TG. Hypoxic augmentation of fast-inactivating and persistent sodium currents in rat caudal hypothalamic neurons. J. Neurophysiol. 2000;84:2572–2581. doi: 10.1152/jn.2000.84.5.2572. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J, Zapata P. Dissociation of hypoxia-induced chemosensory responses and catecholamine efflux in cat carotid body superfused in vitro. J. Physiol. (London) 1996;497:551–564. doi: 10.1113/jphysiol.1996.sp021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Varas R, Alcayaga J. Electrical and pharmacological properties of petrosal ganglion neurons that innervate the carotid body. Respir Physiol Neurobiol. 2007;157:130–139. doi: 10.1016/j.resp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Jiang RG, Eyzaguirre C. Effects of prolonged hypobaric hypoxia on carotid nerve endings and glomus cells. Changes in intercellular coupling. Brain Res. 2006;1076:198–208. doi: 10.1016/j.brainres.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J. Physiol. (London) 1996;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J. Physiol. (London) 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienecker EW, Knoche H. Regeneration of nerves and nerve terminals in rabbit carotid body following carotid nerve sectioning and suturing. Springer-Verlag; Berlin: 1977. [Google Scholar]
- Kienecker EW, Knoche H, Bingmann D. Functional properties of regenerating sinus nerve fibres in the rabbit. Neurosci. 1978;3:977–988. doi: 10.1016/0306-4522(78)90118-5. [DOI] [PubMed] [Google Scholar]
- Marchal F, Bairam A, Haouzi P, Crance JP, Di Giulio C, Vert P, Lahiri S. Carotid chemoreceptor response to natural stimuli in the newborn kitten. Respir. Physiol. 1992;87:183–193. doi: 10.1016/0034-5687(92)90058-5. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis. J. Neurocytol. 1975;4:177–230. [Google Scholar]
- Mitchell RA, Sinha AK, McDonald DM. Chemoreceptive properties of regenerated endings of the carotid sinus nerve. Brain Res. 1972;43:681–685. doi: 10.1016/0006-8993(72)90430-1. [DOI] [PubMed] [Google Scholar]
- Mulligan EM. Discharge properties of carotid bodies: developmental aspects. In: Haddad GG, Farber J.P. (Eds.), editors. Developmental Neurobiology of Breathing. Marcel Dekker, Inc.; New York, NY: 1991. pp. 321–340. [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC, Kumar P. Postnatal development of CO2-O2 interaction in the rat carotid body in vitro. J. Physiol. (London) 1995;485:531–541. doi: 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes EP, Fernandez R, Larrain C, Zapata P. Carotid body chemosensory activity and ventilatory chemoreflexes in cats persist after combined cholinergic-purinergic block. Respir Physiol Neurobiol. 2007a;156:23–32. doi: 10.1016/j.resp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Reyes EP, Fernandez R, Larrain C, Zapata P. Effects of combined cholinergic-purinergic block upon cat carotid body chemoreceptors in vitro. Respir Physiol Neurobiol. 2007b;156:17–22. doi: 10.1016/j.resp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995;74:2220–2224. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- Spadoni F, Hainsworth AH, Mercuri NB, Caputi L, Martella G, Lavaroni F, Bernardi G, Stefani A. Lamotrigine derivatives and riluzole inhibit INa,P in cortical neurons. Neuroreport. 2002;13:1167–1170. doi: 10.1097/00001756-200207020-00019. [DOI] [PubMed] [Google Scholar]
- Stea A, Nurse CA. Whole-cell currents in two subpopulations of cultured rat petrosal neurons with different tetrodotoxin sensitivities. Neuroscience. 1992;47:727–736. doi: 10.1016/0306-4522(92)90180-a. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Traub RD, Buhl EH, Gloveli T, Whittington MA. Fast rhythmic bursting can be induced in layer 2/3 cortical neurons by enhancing persistent Na+ conductance or by blocking BK channels. J Neurophysiol. 2003;89:909–921. doi: 10.1152/jn.00573.2002. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Varas R, Alcayaga J, Iturriaga R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res. 2003;988:154–163. doi: 10.1016/s0006-8993(03)03366-3. [DOI] [PubMed] [Google Scholar]
- Verna A. The mammalian carotid body: morphological data. In: Gonzalez C, editor. The carotid body chemoreceptors. Springer; Heidelberg, Germany: 1997. pp. 1–29. [Google Scholar]
- Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Sterni LM, Bamford OS, Monrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J. Physiol. (London) 1999;514:493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Progress in neurobiology. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Yao C, Williams AJ, Cui P, Berti R, Hunter JC, Tortella FC, Dave JR. Differential pattern of expression of voltage-gated sodium channel genes following ischemic brain injury in rats. Neurotoxicity research. 2002;4:67–75. doi: 10.1080/10298420290007646. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, Song XJ, LaMotte RH. Axotomy increases the excitability of doral root ganglion cells with unmyelinated axons. J. Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J. Physiol. (London) 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Wu L, Wiesner EP. Persistent sodium current contributes to induced voltage oscillations in locomotor-related hb9 interneurons in the mouse spinal cord. J Neurophysiol. 2008;100:2254–2264. doi: 10.1152/jn.90437.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]