Abstract
A reversible neuronal inactivation procedure was used to study the role of the medial orbital cortex (MO) and medial tip of the subthalamic nucleus (mSTN) in maintenance of cocaine self-administration studied under a second-order schedule of drug and cue presentation. Lidocaine or vehicle was infused 5-min before 1-hr self-administration test sessions, using bilateral, asymmetric or unilateral manipulations. The results demonstrated that whether the MO was inactivated bilaterally, unilaterally or asymmetrically (with contralateral mSTN inactivation), cocaine seeking and cocaine intake were reduced. In contrast, bilateral mSTN inactivation did not impact cocaine seeking or cocaine intake, suggesting that the reductions in these measures following asymmetric inactivation may have been due to a unilateral influence of lidocaine in MO. Expression of c-Fos protein was measured in sites downstream of the STN to ensure that the lidocaine inactivation procedure was effective in selectively altering activity of neurons in mSTN. Cocaine-induced c-Fos protein expression was augmented only in the ipsilateral nucleus accumbens core after mSTN lidocaine pretreatment, consistent with the expectation that inactivation of mSTN would disinhibit nucleus accumbens core, but not shell, activity. The present investigation shows the critical importance of the MO for maintaining cocaine seeking and cocaine intake in rats, though its projections to mSTN appear to be unimportant for this purpose. Because cocaine seeking was impacted to such a great extent (45% of baseline, on average), it is likely that MO inactivation exerts its influence on maintenance of cocaine self-administration by interfering primarily with cue-controlled behavior rather than by modifying the reinforcing effects of cocaine.
Keywords: c-Fos protein, Cocaine, Medial orbital cortex, Medial subthalamic nucleus, Nucleus accumbens, Self-administration
1. Introduction
Through work in rodents and other species, the orbitofrontal cortex has been identified as a neurosubstrate important for the cocaine addiction process [1]. In rats, large lesions that encompass the entire extent of this area disrupt acquisition of cocaine seeking studied under a second-order schedule of drug delivery [2], but were shown to facilitate acquisition while having little or no impact on the maintenance of cocaine self-administration studied under a simple fixed-ratio (FR) 1 schedule of drug delivery [3]. Importantly, the rodent orbital area consists of multiple subdivisions, labeled as the medial orbital (MO), ventral orbital, ventral lateral orbital, lateral orbital (LO), dorsal lateral orbital, and agranular insular cortices, and each subdivision has its own pattern of connectivity to cortical and subcortical structures [4, 5]. These different subdivisions may have heterogeneous control over cocaine self-administration behavior. Along these lines, it has been shown that temporary neuronal inactivation of the LO, but not agranular insular subdivision, reduces maintenance of cocaine self-administration studied under a second-order schedule of drug delivery [6, 7]. However, when studied under an FR1 schedule, lesions of the LO or MO do not impact maintenance of cocaine self-administration [8, 9]. Though less well studied than the LO, the MO is implicated in responding to incentive stimuli [10]. It is highly relevant that response-contingent cocaine-paired stimuli that are briefly presented during intervals between periods of cocaine availability are what primarily maintain the very high rates of responding typically observed under a second-order schedule of drug delivery [11]. Thus, an evaluation of how MO inactivation may impact maintenance of cocaine self-administration under a second-order schedule is warranted.
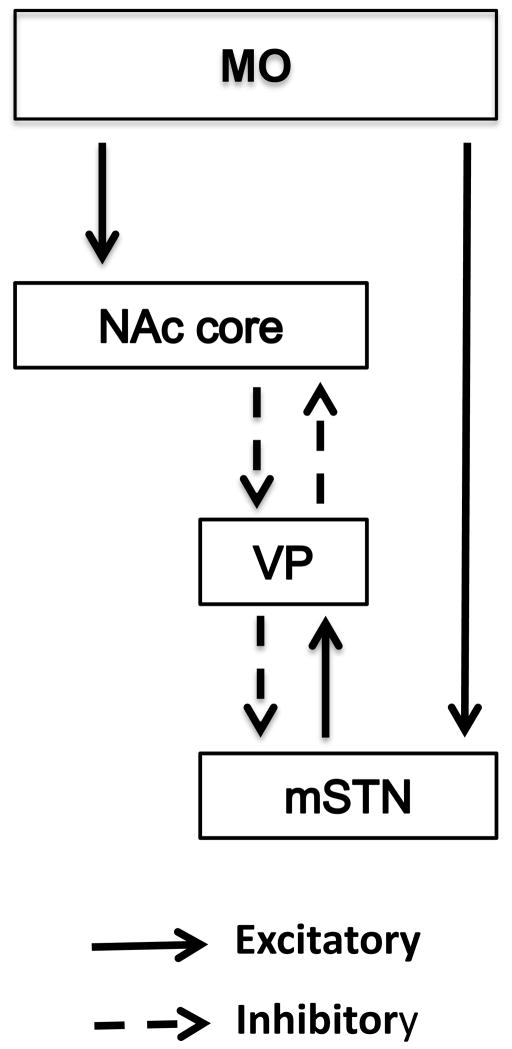
Over the past several years, investigations concerning the neural control of cocaine-seeking and cocaine-taking behavior have begun to focus on serial connections between brain sites rather than conducting only single brain site manipulations, e.g. [12–19]. Of potential relevance to the study of MO control over cocaine self-administration behavior are circuits that engage the subthalamic nucleus (STN). The STN in rat is divided into dorsolateral (motor function), ventromedial (associative function) and medial tip (limbic function) subdivisions, with each having a unique set of inputs and outputs [20]. The MO sends excitatory projections to the medial tip of the STN (mSTN) either through a direct MO-mSTN pathway or through an indirect pathway [21]. For the indirect pathway, excitatory projections from MO first reach the nucleus accumbens (NAc) core that sends inhibitory projections to ventral pallidum (VP), which in turn sends inhibitory projections to mSTN [22]. In this indirect way, MO and NAc core activation disinhibits mSTN activity. There also are excitatory projections from mSTN to the VP that sends reciprocal inhibitory projections to NAc core [23, 24]. In this indirect way, mSTN activation inhibits NAc core activity. A diagram of these connections is shown in Figure 1.
Figure 1.
Diagram showing the excitatory (solid lines) and inhibitory (dashed lines) connections between the medial orbital cortex (MO), nucleus accumbens core (NAc core), ventral pallidum (VP) and medial tip of the subthalamic nucleus (mSTN).
While subdivision-selective manipulation of STN in relation to cocaine self-administration behavior has yet to be reported, research based on damage to the whole STN is conflicting. Both increases and decreases, and even no changes in cocaine self-administration behavior have been reported under FR1 and/or progressive ratio schedules of drug delivery [25, 26]. A more recent study demonstrated that rats with whole STN damage exhibited facilitated sign-tracking behavior toward a cocaine-paired cue, suggesting a role for this site in responding to cocaine-paired incentive stimuli [27]. Given the anatomical connections between MO and mSTN, the present investigation used a temporary neuronal inactivation procedure to determine if bilateral (same site in both hemispheres), asymmetric (different sites in opposite hemispheres), and unilateral (single site in one hemisphere) manipulations of MO or mSTN could modify the maintenance of cocaine self-administration behavior. As each site has a role in mediating responding to incentive stimuli (MO supporting; mSTN inhibiting), we predict that inactivation of the MO will disrupt whereas inactivation of the mSTN will enhance cocaine-seeking behavior under a second-order schedule of drug delivery. As the individual sites are predicted to have opposing influences, asymmetric manipulation may be ineffective.
2. Materials and methods
2.1 Subjects
Male Wistar rats (Crl(WI)BR; 275–300 g; Charles River Laboratories, Wilmington, MA) were housed individually in clear plastic cages (24 cm X 22 cm X 20 cm) inside a temperature-(21–23 °C) and light- (08:00 h on, 20:00 h off) controlled vivarium. All experimental procedures were conduced during the light phase of the cycle. After a 72 hr acclimation period, rats began moderate food restriction (16–20 g/day) to maintain body weight at 85–90% of a growth-adjusted ad libitum weight. Food restriction initially was used to facilitate acquisition of lever responding maintained by food pellet delivery. Food restriction was continued so that rats maintained a more moderate increase in body weight over the course of the experiment. The slower growth preserved longevity of intravenous catheters and prevented large fluctuations in the absolute amount of cocaine (dose is based on body weight) received during self-administration sessions at the beginning relative to the end of the experiment. Water was freely available except during experimental sessions. Rats were maintained in accordance with the 1996 NIH Guide for Care and Use of Laboratory Animals and the Boston University Institutional Animal Care and Use Committee approved research protocols.
2.2 Apparatus
Experimental chambers (model ENV-008CT; Med Associates, St. Albans VT), as previously described in detail [11], were used for all behavioral sessions. Motor-driven syringe pumps were used for intravenous (Model PHM-100; Med Associates) and intracranial (Orion/Sage, Model 341A, Boston, MA) drug delivery.
2.3 Drugs
Cocaine hydrochloride (gift from NIDA, Bethesda, MD) was dissolved in sterile 0.9% saline containing 3 IU heparin/ml to a concentration of 0.81 mg/ml. A 0.3 mg/kg unit dose was delivered intravenously (i.v.) at a rate of 0.03 ml/sec. Infusion volume was adjusted for body weight using a delivery time of 1.2 sec/100 g body weight. On test day, a 20% (200 mg/ml) solution of lidocaine hydrochloride (Sigma Chemical Co., St. Louis, MO) was made fresh in sterile 0.9% saline for intracranial delivery. Sterile 0.9% saline was used as the vehicle control.
2.4 Surgery and daily catheter maintenance
Prior to surgery, rats were anesthetized with an intraperitoneal injection of 80 mg/kg ketamine plus 8 mg/kg xylazine. A silicon catheter (inner diameter, 0.51 mm; outer diameter, 0.94 mm) was attached to an L-shaped pedestal (Plastics One, Roanoke, VA) and implanted into the right jugular vein. Guide cannulae (22 gauge; Plastics One) were implanted 1 mm above the MO (Anterior-Posterior [AP] +5.2 mm, Medial-Lateral [ML] ±2.0 mm [at a 15° angle], Dorsal-Ventral [DV] −4.5 mm,) or mSTN (AP −3.7 mm, ML ±2.0 mm, DV −6.8 mm). Placements, based on the Swanson atlas [28], were either bilaterally positioned in each site, or were asymmetrically positioned into the MO of one hemisphere and the mSTN of the contralateral hemisphere (right and left sides counterbalanced for each site). Three stainless steel screws were inserted into the skull to serve as anchors for the pedestal and guide cannulae that were imbedded in dental acrylic. To manage postoperative pain, rats received subcutaneous buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) twice daily at a dose of 0.05 mg/kg, and children’s acetaminophen (CVS brand, Woonsocket, RI) that was provided in the drinking water (5 ml/250 ml water) for the first 72 hr following surgery. To prevent infection following surgery, wounds were treated daily with furazolidone powder (Veterinary Products Laboratories, Phoenix, AZ) until healed, and Baytril (Bayer Healthcare, Shawnee Mission, KS) was provided in the drinking water for 10 days post-surgery. Animals were allowed one week to recover from surgery before beginning self-administration sessions.
Catheters were maintained by daily flushing (Monday–Friday) with 0.1 ml of a 0.9% saline solution containing 3 IU of heparin and 6.7 mg of Timentin (Glaxo-SmithKline, Research Triangle Park, NC). On Fridays, a locking solution consisting of glycerol and undiluted (1000 IU/ml) heparin (3:1) was used to fill the catheter dead space. This solution remained in the catheters until Monday, when it was removed and replaced with the heparin/saline solution prior to the start of behavioral sessions. Additionally, catheters were checked weekly for patency by infusing i.v. a 1.0 mg/0.1 ml solution of Brevital (King Pharmaceuticals, Bristol, TN) to produce a rapid temporary loss of muscle tone. A new catheter was implanted into the right femoral vein to replace a leaking or non-functional catheter.
2.5 Intracranial drug delivery
On test day, rats received bilateral or asymmetric infusions of vehicle or 100 μg lidocaine (0.5 μl/side) delivered at a rate of 0.59 μl/min. The two infusions that each rat received were completed separately. The 28-gauge stainless steel infusion cannula (Plastics One) extended 1 mm beyond the guide cannula tip. The infusion cannula was left in place for 1 min following each infusion.
2.6 Histology and c-Fos immunohistochemistry
Rats were sacrificed 30 min following the test session by an overdose of sodium pentobarbital and then perfused with 0.9% saline followed by a 4% paraformaldehyde solution. The brains were extracted, post-fixed and stored at −80°C. One set of coronal sections (40 μm) was collected at the level of the MO and/or mSTN. Sections were mounted on gelatin-coated slides and stained with thionin to verify cannulae placements. In rats with bilateral or asymmetric mSTN placements, another set of sections was collected at three locations along the anterior-posterior extent of the NAc (AP +2.7 mm to +1.2 mm), and then stored in ethylene glycol solution at −20°C for later assay of c-Fos protein. Methods for processing c-Fos protein were as previously described in detail [29].
2.7 Experiment 1: Bilateral and asymmetric inactivation of MO and mSTN
Before surgery, rats were trained to lever press for food pellet delivery under an FR 1 schedule. One-week after surgery, rats were trained to self-administer 0.3 mg/kg cocaine. Training began under an FR1 schedule of cocaine delivery and cue light presentation with a 20-sec timeout period (TO) following each infusion. During the TO period, the cue light remained illuminated and the house light was extinguished. Sessions were 1 hr in length. Training requirements were gradually incremented to an FR5 schedule with a 20s-sec TO. Once responding was stable, training continued up to a terminal FI 5-min [FR5:S] second-order schedule, which was in effect for at least 12 sessions and until behavior stabilized (defined as the absence of increasing or decreasing trends and ≤10% variability in an individual rat’s response rate over 5 consecutive sessions). An average of 53±1.2 cocaine self-administration sessions, conducted Monday-Friday, was required to reach this final criterion after training was initiated. For the second-order schedule, a cue light was presented under an FR5 contingency during the entire session. The delivery of cocaine co-occurred with cue light presentation upon completion of the first FR5 after each 5-min fixed interval (FI) elapsed. A 20-sec TO followed each infusion. The cue light was illuminated for 2 sec during the 5-min FI and illuminated for the duration of the infusion and 20-sec TO period upon delivery of cocaine. During the 20-sec TO, the house light was extinguished. After the 20-sec TO, the house light was illuminated and the FI component was again in effect. During the 1-hr sessions under the terminal FI 5-min [FR5:S] second-order schedule, a rat could earn a maximum of 11 cocaine infusions. We have routinely used these second-order schedule parameters in our studies of brain site regulation of cocaine self-administration behavior. A second-order schedule was used because it provides independent measures of drug-seeking behavior and drug intake [11], and a 0.3 mg/kg unit cocaine dose was selected because it typically produces the peak number of responses and infusions under FI 5-min [FR5:S] second-order parameters [7, 30].
After establishing the self-administration baseline under the FI 5-min [FR5:S] second-order schedule, all rats were given a 14-day abstinence period during which time rats received ten 1-hr sessions in operant chambers for which levers were retracted, and without presentation of either the cocaine-paired cue light or cocaine. During these sessions, the rat’s head mount was connected to the catheter leash, as during self-administration sessions. This manipulation allowed the experimental conditions to be identical for all rats, while at the same time this manipulation restored the ability of self-administered cocaine and dampened the ability of the cocaine environment (context) to acutely express c-Fos protein on test day in the subset of rats selected for c-Fos analysis (see experiment 2 below). Rats then received a single 1hr test session under the FI 5-min [FR5:S] second-order schedule to determine if the MO and mSTN, individually or together, regulated the maintenance of cocaine seeking and cocaine intake. A total of 21 rats received vehicle infusions (8 bilateral MO, 7 bilateral mSTN and 6 asymmetric MO/mSTN) and a total of 23 rats received lidocaine infusions (7 bilateral MO, 8 bilateral mSTN and 8 asymmetric MO/mSTN) on test day, 5 min before the test session began.
2.8 Experiment 2: c-Fos protein expression in NAc after inactivation of mSTN
As the mSTN is a relatively small subdivision to target, we measured c-Fos protein expression in sites downstream of the STN to ensure that our lidocaine inactivation procedure was effective in selectively altering the activity of neurons in the mSTN. Therefore, c-Fos protein expression was measured in the NAc core and shell of rats with bilateral or asymmetric mSTN placements. Since the mSTN forms indirect reciprocal connections with the NAc core [23, 24], lidocaine inactivation that primarily targets the mSTN should selectively disinhibit activity in the NAc core. If sufficient mSTN inactivation is achieved, then lidocaine should produce greater c-Fos protein expression than vehicle in NAc core, but not in NAc shell. For this experiment, c-Fos protein expression was measured in NAc core and shell of 5 rats receiving lidocaine into both sides of the mSTN and of 4 rats receiving lidocaine into the ipsilateral side of the mSTN, for a total of 14 observations for each NAc subregion. Cell counts from rats receiving lidocaine were compared to those obtained in 4 rats receiving vehicle into both sides of the mSTN and in 5 rats receiving vehicle into the ipsilateral side of the mSTN, for a total of 13 observations for each NAc subregion. Cell counts from rats receiving lidocaine also were compared to those previously obtained in a group of 5 rats that had received yoked-saline sessions in a separate experiment identical to that described for experiment 1 (n=10 observations for each NAc subregion). During yoked-saline sessions, rats received non-contingent saline infusions and cue presentations that were paired to the performances of rats trained under an FI 5-min [FR5:S] second-order schedule of 0.3 mg/kg cocaine delivery and then given 10 abstinence sessions prior to a single test session. These latter values were used as the basal c-Fos cell counts.
2.9 Experiment 3: Unilateral inactivation of MO
Given that bilateral MO and asymmetric MO/mSTN inactivation, but not bilateral mSTN inactivation, attenuated maintenance of cocaine self-administration behavior in experiment 1, it is possible that the observed reductions after asymmetric lidocaine manipulations were due to a unilateral influence of lidocaine in the MO. Therefore, a total of 6 rats received a lidocaine infusion into the MO and a vehicle infusion into the mSTN on test day (unilateral MO). The rats that had received asymmetric vehicle infusions into the MO and mSTN in experiment 1 (asymmetric MO/mSTN vehicle) were used as the control group for statistical comparisons. The design of experiment 3 was identical to experiment 1.
2.10 Data analysis
Three behavioral measures were calculated: (1) number of active lever responses, (2) number of inactive lever responses and (3) number of cocaine infusions earned. Baseline performance was based on the average of the last five cocaine self-administration training sessions. During the 1hr test session that followed the abstinence period, active lever responses were expressed as percent of baseline active lever responses because baseline values varied 3 to 11-fold in individual rats within the different groups, though overall, groups did not differ in baseline performance (see below). All measures were analyzed by one-factor ANOVA, and the Tukey procedure was used for post-hoc testing.
3. Results
3.1 Histology
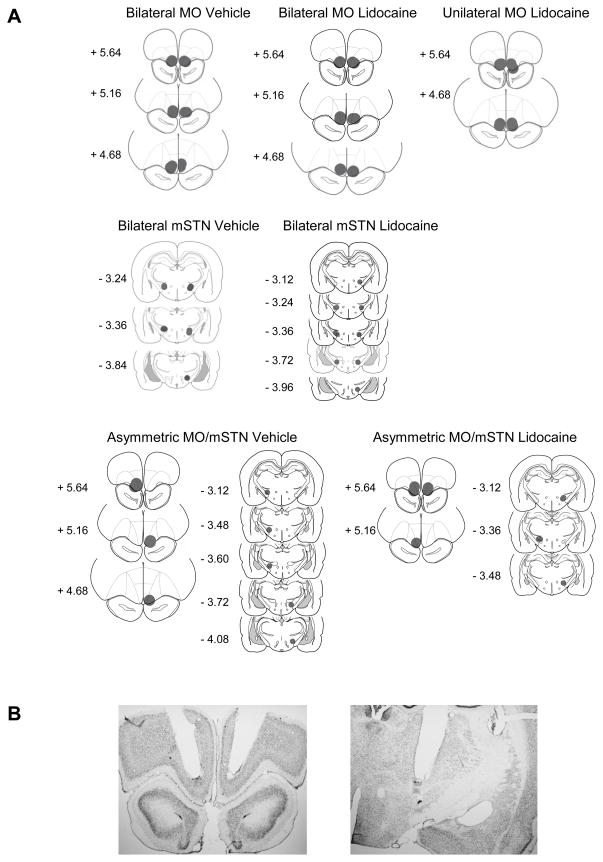
Placements were confirmed for 7 of 8 rats receiving bilateral vehicle in MO, 7 of 7 rats receiving bilateral lidocaine in MO, 5 of 7 rats receiving bilateral vehicle in mSTN, 5 of 8 rats receiving bilateral lidocaine in mSTN, 5 of 6 rats receiving asymmetric vehicle in MO/mSTN, 5 of 8 rats receiving asymmetric lidocaine in MO/mSTN, and 4 of 6 rats receiving unilateral lidocaine in MO. Only rats with accurate cannulae placements were used in the data analysis. Histological reconstructions are shown in Figure 2A, and depict the midpoints of the guide cannulae placements along the AP plane and the theoretical spread of 0.5 μl lidocaine, which is estimated to spread spherically with a radius of 0.5 mm from the infusion site [31]. For MO placements, infusions encompassed the ventral portion of the MO in all cases, but also likely spread to the ventral orbital subdivision in a majority of cases and to the olfactory bulb or piriform cortex in a minority of cases. For mSTN placements, infusions encompassed the medial tip of the STN in all cases, but also likely spread to the zona incerta in a majority of cases and to the more lateral aspects of the STN in a minority of cases. Representative photographs of stained sections are shown in Figure 2B and depict guide cannulae placements positioned 1 mm above the intended sites.
Figure 2.
(A) Schematic drawings representing coronal sections depicting placements in medial orbital cortex (MO) and medial tip of the subthalamic nucleus (mSTN). Circles (0.5 mm radius, drawn to scale) indicate the location and theoretical spread of infused drug, based on the spherical volume equation for lidocaine [31]. In all drawings, the anterior-posterior (AP) reference points are measured in mm from bregma, and each placement is shown at the midpoint of its AP extent. (B) Representative low-magnification (2X) photographs of guide cannulae placements terminating 1 mm above the MO (left) and mSTN (right).
3.2 Experiment 1: Bilateral and asymmetric inactivation of MO and mSTN
Prior to test sessions with lidocaine or vehicle treatments, the bilateral and asymmetric groups had similar baseline levels of cocaine self-administration behavior (Table 1). Analysis of active lever responses (F[5,28] = 1.1; p≤0.39), inactive lever responses (F[5,28] = 1.9; p≤0.12) and cocaine infusions earned (F[5,28] = 1.3; p≤0.27) during baseline sessions revealed no significant differences among the six groups. At baseline, inactive lever responses were 30% or less of active lever responses, indicating that each group discriminated the active from the inactive lever in a ratio greater than 2:1. The baseline data in Table 1 also illustrate the independence of cocaine seeking and cocaine intake when studied under a second-order schedule. That is, the average number of infusions earned (8.4 to 9.8) varied over a narrow range by the various groups, while at the same time, the average number of active lever responses varied over a larger range (283–564).
Table 1.
Mean ± SEM active and inactive responses and infusions earned during cocaine self-administration sessions at baseline.
| Site | Treatment (n) | Active Responses | Inactive Responses | Cocaine Infusions |
|---|---|---|---|---|
|
| ||||
| Bilateral MO | Vehicle (7) | 430±34 | 69±47 | 9.0±0.40 |
| Lidocaine (7) | 351±79 | 30±12 | 8.7±0.45 | |
|
| ||||
| Bilateral mSTN | Vehicle (5) | 515±102 | 67±13 | 9.4±0.23 |
| Lidocaine (5) | 564±125 | 171±56 | 9.8±0.11 | |
|
| ||||
| Asymmetric MO/mSTN | Vehicle (5) | 353±135 | 62±48 | 8.4±0.48 |
| Lidocaine (5) | 437±118 | 24±12 | 8.8±0.57 | |
|
| ||||
| Unilateral MO | Lidocaine (4) | 283±67 | 36±23 | 9.3±0.43 |
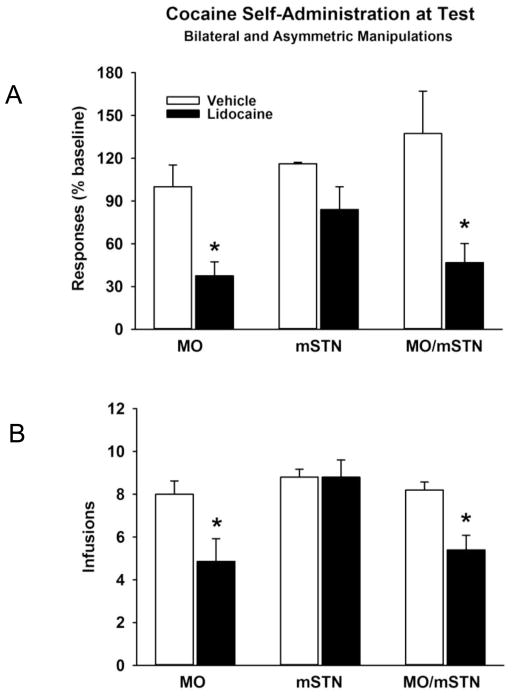
At test, bilateral inactivation of the MO and asymmetric inactivation of the MO and mSTN reduced cocaine self-administration behavior (Figure 3A and 3B). There were significant group differences for both active lever responses (F[5,28] = 5.4; p≤0.001) and cocaine infusions earned (F[5,28] = 5.6; p≤0.001). Relative to vehicle infusions, there were significant reductions in both active lever responses and cocaine infusions earned after bilateral lidocaine infusion into the MO (p≤0.05) and after asymmetric lidocaine infusion into the MO/mSTN (p≤0.05). Bilateral lidocaine infusion into the mSTN was ineffective in altering cocaine self-administration behavior relative to vehicle infusion (p≤0.99). Inactive lever responses were 21% or less of active lever responses (Table 2), and no significant group differences were evident at test (F[5,28] = 1.1; p≤0.39). Thus, rats in each group continued to discriminate the active from inactive lever during the test session. It is important to note that the larger group averages in inactive lever responding for the bilateral mSTN groups was due to a single rat in each group making over 200 inactive lever responses. If these rats are removed from the descriptive statistics, the bilateral mSTN groups made 82±29 (vehicle) and 51±21 (lidocaine) inactive lever responses, inline with the other groups in Table 2. The actual number of active lever responses made by each group at test also is reported in Table 2.
Figure 3.
Cocaine self-administration behavior at test following bilateral and asymmetric vehicle or lidocaine infusions into the medial orbital cortex (MO) and medial tip of the subthalamic nucleus (mSTN). Values are the mean ± s.e.m. (A) Responses on the active lever expressed as % of baseline. (B) Number of infusions earned. * p≤ 0.05 compared to the corresponding vehicle treatment.
Table 2.
Mean ± SEM active and inactive lever responses during cocaine self-administration sessions at test.
| Site | Treatment | Active Responses | Inactive Responses |
|---|---|---|---|
|
| |||
| Bilateral MO | Vehicle | 434±74 | 71±29 |
| Lidocaine | 131±44 | 29±12 | |
|
| |||
| Bilateral mSTN | Vehicle | 597±116 | 106±33 |
| Lidocaine | 500±176 | 100±52 | |
|
| |||
| Asymmetric MO/mSTN | Vehicle | 398±104 | 66±50 |
| Lidocaine | 302±143 | 21±14 | |
|
| |||
| Unilateral MO | Lidocaine | 123±34 | 15±10 |
3.3 Experiment 2: c-Fos protein expression in NAc after inactivation of mSTN
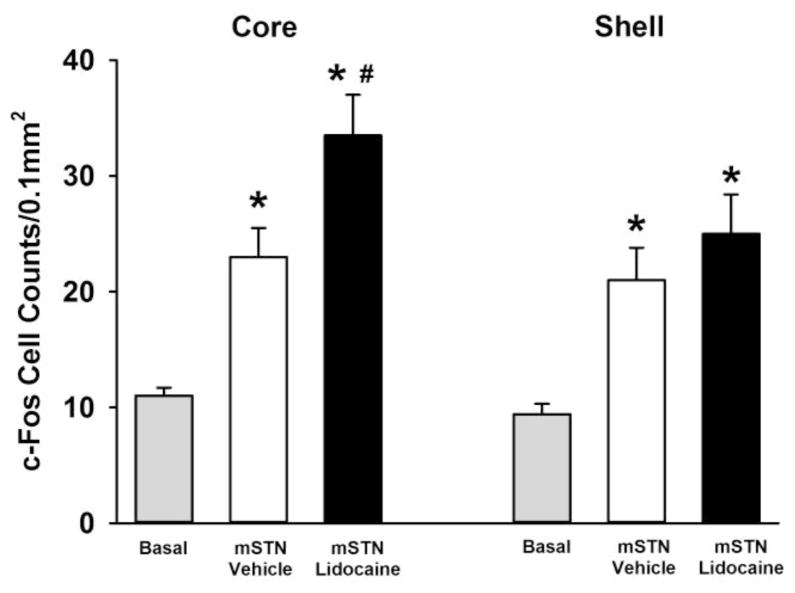
Analysis of c-Fos protein expression showed significant group differences in both the NAc core (F[2,34] = 15.1; p≤0.001) and NAc shell (F[2,34] = 7.2; p≤0.002). In NAc core, c-Fos cell counts were significantly greater in groups self-administering cocaine and receiving mSTN vehicle (p≤0.02) or mSTN lidocaine (p≤0.001) compared to basal c-Fos cell counts obtained in a yoked-saline control group (Figure 4). Additionally, c-Fos cell counts in NAc core were significantly greater after mSTN lidocaine than mSTN vehicle (p≤0.03). In NAc shell, groups self-administering cocaine and receiving mSTN vehicle (p≤0.03) or mSTN lidocaine (p≤0.002) also had significantly greater c-Fos cell counts compared to basal c-Fos cell counts (Figure 4). However, the mSTN lidocaine and mSTN vehicle groups did not differ significantly (p≤0.48).
Figure 4.
Expression of c-Fos protein in ipsilateral nucleus accumbens core and shell after cocaine-self-administration test sessions in groups receiving bilateral or asymmetric vehicle or lidocaine infusions into the medial tip of the subthalamic nucleus (mSTN) or after yoked-saline control sessions (basal). Values are the mean ± SEM number of c-Fos-positive cell counts per 0.1 mm2. * p≤0.05 compared to basal cell counts and # p≤0.05 compared to mSTN vehicle infusion.
3.4 Experiment 3: Unilateral inactivation of MO
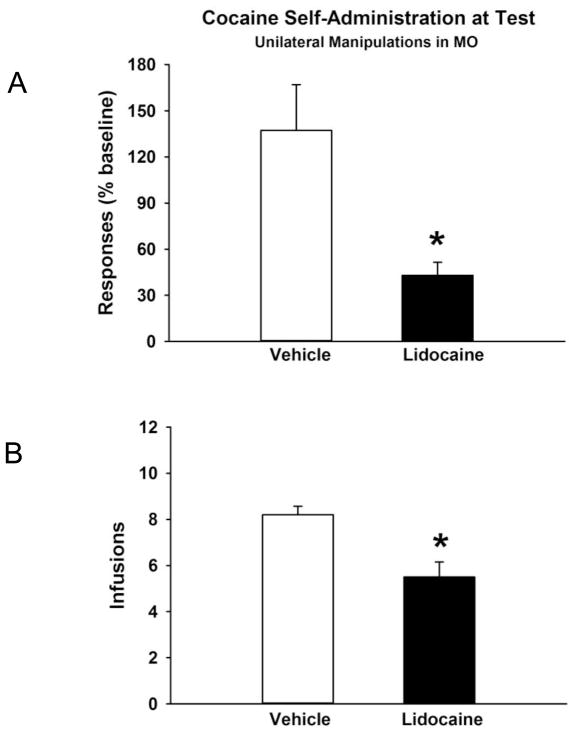
Prior to test sessions with lidocaine or vehicle treatments, the unilateral MO lidocaine group and the asymmetric MO/mSTN vehicle control group had similar baseline cocaine self-administration behavior (Table 1). Analysis of active lever responses (F[1,7] = 0.18; p≤0.68), inactive lever responses (F[1,7] = 0.19; p≤0.67) and cocaine infusions earned (F[1,7] = 1.5; p≤0.26) during baseline sessions showed no significant group differences. At baseline, inactive lever responses were 15% or less of active lever responses. At test, unilateral inactivation of the MO reduced cocaine self-administration behavior (Figure 5A and 5B). There were significant differences between lidocaine and vehicle treatments for both active lever responses (F[1,7] = 7.4; p≤0.03) and cocaine infusions earned (F[1,7] = 14.5; p≤0.007). Inactive lever responses were 16% or less of active lever responses (Table 2), and no significant treatment differences were evident (F[1,7] = 0.76; p≤0.41). The actual number of active lever responses made by the unilateral MO lidocaine group and its control at test also is reported in Table 2.
Figure 5.
Cocaine self-administration behavior at test following unilateral vehicle or lidocaine infusion into the medial orbital cortex (MO). Each rat also received a unilateral vehicle infusion into the contralateral medial tip of the subthalamic nucleus (mSTN). Values are the mean ± s.e.m. (A) Responses on the active lever expressed as % of baseline. (B) Number of infusions earned. * p≤ 0.05 compared to the corresponding vehicle treatment.
4. Discussion
The results of the present investigation demonstrated a critical role for the MO in the maintenance of cocaine self-administration behavior. Whether the MO was inactivated in both hemispheres or in a single hemisphere (with or without concomitant mSTN inactivation), both cocaine seeking and cocaine intake were reduced. Contrary to expectation, inactivation of the mSTN in both hemispheres did not impact cocaine seeking or cocaine intake, suggesting that the reductions in these measures following asymmetric inactivation may have been due to a unilateral influence of lidocaine in the MO. It is unlikely that the lack of an effect of bilateral mSTN inactivation was due to insufficient modulation of neural activity in this site. Given the relatively restricted topographical organization of reciprocal connections between different STN, VP and NAc subdivisions [23, 24, 32], we measured c-Fos protein expression in sites downstream of the STN to determine whether our lidocaine inactivation procedure was selectively altering activity of neurons in the mSTN. We found that lidocaine pretreatment in mSTN selectively augmented cocaine-induced c-Fos protein expression in ipsilateral NAc core above levels observed after vehicle pretreatment in mSTN, consistent with the expectation that lidocaine inactivation of mSTN would disinhibit NAc core activity [23, 24]. Our findings also confirm that acute cocaine self-administration increases c-Fos protein expression above basal levels in both NAc core and shell in rats [33]. However, since cocaine-induced c-Fos protein expression was not augmented further in the NAc shell after lidocaine pretreatment in mSTN, our findings suggest that the influence of lidocaine pretreatment in STN on neural activity was greatest at the medial tip. Despite this finding, changes in the maintenance of cocaine self-administration behavior were not observed after bilateral inactivation of mSTN. Consequently, an important question pertains to how might bilateral and unilateral MO inactivation as well as asymmetric MO/mSTN inactivation lead to reductions in the maintenance of cocaine self-administration behavior but bilateral mSTN inactivation does not, given the circuit-based connections between the MO and mSTN.
4.1 Bilateral MO inactivation
Based on the mechanism of action of lidocaine as a sodium channel blocker and neuronal inactivating agent [31], it is presumed that bilateral inactivation of the MO would inhibit the direct excitatory projections from the MO to the mSTN and from the MO to the NAc core. While such actions could result in opposing influences over neural activity in the NAc core [23, 24], it is possible that direct NAc core inhibition may predominate over indirect NAc core excitation after bilateral MO inactivation. In support of this idea, electrical stimulation of the MO has been shown to activate NAc core neurons in the direct MO-NAc core pathway with a latency of ~11 ms [34] and to activate mSTN neurons in the direct MO-mSTN pathway with a latency of ~8 ms; however, this latter activation is of short duration and is followed by a prolonged inhibition of the mSTN via inhibitory feedback from the VP [22, 24]. Assuming the opposite is true when the MO is inactivated, these actions could lessen the impact of mSTN inhibition for facilitating NAc core activity, allowing NAc core inhibition to predominate in both hemispheres after bilateral MO inactivation. Consistent with this view, past research has shown that cocaine self-administration behavior under a second-order schedule is reduced when neural activity in the NAc core is bilaterally inhibited by the AMPA/KA antagonist LY293558 [35, 36].
4.2 Bilateral mSTN inactivation
In the case of bilateral mSTN inactivation, disinhibition of the NAc core on both sides of the brain might allow cocaine self-administration behavior to proceed at its normal rate or perhaps even be facilitated. We did not observe increases in cocaine self-administration behavior after bilateral mSTN inactivation, but one previous study did show increases under FR1 and progressive ratio schedules following damage encompassing the whole STN [26]. Other studies, however, demonstrated that damage to whole STN decreased cocaine self-administration under progressive ratio and FR1 schedules [25]. One possibility for these divergent findings may relate to differences in baseline rates of responding. Under the second-order schedule of 0.3 mg/kg cocaine delivery used in the present study, rats were already responding at high rates prior to mSTN inactivation (over 500 responses in 1 hr). In the Uslaner et al study [26], control rats emitted substantially fewer responses under FR1 and progressive ratio schedules of 0.3 mg/kg cocaine delivery (approximately 3 and 126 responses, respectively), perhaps making it easier to detect an increase in responding in STN lesioned rats. However, differences in baseline rates of responding may not fully explain these divergent findings because in the Baunez et al study [25], control rats emitted approximately 436 responses under the progressive ratio schedule and approximately 25–40 responses in 1 hr under the FR1 schedule of cocaine delivery. Lesioned rats exhibited decreased cocaine self-administration behavior whether the control rates of responding were high (using a 0.25 mg/kg cocaine dose) or low (using 0.01 and 0.03 mg/kg cocaine doses, but not a 0.25 mg/kg cocaine dose). Consequently, these divergent findings also cannot be explained by differences in the dose of cocaine that was used.
Another possibility for these divergent findings may relate to differences in the subdivision of the STN that was predominantly affected. Based on evaluation of c-Fos protein expression, the medial tip of the STN appeared to be specifically targeted in the present study, given that cocaine-induced c-Fos cell counts were enhanced further only in the NAc core after bilateral lidocaine pretreatment in STN. Evaluation of c-fos mRNA by Uslaner et al [26] revealed that cocaine self-administration caused increased expression in both NAc core and shell, but expression was augmented further only in NAc shell of STN lesioned rats. This latter finding suggests that there may have been more pronounced damage to the ventromedial subdivision than medial tip of the STN. Past work shows that high frequency stimulation of the ventromedial STN (a technique that produces effects similar to STN lesions) causes pronounced increases in extracellular dopamine concentration in NAc shell but does not alter extracellular dopamine concentration in NAc core [37]. A distinction between NAc core and shell activation after STN inactivation may be critical for observing no change vs. an increase in cocaine self-administration after bilateral inhibition of the STN. Notably, rats will self-administer a cocktail consisting of a D1 and D2 receptor agonist directly into the NAc shell but not NAc core [38], and dopamine efflux is greater in NAc shell than core in rats self-administering cocaine under a second-order schedule [39]. These findings suggest that activation of NAc shell is more likely than activation of NAc core to produce an increase in cocaine self-administration behavior. This view, though, is tempered by the decrease in cocaine self-administration behavior produced by whole STN lesions [25] and high frequency stimulation of STN targeting the ventrolateral STN [40], implicating modulation of NAc shell activity in both studies. However, past work has shown that increasing concentrations of dopamine in NAc shell can differentially modulate cocaine self-administration behavior [41]. Small increases (via application of 90 nM dopamine) result in decreased cocaine self-administration, and large increases (via application of 450 nM dopamine) result in increased cocaine self-administration. Though a viable explanation, the discrepancy between the effects of whole STN lesions on cocaine self-administration behavior in the Uslaner et al [26] and Baunez et al [25] studies remains unresolved and further investigation is needed to understand the opposite changes in cocaine self-administration behavior in these two studies.
4.3 Unilateral MO and asymmetric MO/mSTN inactivation
Unilateral MO and asymmetric MO/mSTN inactivation reduced the maintenance of cocaine self-administration behavior to the same degree as bilateral MO inactivation. As MO inactivation on one side of the brain should directly inhibit neural activity in the ipsilateral NAc core while leaving the contralateral NAc core either unaffected (unilateral MO manipulation) or disinhibited (asymmetric MO/mSTN manipulation), one possible explanation is that MO-mediated unilateral inhibition of NAc core activity may be just as effective as MO-mediated bilateral inhibition for reducing cocaine self-administration behavior. Arguing against this view are findings showing that unilateral NAc core lesions and unilateral blockade of the NAc core with the AMPA/KA antagonist LY293558 do not impact responding under a second-order schedule of cocaine delivery [14, 36].
In addition to the NAc core and mSTN, the MO sends dense projections to several cortical (e.g., agranular insular, ventral orbital, anterior cingulate, prelimbic and infralimbic) and subcortical (e.g., amygdala, hippocampus, dorsal striatum) sites [5]. The MO is thought to bridge the lateral and medial prefrontal cortex networks [42], and via dense connections to the thalamus (e.g., reuniens and mediodorsal nuclei), to have a widespread influence on emotional and cognitive aspects of goal-directed behavior [5]. It is possible that inactivation of the MO on one side of the brain is sufficient to produce hemispheric asymmetry in neural activity in several cortical and subcortical brain sites, and in this way reduce maintenance of cocaine self-administration behavior. Indirect support for this idea is shown by findings demonstrating that ipsilateral (different sites in the same hemisphere) inactivation of orbitofrontal cortex + basolateral amygdala [17], dorsomedial prefrontal cortex + basolateral amygdala [13], ventromedial prefrontal cortex + NAc shell [43], and infralimbic prefrontal cortex + NAc shell [44] can modify reinstatement of cocaine-seeking behavior in rats. Based on these observations and the views outlined above, it appears that the neural mechanisms by which bilateral and unilateral MO inactivation influence the maintenance of cocaine self-administration behavior may not be the same, and further research is needed to understand these differences more fully.
4.4 The MO and cue-controlled behavior
It is likely that MO inactivation exerts its influence on the maintenance of cocaine self-administration by primarily interfering with cue-controlled behavior rather than by modifying the reinforcing effects of cocaine. In the present study, cocaine seeking was reduced to 45% of baseline, on average, after bilateral or unilateral MO and asymmetric MO/mSTN inactivation. This finding is consistent with research in human subjects implicating the MO in the processing of drug cues in individuals with a cocaine use disorder [45, 46]. It was somewhat surprising that in a past study in rats, post-training inactivation of MO did not impact cocaine cue-induced reinstatement of cocaine-seeking behavior [8]. An important distinction that may explain our robust reduction in responding maintained by cocaine-paired cues during maintenance testing and the lack of changes in responding maintained by cocaine-paired cues during reinstatement testing in this earlier study is the level at which the MO was manipulated. Our MO placements (AP +4.7 mm to +5.6 mm) were more anterior and ventrally positioned than the MO placements (AP +4.2 mm to +5.2 mm) reported by Fuchs et al [8]. At the more anterior and ventral locations, the MO areas targeted in the present study correspond to those depicted in Hoover and Vertes [5] showing the most intense staining for Phaseolus vulgaris leucoagglutinin injections used to map the projections of the MO to cortical and subcortical structures.
5. Conclusions
Furthering knowledge concerning the neural circuits that control the maintenance of cocaine seeking and cocaine intake may contribute to an understanding of the mechanisms underlying the cocaine addiction process and provide insight for novel treatments. The present investigation shows the critical importance of the MO for maintaining cocaine seeking and cocaine intake in rats, though its projections to the mSTN appear to be unimportant for this purpose. Most research on the control of cocaine seeking and cocaine intake by the orbitofrontal cortex focuses on the role of the LO subdivision. The present study supports further preclinical and clinical investigation of the MO subdivision in the cocaine addiction process, given its role in learning and memory for hedonic experiences [47] and in metabolic responses produced by acute and chronic cocaine self-administration [48].
Inactivation of MO reduces cocaine seeking and cocaine intake
Reductions occur with bilateral, unilateral and asymmetric manipulations
Bilateral inactivation of mSTN does not impact cocaine seeking or cocaine intake
Inactivation of mSTN augments cocaine-induced c-Fos in NAc core but not shell
MO inactivation likely interferes primarily with cue-controlled behavior
Acknowledgments
This study was supported by DA011716. The authors thank Brittany Lovascio for her assistance with c-Fos cell counting.
Footnotes
Author contribution
KK and LY were responsible for the study concept and design. LY and MB collected behavioral data and conducted the histological and c-Fos analyses. KK, LY and MB performed data analysis and interpretation. KK drafted the manuscript, and LY and MB provided important intellectual content. All authors critically reviewed content and approved final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–97. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- 3.Grakalic I, Panlilio LV, Quiroz C, Schindler CW. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience. 2010;165:313–24. doi: 10.1016/j.neuroscience.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 4.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 5.Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- 6.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 7.Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–36. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–10. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–81. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–8. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 14.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2010;96:347–53. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szalay JJ, Morin ND, Kantak KM. Involvement of the dorsal subiculum and rostral basolateral amygdala in cocaine cue extinction learning in rats. Eur J Neurosci. 2011;33:1299–307. doi: 10.1111/j.1460-9568.2010.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2011;36:711–20. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–22. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 21.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the cortico-nigral circuits. J Neurosci. 1999;19:4674–81. doi: 10.1523/JNEUROSCI.19-11-04674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–46. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchill L, Kalivas PW. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol. 1994;345:579–95. doi: 10.1002/cne.903450408. [DOI] [PubMed] [Google Scholar]
- 24.Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Prefrontal cortex-basal ganglia circuits in the rat: involvement of ventral pallidum and subthalamic nucleus. Synapse. 1998;29:363–70. doi: 10.1002/(SICI)1098-2396(199808)29:4<363::AID-SYN8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci. 2005;8:484–9. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 26.Uslaner JM, Yang P, Robinson TE. Subthalamic nucleus lesions enhance the psychomotor-activating, incentive motivational, and neurobiological effects of cocaine. J Neurosci. 2005;25:8407–15. doi: 10.1523/JNEUROSCI.1910-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uslaner JM, Dell’Orco JM, Pevzner A, Robinson TE. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology. 2008;33:2352–61. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- 28.Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 29.Nic Dhonnchadha BA, Lovascio BF, Shrestha N, Lin A, Leite-Morris KA, Man HY, et al. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–6. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–62. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 31.Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- 32.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–42. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 33.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montaron MF, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience. 1996;71:371–82. doi: 10.1016/0306-4522(95)00455-6. [DOI] [PubMed] [Google Scholar]
- 35.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–60. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 36.Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter C, Lemke C, Sohr R, Meissner W, Harnack D, Juckel G, et al. High frequency stimulation of the subthalamic nucleus modulates neurotransmission in limbic brain regions of the rat. Exp Brain Res. 2008;185:497–507. doi: 10.1007/s00221-007-1171-1. [DOI] [PubMed] [Google Scholar]
- 38.Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–7. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107:1196–200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurd YL, Ponten M. Cocaine self-administration behavior can be reduced or potentiated by the addition of specific dopamine concentrations in the nucleus accumbens and amygdala using in vivo microdialysis. Behav Brain Res. 2000;116:177–86. doi: 10.1016/s0166-4328(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 42.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 43.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–91. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–9. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 48.Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]