Abstract
Like other tissue injuries, bone fracture triggers an inflammatory response, which plays an important role in skeletal repair. Inflammation is believed to have both positive and negative effects on bone repair, but the underlying cellular mechanisms are not well understood. To assess the role of inflammation on skeletal cell differentiation, we used mouse models of fracture repair that stimulate either intramembranous or endochondral ossification. In the first model, fractures are rigidly stabilized leading to direct bone formation, while in the second model, fracture instability causes cartilage and bone formation. We compared the inflammatory response in these two mechanical environments and found changes in the expression patterns of inflammatory genes and in the recruitment of inflammatory cells and osteoclasts. These results suggested that the inflammatory response could influence skeletal cell differentiation after fracture. We then exploited matrix metalloproteinase 9 (MMP9) that is expressed in inflammatory cells and osteoclasts, and which we previously showed is a potential regulator of cell fate decisions during fracture repair. Mmp9-/- mice heal stabilized fractures via endochondral ossification, while wild type mice heal via intramembranous ossification. In parallel, we observed increases in macrophages and T cells in the callus of Mmp9-/- compared to wild type mice. To assess the link between the profile of inflammatory cells and skeletal cell fate functionally, we transplanted Mmp9-/- mice with wild type bone marrow, to reconstitute a wild type hematopoietic lineage in interaction with the Mmp9-/- stroma and periosteum. Following transplantation, Mmp9-/- mice healed stabilized fractures via intramembranous ossification and exhibited a normal profile of inflammatory cells. Moreover, Mmp9-/- periosteal grafts healed via intramembranous ossification in wild type hosts, but healed via endochondral ossification in Mmp9-/- hosts. We observed that macrophages accumulated at the periosteal surface in Mmp9-/- mice, suggesting that cell differentiation in the periosteum is influenced by factors such as BMP2 that are produced locally by inflammatory cells. Taken together, these results show that MMP9 mediates indirect effects on skeletal cell differentiation by regulating the inflammatory response and the distribution of inflammatory cells, leading to the local regulation of periosteal cell differentiation.
1. Introduction
The recruitment of skeletal stem/progenitor cells and their differentiation into osteoblasts and chondrocytes is key to the success of bone repair. Many factors can influence skeletal progenitors during the early stages of repair, including mechanical stimuli and inflammatory factors. The mechanical environment is crucial in determining healing via endochondral versus intramembranous ossification [1, 2]. A stabilized environment favors osteogenic differentiation, whereas the loss of stabilization favors chondrogenic differentiation at the fracture site. These cell fate decisions occur during the inflammatory phase of fracture repair [3, 4], however the role of inflammatory signals in skeletal cell fate is not well characterized.
The inflammatory phase of bone repair is marked by the infiltration of inflammatory cells that contribute to formation of the hematoma and removal of damaged tissue. While a controlled inflammatory response is necessary for stimulating tissue regeneration, prolonged inflammation can hinder the completion of the repair process [5-7]. The multiple inflammatory cell types and factors involved makes it difficult to define the specific impacts of inflammation on bone healing. Thus far, several studies have shown negative effects of the adaptive immune system and positive effects of the innate immune system [8-11]. However, these studies mostly revealed inflammatory functions during the remodeling phase of repair. Less is known about the role of inflammation on skeletal progenitors that are recruited at the beginning of the inflammatory phase during the first few days after fracture. Inflammatory mediators such as tumor necrosis factor-α (TNFα) are required for bone formation but can also impair later stages of repair by stimulating cartilage degradation [12-14]. Likewise, apparent opposite results have been reported on the role of nonsteroidal anti-inflammatory drugs (NSAIDs), which target various cell types at different stages of repair including osteoblasts, chondrocytes and osteoclasts [15-18]. In orthopaedic trauma, NSAIDs are given to patients to reduce pain; however, the extent to which they affect repair is not well defined. The lack of Cox2, a target of NSAIDs, inhibits osteoblast differentiation in the periosteum [19, 20]. The role of Cox2 is counterbalanced by 5-lipoxygenase (5-LO) and leukotriene inhibitors can stimulate cartilage and bone formation in the early phase of repair through direct actions on chondrocytes [21]. Interestingly, transplanted mesenchymal stem cells (MSCs), which can improve healing, have systemic anti-inflammatory effects on the cytokines released after fracture including TNFα and interleukin-1β (IL1β) [22]. These results suggest that inflammatory cells and skeletal progenitors influence each other.
Matrix metalloproteinases (MMPs) play important roles in bone development and repair and these enzymes may participate in the interaction between inflammatory cells and skeletal progenitors [3, 23-27]. MMP9, along with other MMPs, is expressed in inflammatory cells and regulates inflammation in other tissues and diseases [28-33]. Our previous work showed that MMP9 operates both during the inflammatory and remodeling phases of repair [3]. During the inflammatory phase, MMP9 may regulate skeletal cell fate, as MMP9-/- but not wild type skeletal progenitors differentiate into chondrocytes in both non-stabilized and stabilized fractures. We hypothesized that MMP9 mediates the inflammatory and progenitor cell responses to mechanical stimuli during bone repair. In this study, we assessed the relationship between inflammation and the differentiation of skeletal progenitors under different mechanical stimuli at the fracture site, and the role of matrix metalloproteinase 9 (MMP9) in coordinating these events. We used bone marrow transplantation and bone grafting to functionally test the role of the mechanical environment and inflammation on cell differentiation within the periosteum, a key source of skeletal progenitors for bone repair [34].
2. Materials and methods
2.1. Non-stabilized and stabilized fractures
All protocols were approved by the Institutional Animal Care and Use Committee of the University of California at San Francisco. Mmp9–/– mice (12- to 16-week-old males) and their wild-type (WT) littermates were anesthetized with an intraperitoneal injection of Ketamine/Medetomidine. Non-stabilized or stabilized tibia fractures were generated in the mid-diaphysis via three point-bending [1, 3]. Mice received analgesics and were monitored for signs of pain as described [26]. Mice were sacrificed by cervical dislocation following an intraperitoneal injection of 2% Avertin 2, 5, 7, 10, and 14 days after fracture.
2.2. Microarrays
One centimeter of the wild type fractured hind limb between knee and ankle was collected free of skin followed by RNA extraction at day 2 (stabilized: n=4; non-stabilized: n=3) and day 7 (non-stabilized: n=4; stabilized: n=4) and from uninjured limbs (n=4) using Trizol (Invitrogen, Carlsbad, CA). Microarrays were performed using Agilent Mouse single-color 4×44K arrays. Image analysis was performed using Agilent's Extraction 9.1. Comparisons of stabilized vs. no fracture, non-stabilized vs. no fracture, and stabilized vs. non-stabilized at each time point were assessed with ANOVA and t-statistics were assigned using R/Bioconductor. Genes with differential expression were analyzed for functional enrichment using DAVID (NIAID/NIH). K-means clustering was performed in MeV4.1.02.
2.3. Fluorescence-activated cell sorting
Wild type and Mmp9–/– mice were euthanized following anesthesia at days 0 (unfractured), 2 and 5 post-fracture (n=5 or 6 per group). The fractured hind limb between the knee and the ankle was collected free of skin. The bone marrow and soft tissues (periosteum, muscle and hematoma) were separated. Bone marrow cells were flushed and harvested in PBS. The minced soft tissue was digested with collagenase/dispase (Roche, Palo Alto, CA) for one hour at 37°C and the cells were filtered through a cell strainer (BD Falcon, Bedford, MA). The cells were incubated with a panel of monoclonal antibodies to identify various inflammatory cell types including anti-F4/80, anti-CD4 and anti-CD8 (Serotec, Raleigh, NC), anti-FcεRIα, anti-CD11b, anti-CD19, anti-Ly6G, and anti-IgM (eBiosciences, San Diego, CA), and anti-CD117 (BD Pharmingen, San Diego, CA). Monoclonal antibodies were conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). Single or double staining was performed to identify the following inflammatory cell populations: macrophages (F4/80+ or CD11b+/Ly6G-), neutrophils (CD11b+/Ly6G+), T-helper lymphocytes and cytotoxic T cells (CD4+ and CD8+, respectively), B cells (CD19+/IgM+), and mast cells (CD117+/FcεRIα+). Cells were washed and analyzed by fluorescence-activated cell sorting (FACS) using a Facscaliber (Becton Dickinson, San Jose, CA). The percentage of inflammatory cells was determined after gating on the entire viable population. Cells were analyzed by flow cytometry using the Cell Quest software (Becton Dickinson, San Jose, CA). The results were analyzed using both ANOVA and unpaired Student's t test to assess the effects of genotype, fracture stability, tissue type (bone marrow versus soft tissue) and time post-fracture. Significance was determined at P < 0.05.
2.4. Bone marrow transplantation
Ten-week-old Mmp9–/– or wild type males were lethally irradiated with two 6 Gy doses of γ-irradiation 3–4 hours apart. Bone marrow cells from wild type and Mmp9–/– mice were transplanted into irradiated wild type (WT-WT and KO-WT) and Mmp9–/– (WT-KO and KO-KO) hosts from the same FVB/N background as previously described [35]. Following a 6-week recovery period, stabilized or non-stabilized fractures were produced. Mice were sacrificed 2 days after fracture to collect cells from bone marrow and soft tissue for FACS analysis of inflammatory cell populations. At 10 and 14 days post-injury, callus tissues from stabilized and non-stabilized fractures respectively were collected and processed for histological and histomorphometric analyses (n=5 or 6 per group).
2.5. Histological and histomorphometric analyses
Fractured tibiae were harvested free of skin, fixed overnight in 4% paraformaldehyde at 4°C, decalcified in 19% EDTA (pH 7.4) for 14 days, then dehydrated and embedded in paraffin. Sections (10 μm) through the entire callus were collected. Histomorphometry was performed as previously described [3, 6]. To determine the volume of cartilage within each callus, every thirtieth section (300 μm) was stained with Safranin-O/Fast Green (SO/FG) and photographed using a Leica DM 5000 B light microscope (Leica Microsystems GmbH, Wetzler, Germany) that was equipped with a camera (Diagnostic Instruments, Inc., Sterling Heights, MI). The captured images were analyzed with Adobe Photoshop to determine the area of cartilage in each section.
2.6. Detection and quantification of inflammatory cells and osteoclasts on tissue sections
Immunohistochemistry was performed every tenth slide throughout the callus using rat anti-F4/80 and rat anti-Ly6G antibodies (eBiosciences, San Diego, CA) to detect macrophages and neutrophils respectively. Sections were treated with 20 μg/mL proteinase K (37°C, 20 minutes), incubated with primary antibodies at 4°C overnight. Endogenous peroxidase activity was blocked by incubating the sections with 0.3% H2O2 in PBS at room temperature for 10 minutes. Sections were then incubated with Biotin Goat Anti-Rat IgG Polyclonal (BD Pharmingen, San Diego, CA) at room temperature for 1 hour followed by streptavidin-horseradish peroxidase conjugate (Amersham Biosciences, Arlington. Heights, IL). Staining was detected using diaminobenzidene and the tissue was counterstained with 0.1% fast green. On adjacent sections, tartrate-resistant acid phosphatase (TRAP) staining was performed to detect osteoclasts using a leukocyte acid phosphatase kit (Sigma, St. Louis, MO) [36].
Macrophages, neutrophils and osteoclasts were quantified using stereology in all tissues of the hind limb between knee and ankle, excluding the bone marrow compartment. This area corresponds the soft tissue compartment analyzed via FACS. Immunoreactive- or TRAP- positive cells were counted using an Olympus CAST system (Olympus, Center Valley, PA) and software by Visiopharm (Visiopharm, Hørsholm, Denmark). The total area was outlined at low magnification (2x) and the cells were counted at high magnification (20x) in counting frames: ten to thirty fields covering approximately 3% of the tissue section were randomly acquired by unbiased uniform random sampling. Four counting frames covering 50% of the area within a field were overlaid on each field. The number of positive cells (q) and the points of area (p) within these fields were determined. Values were expressed as number of cells per mm2 tissue. The results were analyzed using Student's t test. Significance was determined at P < 0.05.
2.7. Detection of BMP-2 expressing cells on tissue sections
Immunohistochemistry was performed using a goat anti-BMP-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to detect BMP-2 expressing cells. After deparaffinization and rehydration, sections were treated with 0.3% Triton X-100 in PBS for 10 minutes to permeabilize the cells and then treated with 0.05% Trypsin in PBS for recovery of antigenicity (37°C, 20 minutes). Endogenous peroxidase activity was blocked by incubating the sections with 0.3% H2O2 in PBS at room temperature for 20 minutes. Potential nonspecific binding sites were blocked with 3% bovine serum albumin in PBS for 1 hour. Sections were incubated with the primary antibody in humidity chambers at 4°C overnight. After washes in PBS, sections were incubated with biotin-conjugated donkey anti-goat polyclonal IgG-B (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour and then incubated with avidin-biotinylated horseradish peroxidase conjugate (Vector Laboratories, Burlingame, CA) for 30 minutes. Staining was detected using diaminobenzidene and the tissue was counterstained with 0.1% Fast Green.
2.8. Bone graft transplantation and histological analyses
Live bone graft transplantation was performed as previously described [34]. Briefly, bone grafts were isolated from adult wild type mice that express GFP and Mmp9–/– mice (males, 10 week–5-month-old). A fragment of cortical bone was cut in the anterior-proximal area of each tibia. The periosteum was kept intact while the endosteum and bone marrow were removed from the graft using a razor blade. Fresh bone grafts were transplanted into FVBN wild type mice and Mmp9–/– hosts (males, 10 week–5-month-old) to obtain four experimental groups: wild type grafts into wild type donors (WT-WT; n=5), Mmp9–/– grafts into Mmp9–/– hosts (Mmp9–/– - Mmp9–/–; n=6), wild type grafts into Mmp9–/– hosts (WT- Mmp9–/–; n=5) and Mmp9–/– grafts into wild type hosts (Mmp9–/– -WT; n=5). Tibias were harvested and fixed for 24 h at 4°C in 4% paraformaldehyde (PFA) at 4°C, then samples were decalcified and cryo-embedded [35]. Safranin-O/Fast Green and trichrome staining were performed to visualize cartilage and bone, respectively. GFP immunostaining was performed on adjacent sections to confirm the origins of donor versus host cells (data not shown) [25].
3. Results
3.1. Inflammatory genes are differentially expressed in stabilized and non-stabilized fractures
To begin understanding how fracture stability may regulate inflammation and the initial phase of fracture repair, we compared the genomic response in stabilized and non-stabilized mechanical environments using microarray analysis. mRNA transcripts of inflammatory genes appeared at days 2 and 7 after injury in stabilized and non-stabilized fracture environments (Table 1 and Supplemental Fig. 1), indicating that inflammatory genes are up-regulated after fracture regardless of the mechanical environment. While inflammatory gene profiles were indiscernible in stabilized and non-stabilized fractures at day 2 after injury (data not shown), inflammatory genes were significantly up-regulated at day 7 after injury in the stabilized fractures as compared to the non-stabilized fractures (Table 1 and Supplemental Fig. 1), indicating that the inflammatory response lasts longer in stabilized fractures.
TABLE 1. Genes related to inflammation are significantly up-regulated after fracture.
Microarray analysis of fracture calluses at day 2 and day 7 post stabilized or non-stabilized fracture identified genes with significant changes in expression
| Comparison | Day 2 vs No Fx | Day 7 vs No Fx | S vs NS Day 7 | ||
|---|---|---|---|---|---|
| Gene Sets (Gene Hits) | up in S | up in NS | up in S | up in NS | up in S |
| Cytokine | ***(15) | ***(15) | **(15) | NS | NS |
| Cytokine activity | ***(17) | ***(16) | ***(16) | NS | NS |
| Cytokine-cytokine receptor interaction | ***(24) | ***(20) | ***(24) | NS | NS |
| Chemokine activity | ***(12) | ***(11) | ***(11) | NS | ***(8) |
| Chemokine receptor binding | ***(12) | ***(11) | ***(11) | NS | ***(8) |
| CXC chemokine | NS | NS | NS | NS | ***(5) |
| Small chemokine, C-C | ***(8) | ***(8) | NS | NS | NS |
| Small chemokine, interleukin-8-like | ***(12) | ***(11) | ***(10) | NS | ***(8) |
| Immune system process | ***(36) | ***(35) | ***(41) | NS | NS |
| Immune response | ***(28) | ***(31) | ***(36) | NS | NS |
| Immune response | NS | NS | **(13) | NS | NS |
| Acute phase | NS | ***(7) | NS | NS | NS |
| Acute-phase response | NS | ***(8) | ***(7) | NS | NS |
| Acute inflammatory response | ***(9) | ***(12) | ***(16) | NS | NS |
| Inflammatory response | ***(22) | ***(27) | ***(29) | ***(19) | ***(16) |
| Inflammatory response | ***(12) | ***(14) | ***(13) | NS | ***(11) |
| Response to stress | ***(34) | ***(34) | ***(41) | NS | **(26) |
| Defense response | NS | ***(30) | **(33) | NS | NS |
| Response to wounding | ***(27) | ***(32) | ***(35) | ***(26) | **(16) |
p < 0.001
p < 0.01
p < 0.05
NS= Not Significant compared with no-fracture limbs. Changes were also found between stabilized and non-stabilized fractures at day 7 after fracture. Note: M>2, B>=0 for all comparisons between fractured and non-fractured limbs; M>1, FDR<.05 for all comparisons between stabilized and non-stabilized limbs; all p-values are Bonferroni-corrected for multiple comparisons.
We identified distinct sets of genes differentially expressed in stabilized and non-stabilized fractures. Although inflammatory genes were up-regulated in stabilized fractures, genes encoding extracellular matrix proteins were up-regulated in non-stabilized fractures (Supplemental Fig. 1), including several members of the Mmp family, such as Mmp2, Mmp9, Mmp10 and Mmp13, that are involved in extracellular matrix remodeling and inflammation. These results suggested that co-regulation of members of clusters that include important candidates for regulating osteogenesis and chondrogenesis occurs during the inflammatory phase of fracture healing.
3.2. Inflammatory cell recruitment differs in stabilized and non-stabilized fractures
To determine whether molecular changes correlate with changes in the recruitment of inflammatory cells in wild type stabilized and non-stabilized fractures, we quantified the inflammatory cell populations within bone marrow and soft tissues around the fracture site using FACS analysis. In unfractured tibias, immune/inflammatory cells were detected in the bone marrow, but not in the soft tissues surrounding the bone (Fig. 1 and data not shown). After fracture, inflammatory cells were detected in bone marrow as well as in soft tissues surrounding the fracture site. In stabilized and non-stabilized fractures, all inflammatory cell types were detected at the fracture site and surrounding soft tissues by day 2, except for CD8+ T cells (Fig. 1 and Supplemental Fig. 2). In both mechanical environments, most inflammatory cells were found around the fracture site from days 2 to 5 (Fig. 1). However, we observed increased proportions of macrophages and CD4+ T lymphocytes at days 2 and 5 in stabilized fractures compared to non-stabilized fractures (Fig. 1B, H; p<0.05), whereas the proportions of neutrophils, mast cells and B cells were unchanged in the two groups (Fig. 1E and supplemental Fig. 2).
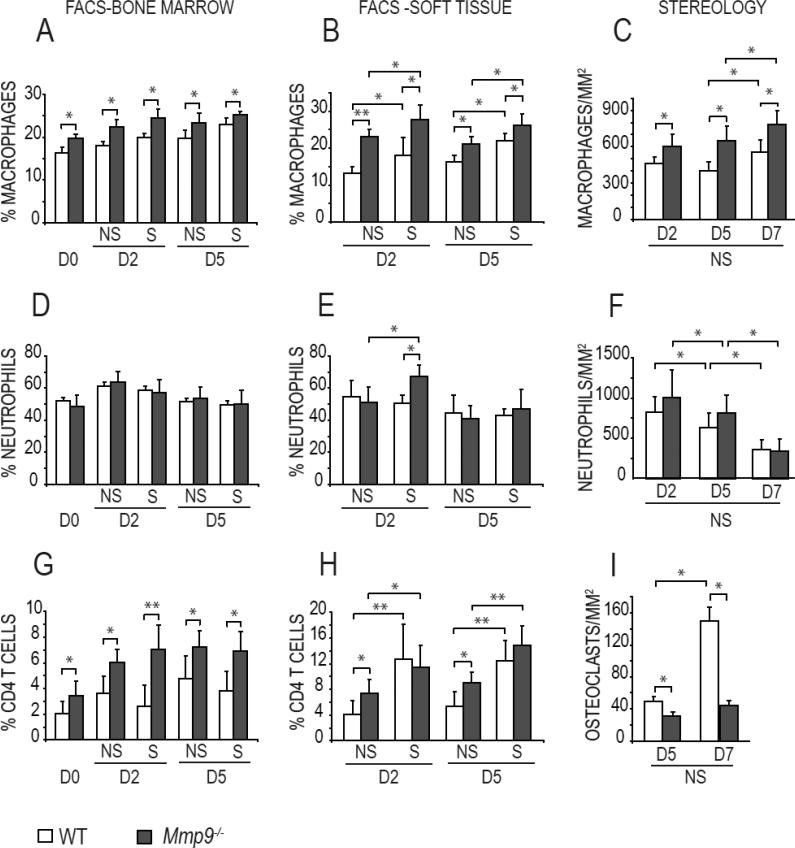
Fig. 1. Mechanical environment and loss of MMP9 alters the inflammatory cell populations in tibial fractures.
Quantification of (A-C) macrophages, (D-F) neutrophils, (G-H) CD4 T cells and (I) osteoclasts in the tibia. FACS analyses of the bone marrow (left) and soft tissue (middle) of Mmp9-/- and wild type (WT) mouse tibia at day 0 (D0, uninjured), and at days 2 (D2) and 5 (D5) following non-stabilized (NS) and stabilized (S) fracture. The percentage of macrophages (Ly6G-negative and CD11b-positive), neutrophils (Ly6G-positive and CD11b-positive) and CD4-positive T cells relative to the total number of cells are reported. (Right) Quantification by stereology of macrophages (C), neutrophils (F) and osteoclasts (I) in the soft tissue of Mmp9-/- and wild type non-stabilized (NS) fractures at days 2 (D2), 5 (D5) and 7 (D7) post-fracture. Bars represent mean ± standard deviation, Student's t-test: * P<0.05, ** P<0.01 (n=5 or 6 per group).
Further analyses of non-stabilized fractures by stereology showed that following this initial phase of inflammation, the proportion of neutrophils decreased in the callus by day 7 compared to days 2 and 5 (Fig. 1F, p<0.05), whereas macrophages and osteoclasts were still found within the callus at day 7 post-fracture and their proportions were increased compared to days 2 and 7 (Fig. 1 C, I, p<0.05).
3.3. Loss of MMP-9 affects inflammatory cell populations in fracture calluses
MMP9 is a known mediator of inflammation and plays a role in fracture repair [3, 29]. The profile of inflammatory cell recruitment differed between Mmp9-/- and wild type mice and changes were mostly observed for macrophages and CD4 T cells between genotypes (p<0.001). In Mmp9-/- stabilized fractures, we observed an increase in the proportions of macrophages at days 2 and 5, and neutrophils at day 2 compared to wild type stabilized fractures (Fig. 1A-B, D-E). Although the proportion of CD4+ T cells was increased in the bone marrow of Mmp9-/- compared to wild type stabilized fractures, this difference was not observed in the surrounding soft tissues (Fig. 1G-H). The proportions of other inflammatory cells were unchanged in Mmp9-/- and wild type stabilized fractures (Supplemental Fig. 2).
In non-stabilized fractures, the proportion of macrophages and CD4+ T cells increased in Mmp9-/- compared to wild type mice (Fig 1 A-C, G-H). In contrast, osteoclast recruitment was markedly decreased in the Mmp9-/- calluses compared to wild type (Fig. 1I). No difference was observed for other inflammatory cell types including neutrophils, mast cells and B cells via flow cytometry (Fig. 1 D-E and supplemental Fig. 2). Thus the absence of MMP9 affected the recruitment of inflammatory cells in the callus of stabilized and non-stabilized fractures.
3.4. Bone marrow transplantation rescues the fracture healing phenotype in Mmp9-/- mice
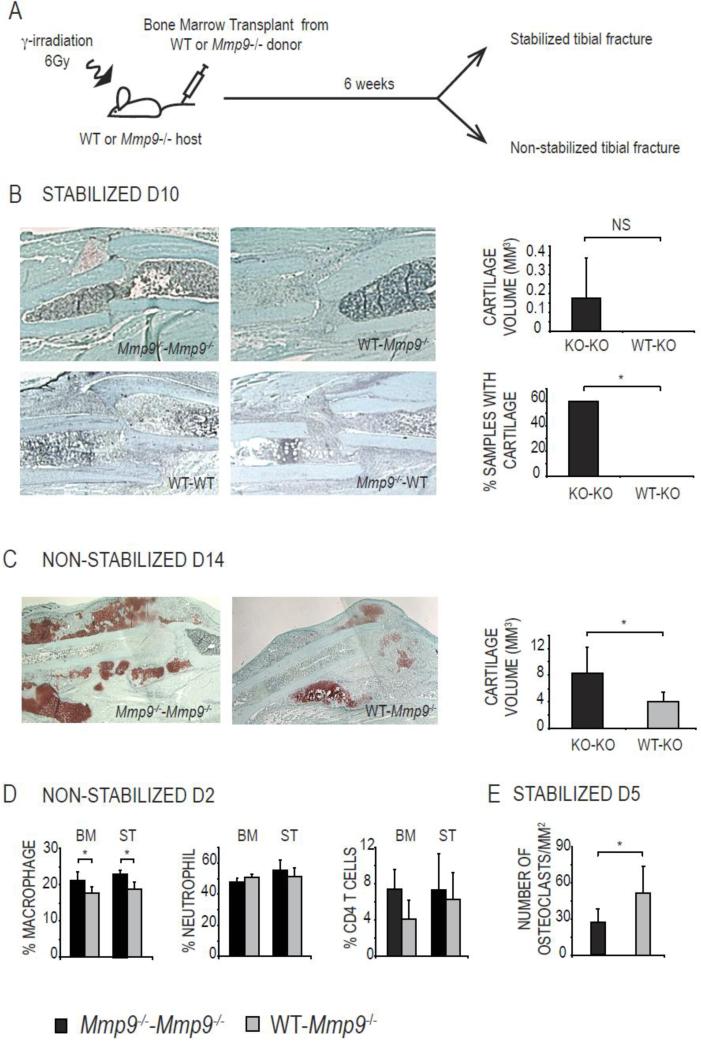
We next tested whether changes in the proportions of inflammatory cells in Mmp9-/- mice impacts skeletal cell differentiation in stabilized and non-stabilized fractures. MMP9 is expressed both by bone marrow-derived myeloid cells and non-myeloid cells [37]. To determine if the production of MMP9 by myeloid cells was responsible for the changes in inflammatory cell recruitment and skeletal cell differentiation during fracture repair, we transplanted Mmp9-/- bone marrow to provide Mmp9-/- hematopoietic cells in a wild type host environment [25, 35]. When we created stabilized fractures in these wild type mice that received Mmp9–/– bone marrow transplants, healing occurred via direct bone formation as observed in wild type mice or in control wild type hosts transplanted with wild type bone marrow (Fig. 2B). Therefore the presence of Mmp9–/– inflammatory cells alone was not sufficient to induce chondrogenic cell differentiation at the fracture site. However, transplantation of wild type bone marrow in Mmp9–/– hosts prevented cartilage formation, which is normally observed in Mmp9–/– calluses as well as in Mmp9–/– controls transplanted with Mmp9–/– bone marrow (Fig. 2B)[3]. Indeed, 0 of 5 Mmp9–/– mice transplanted with wild type bone marrow exhibited cartilage in the callus, while 3 of 5 Mmp9–/– mice transplanted with Mmp9–/– bone marrow exhibited cartilage at day 10 (Fig. 2B). Therefore, wild type bone marrow could rescue the cartilage phenotype in Mmp9–/– stabilized fractures.
Fig. 2. Transplant of wild type bone marrow prevents cartilage formation in Mmp9–/– stabilized fractures and alters inflammatory cell profile.
(A) Experimental design. (B, left) Safranin-O Fast Green staining of day 10 stabilized fracture calluses of Mmp9–/– mice transplanted with Mmp9–/– bone marrow (Mmp9–/–-Mmp9–/–) or with wild type bone marrow (WT-Mmp9–/–), and wild type mice transplanted with wild type bone marrow (WT-WT) or with Mmp9–/– bone marrow (Mmp9–/–-WT). (B, right) Histomorphometric measurements of total cartilage volume at day 10 post stabilized fracture and percentage of samples with cartilage observed at the fracture site. (C, left) Safranin-O Fast Green staining of day 14 non-stabilized fracture calluses of Mmp9–/– mice transplanted with Mmp9–/– bone marrow (Mmp9–/–-Mmp9–/–) or with wild type bone marrow (WT-Mmp9–/–). (C, right) Histomorphometric measurements of total cartilage volume at day 14 post non-stabilized fracture. (D) FACS analyses at day 2 post non-stabilized fracture of macrophages, neutrophils and CD4-positive T cells in bone marrow (BM) and soft tissue (ST) of Mmp9–/– mice transplanted with Mmp9–/– (Mmp9–/–-Mmp9–/–) or wild type bone marrow (WT-Mmp9–/–). (E) Quantification by stereology at day 5 post-stabilized fracture of osteoclasts in Mmp9–/– mice transplanted with Mmp9–/– (Mmp9–/–-Mmp9–/–) or wild-type bone marrow (WT-Mmp9–/–). Bars represent mean ± standard deviation, Student's t-test: * P<0.05; NS=Not Significant (n=5 or 6 per group).
Bone marrow transplantation also rescued the cartilage remodeling defect in Mmp9–/– non-stabilized fractures. By histomorphometric analyses, the cartilage volume in the Mmp9-/- mice transplanted with wild type bone marrow cells was significantly smaller than the Mmp9-/- controls transplanted with Mmp9-/- bone marrow at 14 days post non-stabilized fracture (Fig. 2C). We verified that changes in inflammatory cell profiles inMmp9–/– mice paralleled the cartilage phenotype in stabilized and non-stabilized fractures. The proportions of macrophages decreased in Mmp9–/– mice transplanted with wild type compared to Mmp9–/– bone marrow (Fig. 2D). The proportions of neutrophils remained unchanged and the proportions of CD4+ T cells decreased although not significantly (Fig. 2D). Osteoclast recruitment was also restored as osteoclast number was significantly higher in Mmp9–/– mice transplanted with wild type compared to Mmp9–/– bone marrow (Fig. 2E).
3.5. The periosteal response is influenced by MMP9 and the inflammatory environment
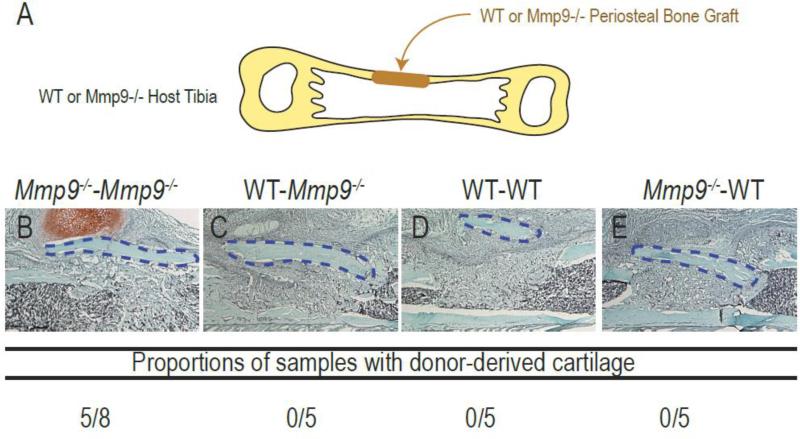
The periosteum is a major contributor of chondrocytes and osteoblasts to bone repair, and cell fate decisions in the periosteum influence healing via endochondral versus intramembranous ossification [34, 38-40]. To determine the role of inflammation and MMP9 on cell differentiation within the periosteum, we used a bone graft approach. Mmp9-/- periosteal bone grafts transplanted in Mmp9-/- hosts healed via endochondral ossification in the absence of mechanical stimuli, compared to Mmp9-/- grafts in wild type hosts, that healed via intramembranous ossification (Fig. 3B-C, p<0.05). This result indicated that a wild type inflammatory environment could prevent cartilage differentiation in the Mmp9-/- periosteum. Conversely, wild type grafts healed via intramembranous ossification in both wild type and Mmp9-/- hosts (Fig. 3D-E). Similar to the bone marrow transplantation results, the Mmp9-/- environment did not induce cartilage differentiation in the wild type periosteum. Thus, the lack of MMP9 in both the periosteum and inflammatory cells was responsible for the cartilage phenotype.
Fig. 3. Analysis of cartilage formation during healing of Mmp9–/– and wild type periosteal bone grafts.
(A) Experimental design. (B-E) Longitudinal sections through the tibia and histological evaluation using Safranin-O staining at day 10 post-surgery of Mmp9–/– periosteal bone grafts transplanted in Mmp9–/– (B, Mmp9–/– - Mmp9–/–) or wild type hosts (C, Mmp9–/–-WT) or wild type periosteal bone grafts transplanted in wild type (D, WT-WT) or Mmp9–/– hosts (D, WT-Mmp9–/–). Cartilage formation is only observed in Mmp9–/– mice transplanted with Mmp9–/– bone grafts. Student's t-test: * P<0.05 (Mmp9–/–-Mmp9–/–compared to WT-Mmp9–/–, WT-WT and Mmp9–/–-WT; n=5 or 6 per group).
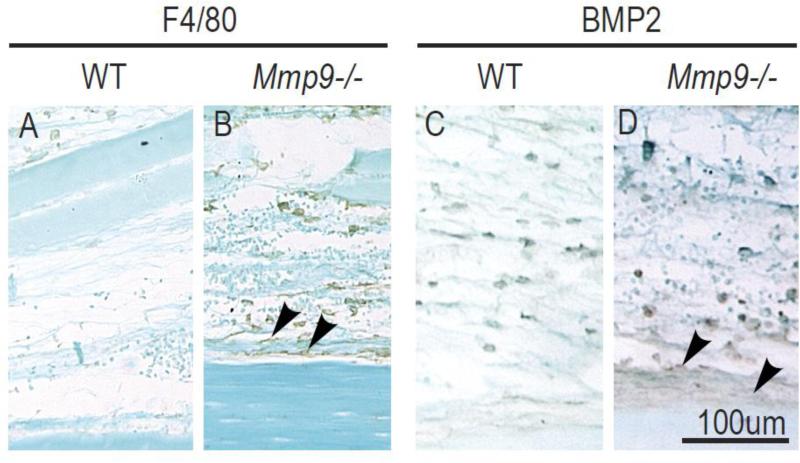
We next examined the distribution of inflammatory cells at the periosteal surface and within the callus more closely. We observed that macrophages remained within the callus as healing progressed, while other inflammatory cell types were found at the periphery of the callus by day 7 (Fig. 1 and data not shown). By immunohistochemistry, macrophages accumulated as early as day 3 near the periosteal surface of the Mmp9–/–compared to wild type stabilized fracture calluses (Fig. 4A-B, arrowheads). The altered distribution of macrophages in Mmp9-/- stabilized fractures affected BMP2 expression. We observed a stronger BMP2 immunostaining in the periosteum of Mmp9-/- stabilized fractures compared to wild type (Fig. 4C-D, arrowheads).
Fig. 4. Distribution of macrophages near the periosteal surface during fracture repair.
Immunodetection of (A-B) macrophages using F4/80 antibody and (C-D) BMP2-expressing cells at the periosteal surface of wild type and Mmp9–/– stabilized tibial fractures at day 3 post-injury. (A-B) Macrophages are found throughout the callus and are concentrated near the periosteum in Mmp9–/– stabilized fractures (B, arrowheads) compared to wild type fractures. (C-D) BMP2 immunostaining is detected in inflammatory cells at the fracture site and near the periosteum with stronger staining found in the periosteum of Mmp9–/– stabilized fractures (D, arrowheads) compared to wild type.
4. Discussion
4.1. The mechanical environment affects the inflammatory response and skeletal cell differentiation during bone repair
Bone repair is initiated by an inflammatory response, which coincides with the recruitment and differentiation of skeletal progenitors. To elucidate the role of inflammation and mechanical signals in skeletal cell differentiation, we compared the inflammatory response between stabilized and non-stabilized tibial fractures. Inflammatory genes were up-regulated by day 2 in both stabilized and non-stabilized fractures, with no major differences in gene expression profiles in the two mechanical environments. This is likely due to the fact that inflammation is first triggered by the same injury in these two fracture models. These results correlate with the timing of inflammatory response that was previously described in several animal models of fracture repair, with a peak of inflammatory genes and cytokines expression reported at day 2 post fracture [41-44]. This coincides with the recruitment of the major inflammatory cell types during the early stages of repair, a crucial stage for skeletal cell fate decisions [3, 45]. Although we detected no differences at the transcriptional level, we observed that stabilization of the fractured bones increased the proportions of macrophages and CD4+ T cells compared to non-stabilized fractures. These results suggested that macrophages and/or T cells play a predominant role in influencing the mode of repair during the inflammatory phase.
In most tissue repair processes, inflammation must resolve to support healing [32]. Following the initial inflammatory response, the expression of inflammatory genes remained high in stabilized fractures indicating a prolonged inflammatory response. In contrast, in non-stabilized factures, the decrease in inflammatory gene expression paralleled cartilage and bone production within the fracture callus. Thus, differences in the inflammatory environment clearly influence the formation of cartilage and bone in the callus, as chondrogenesis and osteogenesis are more robust when inflammation resolves faster. Indeed, systemic inflammation associated with polytrauma has negative effects on fracture repair and has been associated with the accumulation of inflammatory factors [46, 47]. Robust cartilage formation in the non-stabilized fracture calluses could also play a role in suppressing inflammation [48]. Moreover, non-stabilized fractures exhibit an enhanced angiogenic response compared to stabilized fractures, which may facilitate the removal of inflammatory cells [49]. Careful analyses of our histological sections showed that inflammatory cells were present at the fracture site during the peak of inflammatory cell recruitment. Inflammatory cells were then slowly excluded from the newly forming fracture callus, where chondrocytes and osteoblasts are differentiating. However, the macrophages were still located in the callus and their proportions increased until day 7. These data provided further evidence that macrophages are a key inflammatory cell type that may interact with chondrocytes and osteoblasts during callus formation.
4.2. MMP9 regulates macrophage recruitment and periosteal cell fate
MMP9 is expressed both by bone marrow-derived myeloid cells and osteoclasts that are involved in the inflammatory response and extracellular matrix remodeling during bone repair [3, 37]. In the absence of MMP9, the inflammatory cell profile at the fracture site was altered compared to wild type animals, and this was functionally linked to phenotypic changes in Mmp9-/- mutants. Osteoclast recruitment was impaired in Mmp9-/- fracture calluses and bone marrow transplants rescued the cartilage remodeling defects in non-stabilized fractures via providing MMP9-expressing osteoclasts. This result was in concordance with the rescue of the growth plate phenotype in Mmp9-/- developing long bones [50]. Similarly, the recruitment of T cells and macrophages was affected Mmp9-/- fracture calluses and transplantation of wild type bone marrow rescued the cartilage phenotype in Mmp9-/- stabilized fractures. Since bone marrow transplantation mostly provides cells from the hematopoietic lineage with long-term engraftment capacities [22, 25, 35, 51-55], we conclude that the modulations of the inflammatory response can indirectly impact osteogenesis and chondrogenesis in the fracture callus. However, transplantation of Mmp9-/- bone marrow into wild type mice did not induce the cartilage phenotype in stabilized fractures, indicating that cartilage induction was not due to a cell autonomous defect in inflammatory cells. Using periosteal graft transplantation, we uncovered the fact that MMP9 acts indirectly at the level of the periosteum, a target of many signals that regulate skeletal cell fate during the early stages of repair [39, 56-58].
This indirect effect of MMP9 on periosteal cell fate was via the regulation of macrophage localization. Only macrophages were located within the periosteum to locally influence chondrogenic and osteogenic cell differentiation. CD4+ T cells were found away from the periosteum, and so are unlikely to have an effect on chondrogenesis and osteogenesis. Previous studies reported that changes in the infiltration of macrophages within the fracture callus were related to the level of fracture stability, but did not provide a functional link with changes in the osteogenic or chondrogenic response [59]. Another study illustrated the important role of resident macrophages, so-called osteomacs, which are closely associated with bone lining osteoblasts. These osteomacs are also associated with osteoclasts, and their depletion impairs bone repair via intramembranous ossification [10]. Surprisingly, the complete absence of macrophages in CCR2 KO mice does not have consequences on the early stages of repair, but mostly impairs the remodeling phase of repair [9].
While macrophages are not required for the induction of endochondral ossification within the callus, they are required for bone induction in pathological conditions such as heterotopic ossification (HO) [60]. This bone induction may be due to the production of BMPs that are expressed by macrophages and other inflammatory cells, but that are not normally active in the muscle environment [60, 61]. During fracture repair, BMPs are produced by many cell types and are up-regulated in the periosteum of non-stabilized fractures, which heal via endochondral ossification [38, 56, 62]. Ectopic localization of macrophages in the periosteum of Mmp9-/- stabilized fractures may be responsible for the induction of endochondral ossification as we found an increase in BMP2 expression. There are other examples of indirect mechanisms of action of MMP9. MMP9 could regulate the mechanical properties of the extracellular matrix, which may directly impact periosteal stem cells [57, 63, 64]. During bone development and during the replacement of cartilage by bone in the fracture callus, MMP9 indirectly regulates angiogenesis by regulating the bioavailability of VEGF [3, 37, 50]. Thus, MMP9 can modulate the function of important growth factors by controlling their release from the extracellular matrix or by controlling the spatial distribution of cells that secrete these growth factors [39, 56].
In conclusion, by elucidating the indirect effects of MMP9 on periosteal cell fate, we have unraveled one of the multiple mechanisms by which inflammatory cells and mechanical stimuli may influence skeletal cell differentiation during bone repair. It is clear that specific inflammatory cell types such as macrophages can be targeted to stimulate bone repair, and that other cell types including T cells may also be targeted. To improve the management of inflammation following bone injury clinically, more studies will be required to dissect the roles of numerous inflammatory and mechanical factors, and their impact on skeletal stem cells [65-67]. These factors act in a complex healing environment where major signaling pathways including BMPs, Wnt, Hedgehogs and Parathyroid Hormone also regulate skeletal stem cells, and may be modulated by mechanical stimuli and inflammatory cytokines [68-70].
Supplementary Material
Highlights.
We assessed the role of inflammation during the early stages of bone repair in mice
The mechanical environment impacts the inflammatory response
Lack of MMP9 affects the inflammatory response
MMP9 regulates the periosteal cell response in distinct mechanical environments
MMP9 regulates the distribution of macrophages at the periosteal surface
Acknowledgements
This work was funded by NIH-NIAMS R01 AR053645 to TM, R01 AR057344 to CC and TM, INSERM ATIP-AVENIR and Marie Curie International Reintegration grant to CC, R01 AR046238 and R01 CA057621 to ZW. Microarray analysis was performed at the UCSF Lung Biology Microarray Core Facility. We thank Jesse Shantz for help with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson Z, Miclau T, Hu D, et al. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20(5):1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 2.Le AX, Miclau T, Hu D, et al. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19(1):78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 3.Colnot C, Thompson Z, Miclau T, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miclau T, Lu C, Thompson Z, et al. Effects of delayed stabilization on fracture healing. J Orthop Res. 2007;25(12):1552–8. doi: 10.1002/jor.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape HC, Marcucio R, Humphrey C, et al. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 2010;24(9):522–5. doi: 10.1097/BOT.0b013e3181ed1361. [DOI] [PubMed] [Google Scholar]
- 6.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005 doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–43. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 8.Toben D, Schroeder I, El Khassawna T, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 2011;26(1):113–24. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- 9.Xing Z, Lu C, Hu D, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7-8):451–8. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander KA, Chang MK, Maylin ER, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517–32. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 11.Colburn NT, Zaal KJ, Wang F, et al. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum. 2009;60(6):1694–703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169(3):285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 13.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 14.Alblowi J, Kayal RA, Siqueira M, et al. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175(4):1574–85. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radi ZA. Pathophysiology of cyclooxygenase inhibition in animal models. Toxicol Pathol. 2009;37(1):34–46. doi: 10.1177/0192623308329474. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Hadi O, Parvizi J, Austin MS, et al. Nonsteroidal anti-inflammatory drugs in orthopaedics. J Bone Joint Surg Am. 2009;91(8):2020–7. [PubMed] [Google Scholar]
- 17.Chang JK, Wu SC, Wang GJ, et al. Effects of non-steroidal anti- inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology. 2006;228(2-3):111–23. doi: 10.1016/j.tox.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Simon AM, O'Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89(3):500–11. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 19.Xie C, Ming X, Wang Q, et al. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43(6):1075–83. doi: 10.1016/j.bone.2008.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wixted JJ, Fanning PJ, Gaur T, et al. Enhanced fracture repair by leukotriene antagonism is characterized by increased chondrocyte proliferation and early bone formation: a novel role of the cysteinyl LT-1 receptor. J Cell Physiol. 2009;221(1):31–9. doi: 10.1002/jcp.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–98. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 24.Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131(23):5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behonick DJ, Xing Z, Lieu S, et al. Role of Matrix Metalloproteinase 13 in Both Endochondral and Intramembranous Ossification during Skeletal Regeneration. PLoS ONE. 2007;2(11):e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieu S, Hansen E, Dedini R, et al. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis Model Mech. 2011;4(2):203–11. doi: 10.1242/dmm.006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dan H, Simsa-Maziel S, Hisdai A, et al. Expression of matrix metalloproteinases during impairment and recovery of the avian growth plate. J Anim Sci. 2009;87(11):3544–55. doi: 10.2527/jas.2009-2068. [DOI] [PubMed] [Google Scholar]
- 28.Esparza J, Kruse M, Lee J, et al. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J. 2004;18(14):1682–91. doi: 10.1096/fj.04-2445com. [DOI] [PubMed] [Google Scholar]
- 29.Corry DB, Kiss A, Song LZ, et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;18(9):995–7. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenlee KJ, Corry DB, Engler DA, et al. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177(10):7312–21. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu JY, McKeon R, Goussev S, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26(39):9841–50. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez-Fernandez A, Inada M, Balbin M, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). Faseb J. 2007;21(10):2580–91. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reif S, Somech R, Brazovski E, et al. Matrix metalloproteinases 2 and 9 are markers of inflammation but not of the degree of fibrosis in chronic hepatitis C. Digestion. 2005;71(2):124–30. doi: 10.1159/000084626. [DOI] [PubMed] [Google Scholar]
- 34.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–82. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350(3):557–61. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 36.Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100(2):245–50. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 37.Ortega N, Wang K, Ferrara N, et al. Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Model Mech. 2010;3(3-4):224–35. doi: 10.1242/dmm.004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu YY, Lieu S, Lu C, et al. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46(3):841–51. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Yu YY, Lieu S, Lu C, et al. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010;47(1):65–73. doi: 10.1016/j.bone.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Huang C, Zeng F, et al. Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: implication for postnatal bone repair. Am J Pathol. 2010;177(6):3100–11. doi: 10.2353/ajpath.2010.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Einhorn TA, Majeska RJ, Rush EB, et al. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10(8):1272–81. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 42.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 43.Einhorn TA. The cell and molecular biology of fracture healing. Clinical Orthopaedics and Related Research. 1998;46(355 Suppl):S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 44.Rundle CH, Wang H, Yu H, et al. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38(4):521–9. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Heiner DE, Meyer MH, Frick SL, et al. Gene expression during fracture healing in rats comparing intramedullary fixation to plate fixation by DNA microarray. J Orthop Trauma. 2006;20(1):27–38. doi: 10.1097/01.bot.0000184143.90448.aa. [DOI] [PubMed] [Google Scholar]
- 46.Claes L, Ignatius A, Lechner R, et al. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop. 2011;82(2):223–7. doi: 10.3109/17453674.2011.570677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Recknagel S, Bindl R, Kurz J, et al. Experimental blunt chest trauma impairs fracture healing in rats. J Orthop Res. 2011;29(5):734–9. doi: 10.1002/jor.21299. [DOI] [PubMed] [Google Scholar]
- 48.Ulivi V, Lenti M, Gentili C, et al. Anti-inflammatory activity of monogalactosyldiacylglycerol in human articular cartilage in vitro: activation of an anti-inflammatory cyclooxygenase-2 (COX-2) pathway. Arthritis Res Ther. 2011;13(3):R92. doi: 10.1186/ar3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu C, Saless N, Hu D, et al. Mechanical stability affects angiogenesis during early fracture healing. J Orthop Trauma. 2011;25(8):494–9. doi: 10.1097/BOT.0b013e31822511e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211(1):27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 52.Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13(11-12):4385–402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing Z, Lu C, Hu D, et al. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28(8):1000–6. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons PJ, Przepiorka D, Thomas ED, et al. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328(6129):429–32. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 55.Rieger K, Marinets O, Fietz T, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33(5):605–11. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Huang C, Xue M, et al. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone. 2011;48(3):524–32. doi: 10.1016/j.bone.2010.10.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colnot C, Zhang X, Tate ML. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012 doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Y, Zhang X, Du K, et al. Inhibition of beta-catenin signaling in chondrocytes induces delayed fracture healing in mice. J Orthop Res. 2012;30(2):304–10. doi: 10.1002/jor.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hankemeier S, Grassel S, Plenz G, et al. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. Journal of Orthopaedic Research. 2001;19(4):531–538. doi: 10.1016/S0736-0266(00)00044-9. [DOI] [PubMed] [Google Scholar]
- 60.Kan L, Liu Y, McGuire TL, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27(1):150–6. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Y, Lu C, Lieu S, et al. Expression of Bone Morphogenetic Proteins activation pathway and its antagonists during tibial fracture healing. Orthopaedic Research Society; 2009. Paper #785. [Google Scholar]
- 62.Tsuji K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 63.Karamichos D, Skinner J, Brown R, et al. Matrix stiffness and serum concentration effects matrix remodelling and ECM regulatory genes of human bone marrow stem cells. J Tissue Eng Regen Med. 2008;2(2-3):97–105. doi: 10.1002/term.69. [DOI] [PubMed] [Google Scholar]
- 64.McBride SH, Dolejs S, Brianza S, et al. Net change in periosteal strain during stance shift loading after surgery correlates to rapid de novo bone generation in critically sized defects. Ann Biomed Eng. 2011;39(5):1570–81. doi: 10.1007/s10439-010-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mountziaris PM, Spicer PP, Kasper FK, et al. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17(6):393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB J. 2010;24(10):3625–32. doi: 10.1096/fj.10-157370. [DOI] [PubMed] [Google Scholar]
- 67.Palomares KT, Gleason RE, Mason ZD, et al. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J Orthop Res. 2009;27(9):1123–32. doi: 10.1002/jor.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosen V. Harnessing the parathyroid hormone, Wnt, and bone morphogenetic protein signaling cascades for successful bone tissue engineering. Tissue Eng Part B Rev. 2011;17(6):475–9. doi: 10.1089/ten.TEB.2011.0265. [DOI] [PubMed] [Google Scholar]
- 69.Sibai T, Morgan EF, Einhorn TA. Anabolic agents and bone quality. Clin Orthop Relat Res. 2011;469(8):2215–24. doi: 10.1007/s11999-010-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4(7):e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.