Abstract
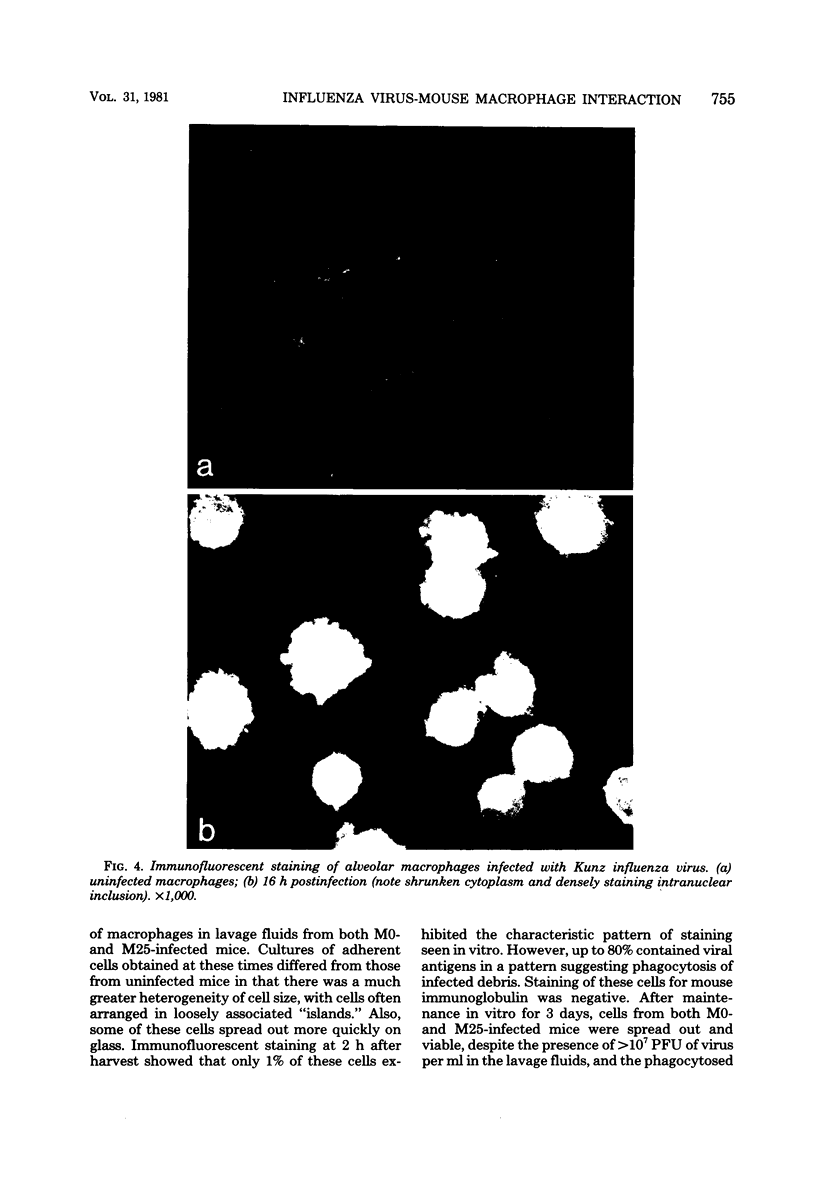
Mouse peritoneal and alveolar macrophages differed substantially in their response to influenza in vitro. Immunofluorescent and infectious-center techniques showed that viral proteins were produced in only a small subpopulation (17%) of peritoneal macrophages and that these infected cells were removed from culture by 3 days postinfection. In contrast, alveolar macrophages were highly susceptible to influenza, and viral antigens were produced in all cells. This was accompanied by a cytopathic effect and cell death. However, no infectious virus was released and the infection was considered abortive. With mouse cytomegalovirus, however, both alveolar and peritoneal macrophages were equally restrictive, and viral antigens were produced in only 1 to 5% of either cell population. No significant differences were observed between mouse-virulent and -avirulent strains of influenza in their interaction with macrophages either in vitro or in vivo. In vivo, both strains induced an influx of cells to the alveolar spaces by 3 to 4 days postinfection, and this was reflected by a 5- to 10-fold increase in the number of "macrophages" in harvest fluids at this time. Many of these cells had an altered morphology compared with alveolar macrophages from uninfected mice, and the cell population as a whole was not susceptible to influenza. However, this resistance was lost by 7 days of in vitro culture.
Full text
PDF



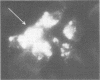
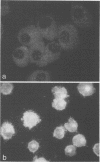
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchmeier N. A., Gee S. R., Murphy F. A., Rawls W. E. Abortive replication of vaccinia virus in activated rabbit macrophages. Infect Immun. 1979 Oct;26(1):328–338. doi: 10.1128/iai.26.1.328-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Gaush C. R., Smith T. F. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol. 1968 Apr;16(4):588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J., Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980 Feb 14;283(5748):660–662. doi: 10.1038/283660a0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
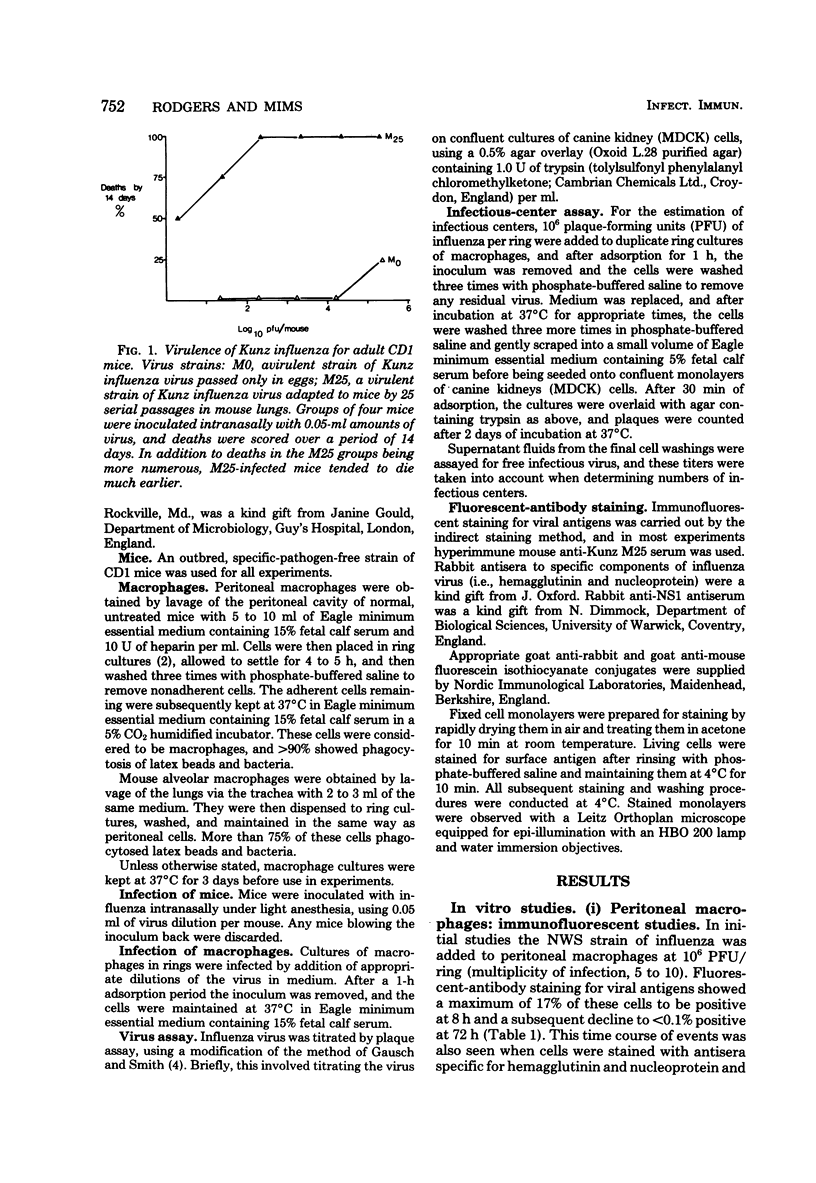
- Hurd J., Heath R. B. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975 May;11(5):886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Sannes P. L. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980 Mar;27(3):960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S. Macrophage nomenclature: where are we going? J Reticuloendothel Soc. 1980 Feb;27(2):223–245. [PubMed] [Google Scholar]
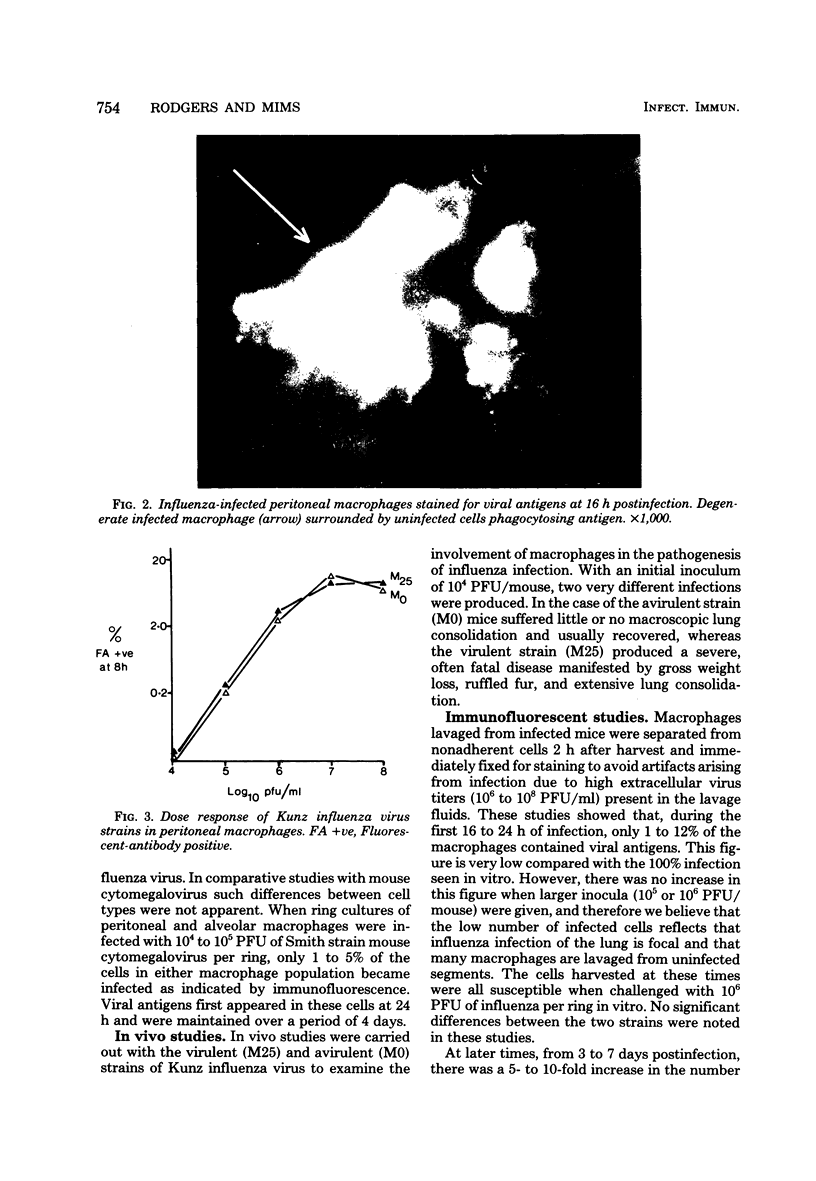
- Nugent K. M., Pesanti E. L. Effect of influenza infection on the phagocytic and bactericidal activities of pulmonary macrophages. Infect Immun. 1979 Nov;26(2):651–657. doi: 10.1128/iai.26.2.651-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut S., Hurd J., Blandford G., Heath R. B., Cureton R. J. The pathogenesis of infections of the mouse caused by virulent and avirulent variants of an influenza virus. J Med Microbiol. 1975 Feb;8(1):127–136. doi: 10.1099/00222615-8-1-127. [DOI] [PubMed] [Google Scholar]
- SELLERS T. F., Jr, SCHULMAN J., BOUVIER C., McCUNE R., KILBOURNE E. D. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961 Aug 1;114:237–256. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Lief F. S., Mudd S. Specific and nonspecific cell-mediated resistance to influenza virus in mice. Infect Immun. 1974 Jun;9(6):991–998. doi: 10.1128/iai.9.6.991-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg B. A., Jakab G. J., Thomson R. G., Warr G. A., Boo K. S. Ultrastructural alterations in phagocytic functions of alveolar macrophages after parainfluenza virus infection. J Reticuloendothel Soc. 1979 Apr;25(4):405–416. [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Albrecht P., Daniel S., Ennis F. A. Host defense mechanisms against influenza virus: interaction of influenza virus with murine macrophages in vitro. Infect Immun. 1978 Dec;22(3):758–762. doi: 10.1128/iai.22.3.758-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Peavy D. L., Cate T. R. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect Immun. 1978 Jul;21(1):140–146. doi: 10.1128/iai.21.1.140-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]