Abstract
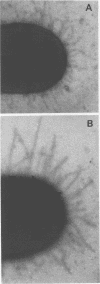
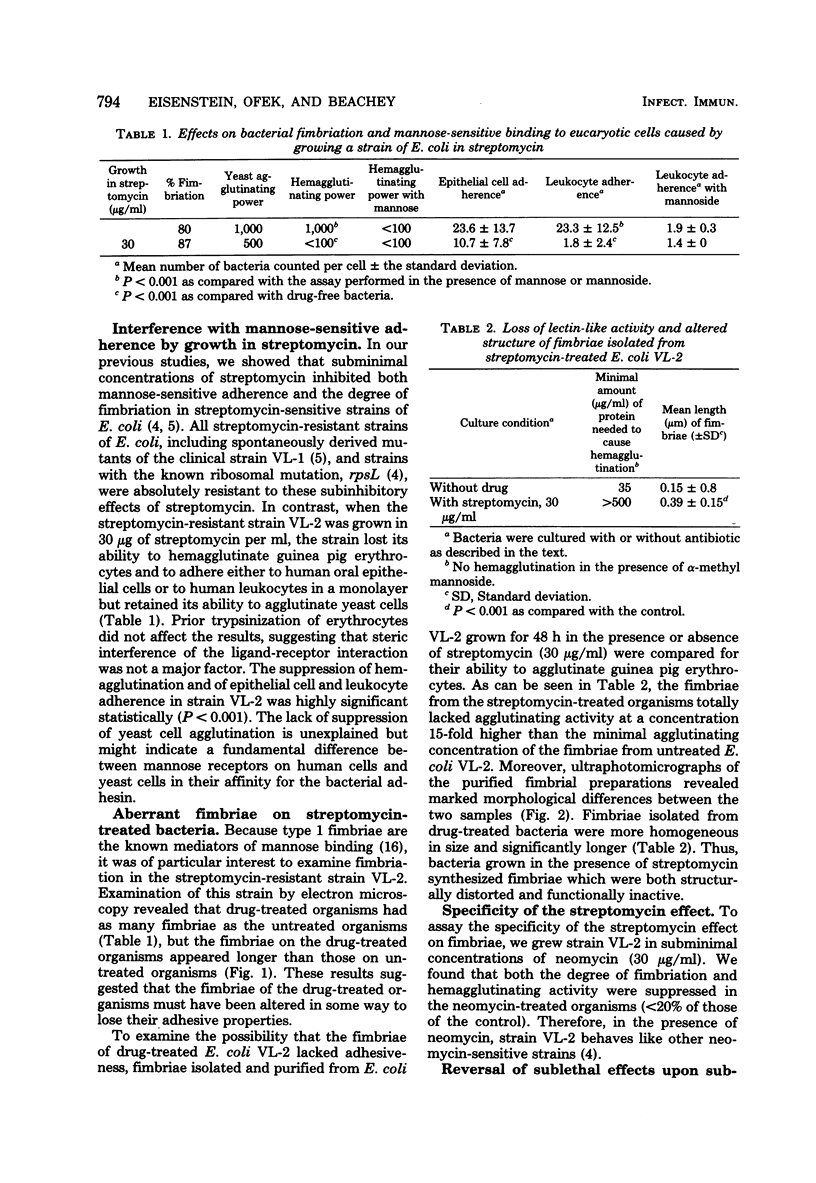
Growth of a streptomycin-resistant strain of Escherichia coli (VL-2) in the presence of 30 microgram of streptomycin per ml resulted in the production by these bacteria of structurally altered, nonfunctional type 1 fimbriae. This strain, when grown in this subinhibitory concentration of streptomycin, became incapable of producing mannose-sensitive hemagglutination (<1% of that of the control). Adhering ability to epithelial cells and human leukocytes was also diminished (42 and 7% of that of the control, respectively). Although these streptomycin-treated bacteria were as heavily fimbriated as untreated bacteria, their fimbriae were significantly longer. Furthermore, in contrast to the fimbriae of the untreated bacteria, those isolated from the drug-treated bacteria were found to lack mannose binding activity as measured by hemagglutination. It appears, therefore, that streptomycin can cause even resistant bacteria to produce an aberrant fimbrial protein, possibly by causing misreading of messenger RNA. These studies indicate that the use of sublethal doses of certain antibiotics whose mode of action is well known may shed light on the genetic and chemical modulation of bacterial factors involved in mucosal colonization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Ofek I. Influence of sublethal concentrations of antibiotics on the expression of the mannose-specific ligand of Escherichia coli. Infect Immun. 1980 Apr;28(1):154–159. doi: 10.1128/iai.28.1.154-159.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Interference with the mannose binding and epithelial cell adherence of Escherichia coli by sublethal concentrations of streptomycin. J Clin Invest. 1979 Jun;63(6):1219–1228. doi: 10.1172/JCI109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Sparling P. F. Mutations to increased antibiotic sensitivity in naturally-occurring gonococci. Nature. 1978 Jan 19;271(5642):242–244. doi: 10.1038/271242a0. [DOI] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eisenstein B. I., Alkan M. L., Sharon N. Suppression of bacterial adherence by subminimal inhibitory concentrations of beta-lactam and aminoglycoside antibiotics. Rev Infect Dis. 1979 Sep-Oct;1(5):832–837. doi: 10.1093/clinids/1.5.832. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Ultraviolet irradiation disrupts somatic pili structure and function. Infect Immun. 1979 Sep;25(3):1060–1065. doi: 10.1128/iai.25.3.1060-1065.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]