Abstract
Respiratory syncytial virus (RSV) is one of the most important causes of upper and lower respiratory tract infections in infants and young children, for which no effective treatment is currently available. Although the mechanisms of RSV-induced airway disease remain incompletely defined, the lung inflammatory response is thought to play a central pathogenetic role. In the past few years, we and others have provided increasing evidence of a role of reactive oxygen species (ROS) as important regulators of RSV-induced cellular signaling leading to the expression of key proinflammatory mediators, such as cytokines and chemokines. In addition, RSV-induced oxidative stress, which results from an imbalance between ROS production and airway antioxidant defenses, due to a widespread inhibition of antioxidant enzyme expression, is likely to play a fundamental role in the pathogenesis of RSV-associated lung inflammatory disease, as demonstrated by a significant increase in markers of oxidative injury, which correlate with the severity of clinical illness, in children with RSV infection. Modulation of ROS production and oxidative stress therefore represents a potential novel pharmacological approach to ameliorate RSV-induced lung inflammation and its long-term consequences. Antioxid. Redox Signal. 18, 186–217.
I. Introduction
Respiratory syncytial virus (RSV) is an enveloped negative-sense single-stranded ribonucleic acid (RNA) virus of the Paramyxoviridae family, which is recognized as a leading cause of respiratory illness in young infants and children. No vaccine is currently licensed to prevent RSV infections. Each year in the United States, 75,000–125,000 hospitalizations related to RSV occur among children aged <1 year, and RSV infection results in ∼1.5 million outpatient visits among children aged <5 years (97, 210), with an annual economic burden estimated to be >$500 million for hospitalizations, and a considerable additional amount for outpatient care (188). A recent systematic review of published studies has estimated that for a given year (2005), 33.8 million new episodes of RSV-associated acute lower respiratory tract infections (ALRI) occurred worldwide in children younger than 5 years of age, with at least 3.4 million episodes representing severe ALRI necessitating hospital admission, and an estimated 66,000–199,000 fatal cases, 99% of which occurred in developing countries (176). Short-lasting and incomplete immunity results in recurrent, although milder, infections with RSV throughout life. Nonetheless, RSV infection is recognized as a cause of serious disease in several adult populations that include the elderly, subjects with chronic heart and lung diseases, and immunocompromised patients (71). Indeed, RSV infection is responsible for an estimated 23/10,000 person hospitalizations (175), and 1500–7000 deaths due to RSV infection occur in the United States in people >65 years (98).
The treatment of RSV infection is primarily symptomatic, except possibly in immunocompromised individuals (248). The administration of supplemental oxygen to maintain oxygen saturations of ≥93% and the replacement of fluid deficits are sufficiently effective, so that the majority of infants hospitalized with RSV infection may be discharged within 72 h. Bronchodilators, corticosteroids, and other modalities are ineffective in reducing the rate of hospitalization when administered to outpatients with RSV infection and in shortening the duration of hospitalization among inpatients. As far as specific antiviral agents, ribavirin is the only licensed antiviral drug for treating RSV infection, but its use is restricted to high-risk or severely ill infants, and its utility has been limited by cost, variable efficacy, and tendency to generate resistant viruses (195). Postinfection (p.i.) treatment of RSV with anti-RSV immunoglobulin shows no benefit (187), and the current need for additional effective anti-RSV agents is well acknowledged (234).
RSV vaccines with immunogenic, protective, and nonreactogenic properties are currently under investigation, but a significant obstacle to the development of vaccines for RSV is our still limited understanding of the pathogenetic mechanisms that determine the severity of ALRI caused by the virus. Paradoxically, intense research efforts boosted by the unfortunate experience with the first RSV formalin-inactivated vaccine (136) has significantly contributed to our understanding of the immune-mediated mechanisms responsible for the enhanced disease that occurred in a subset of vaccinated infants, but has incompletely identified the immunopathogenic components in naturally acquired infections (81). In this regard, although infants with certain risk factors (prematurity, chronic lung disease, congenital heart disease, or immunodeficiencies) have an increased risk for more severe RSV disease, the large majority of infants with RSV infections that require hospitalization were previously healthy (246). Therefore, the spectrum of RSV disease severity in otherwise previously healthy infants points to both host determinants and viral-specific factors that can influence the outcome of infection, and while different circulating RSV strains may in part explain differences in disease severity, these differences are relatively minor and do not at the moment provide convincing proof of the large range of disease manifestations (68, 170). On the other hand, more than 50 years of research on RSV-mediated disease has favored the role of the host response and the immunopathogenesis hypothesis, either as causing excessive/enhanced immune/inflammatory responses in the lower airways or as failing to restrict and terminate viral replication as a result of impaired innate immune response. These only apparently contrasting pathways to disease, likely to be genetically determined, may coexist and/or represent temporally distinct aspects of the host response against RSV infections, which are triggered by the initial infection of respiratory epithelium. Indeed, after entering the respiratory tract primarily through fomite or hand-to-nasal transmission after contact with infectious secretions, RSV infects the local respiratory epithelium. At that point, infection may be self-limited or may spread to the lower respiratory tract in part by a cell-to-cell transfer of the virus along intracytoplasmic bridges (96), although the mechanism by which RSV reaches the lower airway is not clearly defined, as the distribution of infected cells in LRTI is patchy. Thus, the epithelium of the respiratory mucosa, the main function of which is to provide a protective physical barrier against injurious inhaled stimuli, is clearly the main target of RSV replication, as shown by studies of infected patients (178, 250), experimental murine models (224), and by a variety of different in vitro culture systems (75, 185, 203, 258) (Fig. 1). Release of viral particle progeny occurs by a budding process, which takes place from the apical surface when assessed in cultures of polarized epithelial cells (258). As the infection progresses, necrosis and disorganized proliferation of the bronchiolar epithelium and destruction of ciliated epithelial cells become prominent features of RSV ALRI (mainly bronchiolitis) (4), with a significant amount of sloughed epithelial cell debris accumulated in the airway spaces. Influx of polymorphonuclear cells and eosinophil degranulation within the airways cells are also well-recognized features of RSV bronchiolitis (69, 137, 250), along with the peribronchial infiltrate of mononuclear cells. The exact type of such mononuclear cells has not been fully characterized, but paucity of T cells, either CD4 or CD8, in postmortem samples of RSV-infected lung suggests that lymphocytes may be absent at the time when infants with bronchiolitis are experiencing their most severe symptoms (250). Another important pathological feature of RSV bronchiolitis is submucosal edema and excess mucus secretion, which combined with cell debris and inflammatory cells cause bronchiolar obstruction, air trapping, and emphysema (4, 178). Gas exchange becomes compromised, resulting in hypoxemia, and in more severe cases the need for supportive respiratory therapy. Overall, severe RSV infections are associated with recruitment of inflammatory cells to the airway mucosa and release of potent inflammatory mediators, processes that are initiated by viral replication in epithelial cells.
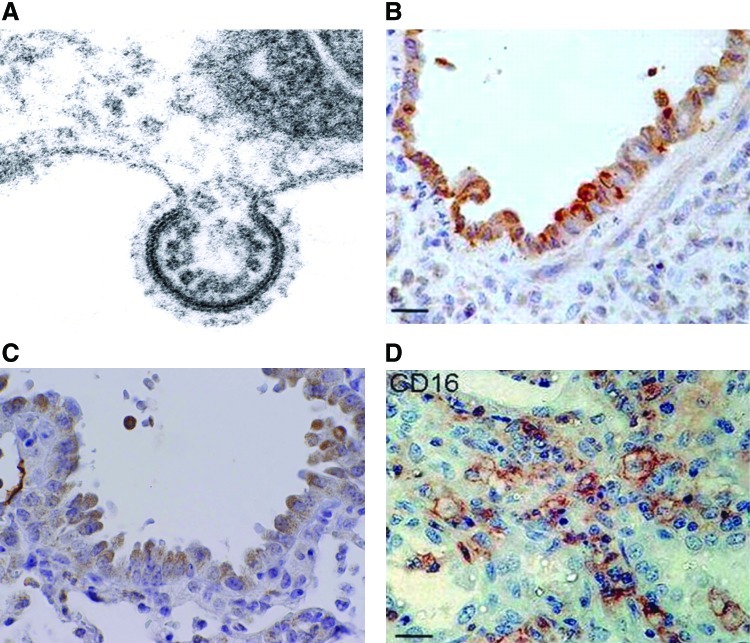
FIG. 1.
RSV replication and pathology. (A) Electron micrograph of RSV budding from an infected HEp-2 cell (Garofalo RP, personal observation). (B) IHC staining for RSV antigen of bronchiolar tissue from autopsy tissue of an infant with bronchiolitis. Brown staining indicates the presence of viral antigen in epithelial cells (reprinted by permission from Welliver et al.) (250). (C) IHC staining for RSV antigen in bronchoalveolar epithelial cells of the mouse lung. Mouse was inoculated with RSV (107 PFU), and lungs were collected at day 5 p.i. for staining (Garofalo RP, personal observation). (D) IHC staining of lung tissue for CD16 antigen (expressed primarily on neutrophils). Tissue was obtained from autopsy tissue of an infant with bronchiolitis (reprinted by permission from Welliver et al.) (250). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) IHC, immunohistochemical; p.i. postinfection; RSV, respiratory syncytial virus.
Although the mechanisms responsible for recruitment of circulating leukocytes into the lung as a consequence of RSV infection are largely unknown, chemokines are recognized as critical molecules in the recruitment and activation of leukocytes in a variety of inflammatory conditions of the lung [reviewed in (14)]. Chemokines are a superfamily of proteins divided into functionally distinct groups: three groups of small basic (heparin-binding) proteins, termed the C, CC, and CXC chemokines (based on the number and spacing of highly conserved NH2-terminal cysteine residues), and a fourth, distantly related group, the CX3C chemokines, composed of large, membrane-bound glycoproteins attached through a COOH-mucin-like domain. Chemokine receptors are expressed in a cell type-restricted fashion, allowing specificity of chemokine activity; for example, members of the C group primarily activate lymphocyte chemotaxis; members of the CXC group induce neutrophil chemotaxis, and the CC group stimulates monocyte, lymphocyte, and eosinophil chemotaxis. These data suggest that pulmonary inflammation associated with neutrophilic and monocytic infiltration is the result of coordinate expression of diverse chemokines with distinct cellular specificities. To identify chemokines produced in the course of RSV infection, a number of clinical studies have been conducted in children with RSV bronchiolitis. These studies have shown that RSV-infected infants have increased production of chemokines, including CXCL8/IL-8, CXCL10/interferon gamma (IFN-γ)-induced protein 10 (IP-10), chemokine (C-C motif) ligand 5 (CCL5)/RANTES, CCL3/macrophage inflammatory protein (MIP)-1α, and CCL2/monocyte chemotactic protein 1 (MCP-1), as measured in nasopharyngeal secretions (NPS) and tracheal aspirates or bronchoalveolar lavage (BAL) of mechanically ventilated patients (82, 167, 184, 211, 232). In some studies, the concentration of chemokines (MIP-1α and MCP-1) has been shown to significantly and inversely correlate to the degree of oxygen saturation, an objective measure of disease severity in RSV-infected infants (82). In some studies, plasma samples of RSV-infected infants with bronchiolitis have been shown to contain higher levels of IL-8 compared to infants with the milder form of disease (26). Interestingly and in contrast with all other studies, analysis of RSV-infected children at the time of visit in the emergency room has shown that those with increased levels of the cytokines IL-6, IL-10, IFN-γ, and the chemokines IL-8 and MIP-1β required less-extended supplemental oxygen therapy, suggesting that a robust immune response may play a protective role at the early phases of infection (23).
Functional studies addressing the role of certain chemokines in RSV infection have been possible only in animal models using mice genetically deficient for chemokines or chemokine receptors, or by neutralizing antibodies. These studies have identified a critical role of chemokines and chemokine receptors, including CCL5/RANTES (231), KC and its receptor CXCR2 (169), CCR1 (the receptor that binds CCL5), and CCL3/MIP-1α (95), in mediating different aspects of RSV-mediated immunopathology and airway pathophysiology. Studies of genetic polymorphism (mostly single-nucleotide polymorphism [SNP]) for single chemokine and chemokine receptor genes, including IL-8, IL-8 receptor, RANTES, CCR5 receptor (for RANTES and MIP-1α), and their association with RSV disease severity have been so far inconclusive, based on the fact that these studies have not been confirmed in more than one population or have reported conflicting results [reviewed in (170)]. Greater scientific consensus on the other hand exists in the recognition of respiratory epithelial cells as a major initiator and a modifier of host innate responses and inflammation by secreting chemokines in response to RSV infection (75, 81, 95, 183, 203). Most of these studies have been performed in cell culture, but some studies have shown RSV-mediated expression of chemokines in airway epithelial cells in vivo (95). Some chemokines appear to be selectively expressed only in epithelial cells from the lower respiratory tract, after RSV infection (185). In the most comprehensive study so far conducted to examine chemokine production, the kinetics and patterns of chemokine expression in RSV-infected lower-airway epithelial cells (A549 and normal human small-airway epithelial cells [SAECs]) have been investigated by membrane-based cDNA microarrays and high-density oligonucleotide probe-based microarrays (257). In A549 cells, RSV induced expression of a complex network of CC (I-309, Exodus-1, thymus and activation regulated chemokine (TARC), RANTES, MCP-1, macrophage-derived chemokine (MDC), and MIP-1α and -1β), CXC (GRO-α, β, and γ, ENA-78, IL-8, and I-TAC), and CX3C (Fractalkine) chemokines. Some chemokines were independently confirmed by multiprobe RNase protection assay, Northern blotting, and reverse transcription–PCR. High-density microarrays performed on SAECs confirmed a similar pattern of RSV-inducible expression of CC chemokines (Exodus-1, RANTES, and MIP-1α and −1β), CXC chemokines (I-TAC, GRO-α, β, and γ, and IL-8), and Fractalkine. In contrast, TARC, MCP-1, and MDC were not induced, suggesting the existence of distinct genetic responses for different types of airway-derived epithelial cells.
In summary, infection of respiratory epithelial cells is the first event occurring after RSV inhalation or inoculation into the nasal mucosa. This is rapidly followed by the induction of a network of epithelial cell cytokines and chemokines that have profound immune and inflammatory regulatory functions. These early elements of the host response to RSV are major determinants of the elimination or the progression of the infection, significantly affect airway mucosa inflammation, and ultimately may dictate the nature of the specific adaptive immune response to the virus (Fig. 2). Thus, understanding the mechanisms that control viral-induced gene transcription in airway epithelium is critically important to identify new therapeutic opportunities to treat ALRI caused by RSV and other respiratory viral pathogens.
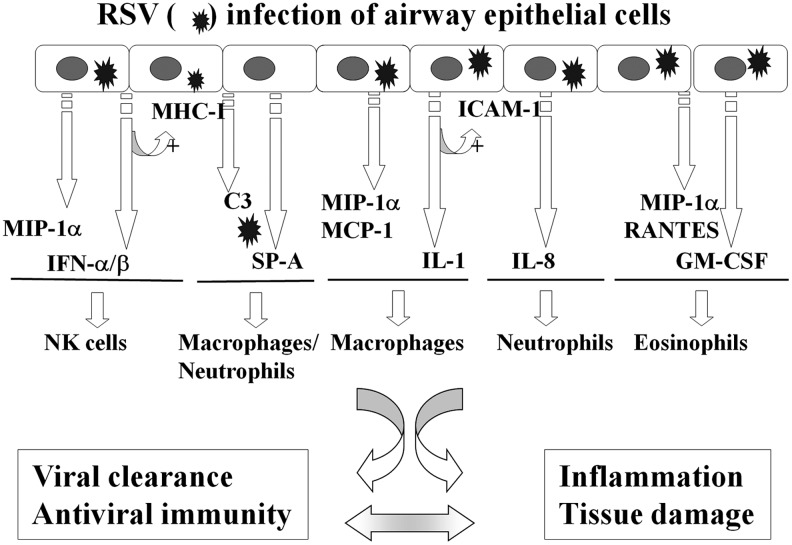
FIG. 2.
Schematic representation of cytokines/chemokines and immunomodulatory mediators produced by RSV-infected epithelium that regulates innate immune cell functions. RSV infection of airway epithelial cells induces the secretion of a variety of proinflammatory and chemoattractant molecules, which leads to the recruitment and activation of innate immune cells. IFN-α/β and IL-1 produced in response to the virus upregulate epithelial expression of MHC-I and ICAM-1, respectively. These elements determine elimination of the virus and/or inflammation and tissue damage of the airway mucosa (reprinted with permission of the American Thoracic Society. Copyright © 2011 American Thoracic Society) (81). ICAM-1, intercellular adhesion molecule 1; IFN, interferon; IL, interleukin; MHC-I, major histocompatibility complex-I.
II. Redox-Sensitive Transcription Factors in RSV Infection
Starting with the discovery of the complex network of cytokines and chemokines produced in response to RSV infection, a number of studies have characterized in great detail the transcriptional mechanisms that control gene expression in respiratory epithelial cells, the major target of RSV infection. RSV replication in these cells results in the activation of multiple cellular signaling pathways involved in the expression of early response genes, such as cytokines, chemokines, and type I IFN, which is coordinated by a small subset of transcription factors. Most of the studies investigating the transcriptional regulation of RSV-induced gene networks have utilized A549 cells, an alveolar type II-like cell line, as a prototype of lower airway respiratory cell, with confirmation of some of the major findings in primary human airway epithelial cells, and many have focused on gene promoter analysis of proinflammatory molecules, such as the C-X-C chemokine IL-8 and the C-C chemokine RANTES. In the following sections, we will discuss the major transcription factors that have been shown to regulate RSV-induced gene expression in epithelial cells and are known to be redox sensitive, together with some of the major signaling pathways leading to their activation. Figure 3 illustrates a part of the signaling network induced by RSV infection in airway epithelial cells.
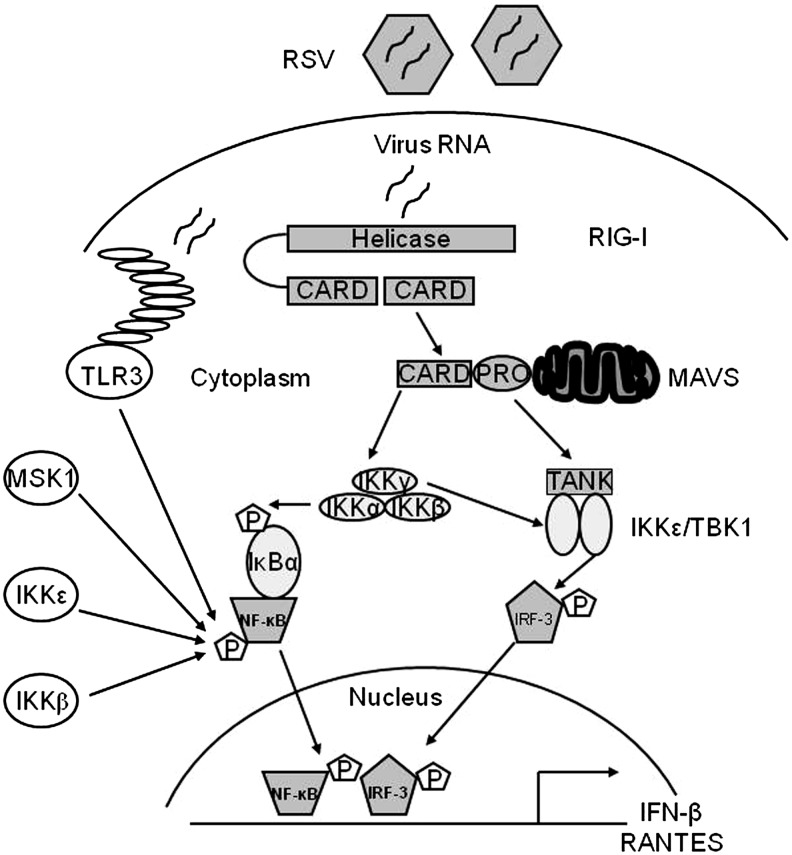
FIG. 3.
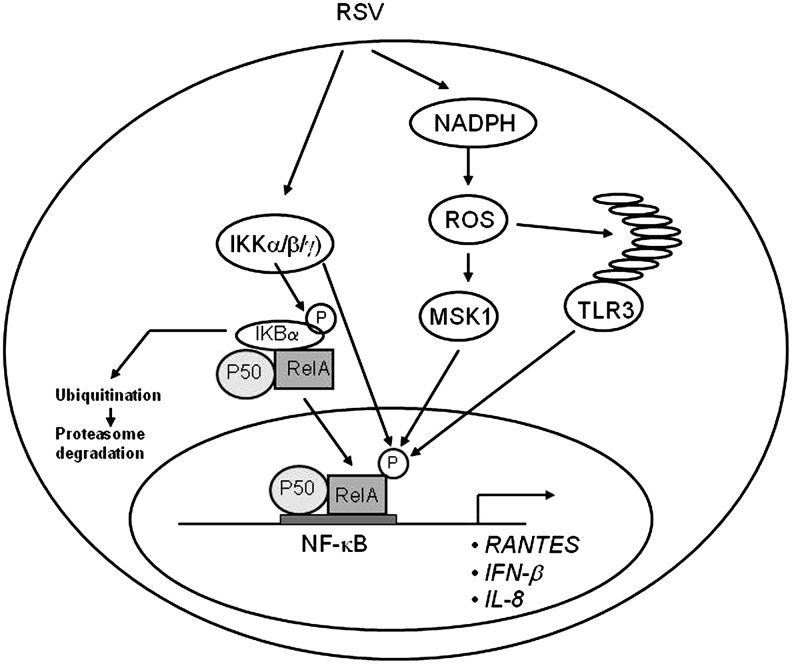
Major signaling pathways regulating RSV-induced NF-κB and IRF activation in airway epithelial cells. Infection of airway epithelial cells with RSV triggers a multitude of signaling pathways, some of which have been shown to be redox sensitive. For lack of space, only the pathways leading to the redox-sensitive transcription factors NF-κB and IRF are illustrated. Production of specific RNA moieties during viral replication leads to activation of the RIG-I-MAVs pathway, which subsequently activates the IKK complex, which is upstream NF-κB activation and of the TBK1/IKKɛ complex, which regulates IRF-3 activation. Activation of these pathways has been shown to be affected by antioxidant treatment in the context of RSV infection. IRF, interferon regulatory factor; NF-κB, nuclear factor kappa-B; IKK, IκB kinase.
A. Nuclear factor-IL6
The CCAAT/enhancer-binding proteins (C/EBP) are basic domain/leucine zipper-containing transcription factors involved in the inducible expression of cytokine and adhesion molecule genes, as well as in cell differentiation (8). C/EBPα, C/EPBβ/NF-IL6, and C/EPBδ are the three major members of this family. RSV infection of A549 cells induces the rapid synthesis of a single 45.7-kDa isoform of nuclear factor (NF)-IL6 in a time-dependent manner (119). NF-IL6 is first detectable after 3 h of infection and continues to accumulate until 48 h. NF-IL6 production could not be induced by ultraviolet (UV)-inactivated virus, demonstrating the requirement of viral replication for NF-IL6 synthesis. Immunoprecipitation studies after [35S]-methionine metabolic cell labeling were performed to investigate the mechanism for NF-IL6 production. In that study, there was robust NF-IL6 protein synthesis within RSV-infected cells. Protein synthesis occurred without detectable changes in the abundance or size of the single 1.8-kb NF-IL6 mRNA. RNase protection assay of transfected chloramphenicol acetyltransferase reporter genes driven by either wild-type or mutated NF-IL6 binding sites showed a virus-induced increase in NF-IL6-dependent transcription. The mechanism for enhanced translation of preformed mRNA used by RSV appears to be distinct from that used by influenza virus (241). Influenza virus infection increased rapidly and transiently the DNA-binding activity of preformed NF-IL6 protein without changing its steady-state levels. Thus, the NF-IL6 activity appears to be inducible by several distinct post-translational mechanisms in viral infections. In studies of RANTES promoter activation by RSV infection, deletions/site mutations of the NF-IL6-binding site decreased RSV-induced reporter (luciferase) activity by ∼50% (38). Similar results have been reported in studies of the intercellular adhesion molecule (ICAM-1) promoter in RSV-infected epithelial cells (45). C/EBP-β and C/EBP-δ appear to be the major components of the NF-IL6 nucleoprotein complex formed on the RANTES promoter (38).
B. Nuclear factor-kappa B
Nuclear factor kappa B (NF-κB) is a family of inducible transcription factors related by a common NH2-terminal Rel homology domain (RHD). This family includes the proteolytically processed DNA-binding subunits NF-κB1 and NF-κB2, also known as p50 and p52, and the transcriptional activators p65/RelA, cRel, and RelB [reviewed in (126)]. NF-κB is complexed and inactivated in the cellular cytoplasm by binding inhibitors of NF-κBs IκBs, which are ankyrin repeat domain-containing proteins that bind the RHD and block nuclear translocation and DNA-binding activity. The major IκBs include the isoforms α/β/ɛ and the 100-kDa NF-κB2 precursor.
RSV is a potent activator of NF-κB in airway epithelial cells (27, 38, 83, 118). A number of RSV-inducible inflammatory and immunoregulatory genes require NF-κB for their transcription and/or are dependent on an intact NF-κB-signaling pathway. Transient transfection of the human IL-8 promoter mutated in the binding site for NF-κB demonstrated that this sequence was essential for RSV-activated transcription (27, 83, 161). Gel mobility shift assays demonstrated RSV induction of sequence-specific binding complexes, and these complexes were supershifted by antibodies directed to the NF-κB subunit RelA. Both by Western immunoblot and indirect immunofluorescence assays, it was shown that cytoplasmic RelA in uninfected cells became localized to the nucleus after RSV infection. Moreover, RelA activation requires replicating RSV, because neither a conditioned medium nor UV-inactivated RSV was able to stimulate its translocation. In addition to IL-8, NF-κB activation is essential for RSV-induced expression of RANTES (38), as well as other chemokines, cytokines, secreted proteins, and signaling molecules (27, 233). In vivo, in a mouse model of infection, RSV activates NF-κB early in the course of infection (92), and inhibition of NF-κB activation reduces cytokine production and clinical disease, without decreasing viral replication (93). Together, these findings suggest that activation of the host inflammatory response via NF-κB is a central step in the immunopathogenesis of RSV infection. As a result, the mechanisms by which RSV activates RelA have been intensively studied, particularly in the context of chemokine gene transcription.
In response to RSV infection, NF-κB translocation is mediated by two distinct pathways termed the canonical and the cross-talk pathways (118, 153). The canonical pathway is induced by cytokines, Toll-like receptor (TLR) ligands, and viral double-stranded RNA (dsRNA), and it requires a multiprotein signaling complex composed of two highly homologous serine/threonine kinases, IκB kinase (IKK)-α and β, and a regulatory subunit, IKK-γ, which exists in a precise stoichiometric relationship of two catalytic subunits to two regulatory subunits. Gene knockout studies have shown that IKK-β is the major IκB-α kinase. IKK-γ, also known as the NF-κB essential modulator (NEMO), is essential for inducible IKK activity, as IKK-γ-deficient cells are unable to activate IKK in response to all stimuli tested. IKK-β phosphorylation in its activation loop induces IκB-α NH2-terminal phosphorylation, ubiquitination, and degradation, releasing RelA to translocate into the nucleus [reviewed in (29)]. Infection of airway epithelial cells with RSV induces a progressive degradation of IκB-α and IκB-β, paralleled by the increase in RelA DNA-binding activity, through a mechanism that is partially independent of the proteasome pathway (118). RSV infection also induces IKK-β activation, demonstrated by kinase assays, starting at 3 h p.i. and peaking between 6 and 12 h p.i. with a gradual decrease at later timepoints of infection. IKK-β is necessary for RSV-induced NF-κB activation, as demonstrated using IKK-β dominant-negative (DN) mutants in reporter gene assays, or the NEMO-binding domain peptide (a known inhibitor of IKK-β catalytic activity) (59).
Upstream of the IKK complex, the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase kinase 1 (MEKK1) and transforming growth factor-beta-activated kinase 1 (TAK1), which are serine/threonine kinases in the MAP kinase kinase kinase (MAPKKK) family, have been shown to activate intracellular kinases such as p38 MAPK, JNK, and IKKs, leading to NF-κB and AP-1 induction (182). Inhibition of TAK1 activation, by overexpression of kinase inactive TAK1 or using cells lacking TAK1 expression, significantly reduces RSV-induced NF-κB nuclear translocation and DNA-binding activity, as well as NF-κB-dependent gene expression, identifying TAK1 as an important upstream signaling molecule regulating RSV-induced NF-κB activation (59).
Infected host cells detect and respond to RNA viruses using different mechanisms in a cell-type-specific manner, including the retinoic acid-inducible gene I (RIG-I)-dependent and TLR-dependent pathways. In studies addressing the upstream pathways of canonical NF-κB activation, we have shown that RIG-I helicase binds RSV transcripts within 12 h of infection (152). Short interfering RNA (siRNA)-mediated RIG-I knockdown significantly inhibited NF-κB nuclear translocation and DNA binding. Consistent with this finding, RSV-induced IFN-β, IP-10, CCL5, and IFN-stimulated gene 15 (ISG15) expression levels were decreased in RIG-I-silenced cells, indicating that the RIG-I-mitochondrial antiviral signaling protein (MAVS) complex is upstream of the NF-κB canonical pathway. In addition, TLR3 (which binds dsRNA) also seems to play a role in RSV-induced NF-κB activation in airway epithelial cells, as siRNA-dependent knockdown of its expression results in the inhibition of RSV-induced chemokines and antiviral cytokines, including IP-10 and IFN-β (152, 202).
The noncanonical pathway induces processing of the 100-kDa NF-κB2 precursor into its mature 52-kDa DNA-binding form (NF-κB2) and liberation of sequestered p100-associated complexes [reviewed in (29)]. In the noncanonical pathway, p100 processing is initiated by IKK-α-mediated serine phosphorylation of the p100 COOH-terminus. IKK-α activation in the noncanonical pathway is controlled by NF-κB-inducing kinase (NIK). NIK serves as a rate-limiting upstream activator that phosphorylates IKK-α and also serves as a docking protein to recruit both p100 NF-κB2 and IKK-α into a complex. Although the focus of the noncanonical pathway has been on the RelB-NF-κB2 complexes, recent work has shown the existence of a p100-sequestered RelA-NF-κBl complex whose liberation can be induced by lymphotoxin-beta. The latter represents a cross-talk pathway where prototypical ReIA-NF-κBl DNA-binding complex is released as a consequence of activating the noncanonical NIK-IKK-α kinases. A part of the RelA activation and RelA-dependent chemokine response to RSV infection also appears to be induced through a noncanonical/cross-talk pathway involving the NIK-IKK-α complex downstream of RIG-I-MAVS activation (49, 152). In airway epithelial cells, RSV infection induces NIK kinase activity, processing of p100 to p52, and nuclear translocation of a ternary complex with IKK-α and processed p52, leading to the expression of a subset of NF-κB-dependent genes (49). Upon RSV infection, NIK associates with the RIG-I-MAVS complex via the RIG-I NH2-terminal caspase activation and recruitment domain (CARD) and the COOH-terminus of NIK, and RIG-I silencing in RSV-infected epithelial cells inhibits p100 processing to p52, suggesting that RIG-I is functionally upstream of the noncanonical regulatory kinase complex composed of NIK-IKK-α subunits (153).
Optimal NF-κB activity also requires signal-induced phosphorylation. Several NF-κB family members, in particular RelA, have been shown to be phosphorylated on specific serine residues, either constitutively or in an inducible manner, by stimuli such as lipopolysaccharide (LPS), IL-1, and tumor nuclear factor (TNF) (244, 245, 259, 260). This event is associated with an increase in p65 transcriptional activity, without modification of nuclear translocation or DNA-binding affinity (260). Among the inducible serine phosphoacceptor sites that regulate NF-κB transcriptional activity, serines 276 (Ser276) and 536 (Ser536) have been shown to be potential targets for protein kinase A (PKA) and mitogen- and stress-activated protein kinase 1 (MSK1) (239, 260) or casein kinase II and IKK-β kinases, respectively (204, 245). In addition, the IKK-like molecule IKKɛ, a critical component of the virus-activated kinase complex responsible for interferon regulatory factor (IRF)-3 activation (73, 209), has also been shown to modulate NF-κB activation in response to LPS and PMA stimulation (191, 212). IKKɛ mediates p65 phosphorylation in response to IL-1 and TNF stimulation (33, 251), and regulates constitutive NF-κB activity in cancer cells through a similar mechanism (2).
In airway epithelial cells, RSV induces a time-dependent RelA phosphorylation on both the residues Ser-276 and Ser-536 (16, 120). RelA Ser-276 phosphorylation in infected cells depends on activation of MSK1 (120), a serine/threonine kinase downstream of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38 MAPKs that play key roles in the immune response, cell proliferation, and apoptosis (63). Inhibition of MSK1 using H89 and siRNA knockdown reduces both RSV-induced phospho-Ser276 RelA formation and expression of a subset of NF-κB-dependent genes (120). In addition, siRNA-mediated knockdown of TLR3 (which binds dsRNA) in infected epithelial cells also results in the reduction of RSV-induced phospho-Ser276 RelA formation and inhibition of RSV-induced chemokines and antiviral cytokines, including IP-10 and IFN-β (152, 202).
The mechanism by which RSV-induced RelA Ser276 phosphorylation induces transcriptional activation is not completely understood. In other contexts, activated NF-κB mediates promoter-specific recruitment of coactivators to produce chromatin remodeling, transcription factor acetylation, and stable enhanceosome formation on inflammatory gene promoters (121). In recent investigations, we found that RSV-induced RelA Ser276 phosphorylation is required for acetylation at lysine 310, an event required for transcriptional activity and stable association of RelA with the activated positive transcriptional elongation factor-b complex proteins, bromodomain-4 (Brd4) and CDK9 (29).
The mechanisms leading to RSV-induced RelA Ser536 phosphorylation have also been investigated. We have recently shown that IKKɛ, a kinase known to regulate IRF-3 activation in response to viral infections (73, 114), also controls NF-κB transcriptional activity in response to RSV infection, by inducing RelA Ser536 phosphorylation (16). Expression of catalytically inactive IKKɛ significantly inhibits RSV-induced IL-8 secretion, promoter activation, and NF-κB-driven gene transcription, indicating a fundamental role of this kinase in the pathway leading to RSV-induced NF-κB activation. Lack of IKKɛ does not affect RelA nuclear translocation and DNA binding, but it greatly reduces RelA Ser536 phosphorylation, a post-translational modification important for RSV-induced NF-κB-dependent gene expression, as indicated by reconstitution experiments of RelA−/− MEFs (using a wild-type or Ser536Ala RelA mutant). In these cells, expression of wild-type RelA, but not Ser536Ala RelA mutant, results in enhanced expression of both basal and RSV-inducible expression of growth-regulated protein beta (Gro-β), an RSV-inducible NF-κB-dependent gene, indicating that phosphorylation of Ser536 plays an important role in RSV-induced NF-κB transcriptional activity (16). Similar results have been reported by a different group who showed that this RelA phosphorylation site is important in modulating NF-κB-dependent gene transcription in A549 cells infected with RSV (72). The presence of residual phospho-Ser536 p65 levels in cells lacking IKKɛ expression suggests the presence of potential additional pathways involved in viral-induced Ser536 p65 phosphorylation. Recent work from Yoboua et al. has shown that in airway epithelial cells, initial RSV-induced p65 Ser536 phosphorylation is dependent on RIG-I, through a pathway involving MAVS, TNF receptor-associated factor 6 (TRAF6), and the IKK-β kinase (254).
C. Activator protein-1
Studies addressing the mechanisms of inducible IL-8 and RANTES gene expression by RSV infection in epithelial cells have identified other critical transcription factors that contribute to the expression of these chemokines. Using transfection studies with reporter plasmids containing mutations in the binding sites for different transcription factors in the 5′-flanking region of the IL-8 gene promoter, it was first shown that mutation in the region of the activator protein-1 (AP-1)-binding site resulted in a diminished response to RSV, even in the presence of intact binding sites for NF-κB and NF-IL6 (163). Interestingly, when the AP-1 site is removed, but not when it is present, mutation of the NF-IL6-binding site significantly decreases responsiveness to RSV. Increased binding of AP-1 after RSV infection of A549 cells was also shown by electrophoretic mobility shift assay in the same studies. Other studies of the IL-8 promoter have shown that the presence of an AP-1-binding site is necessary for RSV inducibility, whereas TNF inducibility of the promoter stimulation mainly requires an intact NF-κB/NF-IL6 binding (39). Indeed, site mutations of the AP-1 site affect both the basal and RSV inducibility of the promoter, the latter being reduced to ∼50%. The AP-1 family of transcription factors consists of homodimers and heterodimers of Jun (c-Jun, JunB, and JunD), Fos (c-Fos, FosB, Fra-1, and Fra-2), or the activating transcription factor (ATF-2 and ATF-3) proteins (127). RSV infection induces the activation of several members of the AP-1 family, including c-Jun and ATF-2, and overexpression of c-Jun DN significantly inhibits IL-8 gene transcription (Casola A, personal communication), as well as RANTES gene transcription (38), indicating an important role of this family of transcription factors in RSV-induced gene expression.
D. Interferon regulatory factor
Other detailed studies have subsequently compared the mechanisms for induction of IL-8 in A549 cells by RSV infection and by stimulation with TNF (39). Promoter deletion and mutagenesis experiments indicated that although the region from −99 to −54 nt is sufficient for TNF-induced IL-8 transcription, this region alone is not sufficient for RSV-induced IL-8 transcription. Instead, RSV requires participation of the previously characterized element at −132 to −99 nt, containing an AP-1-binding site and of a previously unrecognized element, spanning from −162 to −132 nt, which was termed the RSV-response element (RSVRE). Analysis of the RSVRE sequence identified two potential transcription factor-binding sites: a GATA site between −151 and −147 nt, and an IFN-stimulated responsive element (ISRE)-like site between −144 and −132 nt. Mutation of the GATA site did not affect RSVRE binding, and competition assays, using oligonucleotides corresponding to consensus sequences of GATA, could not identify the RSVRE as one of them. On the other hand, Western blot analysis performed on cytoplasmic and sucrose cushion-purified nuclear extracts of A549 cells control and infected for various lengths of time demonstrated that IRF-1 protein, which modulates transcription of ISRE-containing genes, was highly inducible after RSV infection, starting between 3 and 6 h p.i. By a two-step microaffinity isolation/Western blot assay, we confirmed that RSV-induced IRF-1 was indeed binding to the IL-8 RSVRE (39).
IRF-1 belongs to a growing family of transcription factors, the IRFs [reviewed in (102)]. Nine human IRFs have been identified (IRF-1–IRF-9). Each member shares extensive homology in the N-terminal DNA-binding domain (DBD), characterized by five tryptophan-repeat elements, located within the first 150aa of the protein. The IRF DBD mediates specific binding to GAAANN and AANNNGAA sequences, termed ISRE, present in IFN-stimulated genes (ISGs). Each IRF contains a unique C-terminal domain, termed the IRF-association domain (IAD); the unique function of a particular IRF is accounted for by the ability of the IAD to interact with other members of the IRF family and other factors, its intrinsic transactivation potential, and cell-type-specific expression of the IRFs. IRF-1 is of particular interest, as this virus-inducible protein activates IFN-β, a gene highly expressed in RSV-infected epithelium. Interestingly, on the IFN-β gene, IRF-1 activates transcription only when NF-κB is coexpressed (85), indicating a common mechanism of activation with our studies on IL-8. Several studies have investigated the promoter elements involved in the regulation of IRF-1 gene expression and have identified both the NF-κB site and the gamma-IFN-activated sequence (GAS), which bind transcription factors belonging to the signal transducers and activators of transcription (STAT) family, as promoter regulatory elements necessary for inducible IRF-1 gene transcription (99). IRF-3, a critical player in the induction of type I IFNs after virus infection, is a constitutively expressed phosphoprotein. Transcriptional activity of IRF-3 is controlled by carboxyl-terminal phosphorylation events on serines 385 and 386, as well as the serine/threonine cluster between aa 396 and 405 mediated by the IKK-related kinases TBK-1 and IKKɛ. These events induce a conformational change in IRF-3 that allows homo- and heterodimerization, nuclear localization, and association with the coactivator CREB-binding protein (CBP)/p300, which retains IRF-3 in the nucleus and induces transcription of IFN-β and other genes. IRF-7 is a multifunctional protein with transcriptional activity that, like IRF-3, depends on C-terminal phosphorylation. While constitutive IRF-7 expression is restricted to B cells and dendritic cells, in the majority of the other cell types IRF-7 is virus- and IFN-inducible. IRF-7 gene transcription is controlled mainly through activation of the promoter ISRE site, which binds transcription factors of the STAT and IRF families (157). In addition, cytokine stimulation and viral infections lead to IRF-7 phosphorylation of the C-terminal region between aa 471 and 487. Transient transfection studies of the RANTES promoter using a series of 5′-deletion show that a deletion of the RANTES promoter to nt −120 completely abolishes RSV-induced RANTES transcription (38). The promoter region spanning nt −138 to −117 contains a functional ISRE site, and site-directed mutagenesis experiments clearly show that this site plays a fundamental role in promoter activation after RSV infection, since the ISRE mutant is no longer RSV inducible. Supershift assays and microaffinity isolation experiments have shown that IRF-1, 3, and 7 are components of the RSV-inducible complex formed on the RANTES ISRE site. Among the IRF proteins, IRF-3 seems to be essential for RSV-induced RANTES transcription, since overexpression of a transcription-inactive protein completely abolished RANTES promoter activation. Furthermore, Western blot analysis of cytoplasmic and nuclear proteins extracted from A549 cells infected with RSV for various lengths of time have shown that RSV infection induced de novo synthesis of IRF-7 and its nuclear translocation starting around 12 h p.i. By contrast, IRF-3 was constitutively expressed, and RSV infection induced its nuclear translocation starting around 6 h p.i. (37).
E. Signal transducers and activators of transcription
STAT proteins are constitutively expressed and, in unstimulated cells, are located in the cytoplasm. Seven STAT proteins have been identified in mammalian cells: STAT1, 2, 3, 4, 5a, 5b, and 6. Upon activation, they are phosphorylated on specific tyrosine residues, a post-translational modification necessary for dimerization and nuclear translocation, both of which are required for DNA binding [reviewed in (113)]. Upon IFN-α/β stimulation, STAT1/STAT2 heterodimers bind to the ISRE promoter sites, in the presence of an additional DNA-binding protein, p48/IRF-9, to form the ISGF3 complex. In response to IFN-γ or other cytokines, STAT dimers bind to GAS motifs on target genes (113). A number of receptor-associated and nonreceptor-associated tyrosine kinases have been shown to phosphorylate STAT proteins. Among them, the best-characterized ones are the Janus-activated kinases (JAKs). JAKs are a family of tyrosine kinases that includes JAK1, 2, 3, and Tyk2. JAK1, JAK2, and Tyk2 are ubiquitously expressed, whereas JAK3 is tissue specific. JAK1 can form heterodimers with JAK2, JAK3, or Tyk2, depending on the activating stimulus (149). In one of the first reports, RSV infection was shown to induce a rapid (within 30 min) phosphorylation and nuclear translocation of STAT1 in A549 cells and in normal human bronchial cells after RSV infection (142). Treatment of cells with heparin or heparinase blocked RSV cell attachment/infection and RSV-induced activation of STAT1. When A549 cells were preincubated with AG490, an inhibitor of JAK, decreased RSV-induced STAT1 phosphorylation was observed. In the same study, RSV also activated STAT3 via an IL-6-dependent pathway. We have also shown that RSV infection of A549 cells leads to a time-dependent activation of STAT1, STAT2, and STAT3 and their binding to the IRF-1 GAS site and to the IRF-7 ISRE site (155). STAT1 and STAT3 bind IRF-1 GAS, whereas STAT1, STAT2, IRF-1, and IRF-9 bind IRF-7 ISRE, leading to induction of gene expression. In our study, we were not able to consistently show a role of JAK in RSV-induced STAT activation; however, we found that RSV infection inhibits intracellular tyrosine phosphatase activity, which is critical for STAT activation, suggesting that modulation of phosphatases could be an important mechanism of virus-induced STAT activation (155).
F. Hypoxia-inducible factor
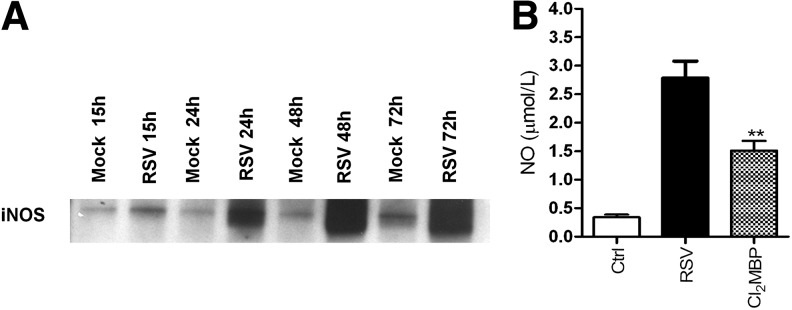
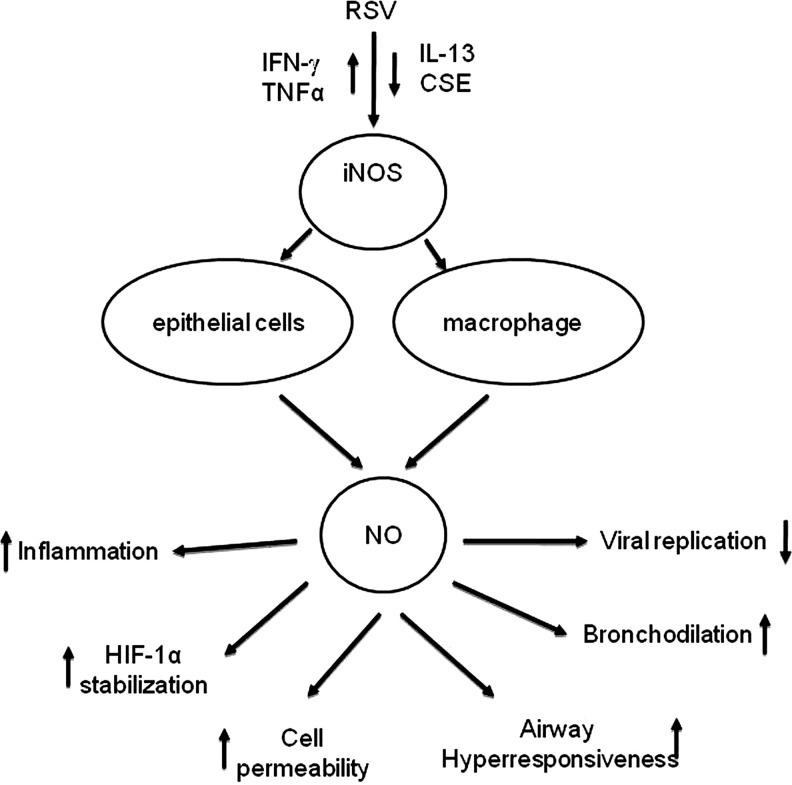
Hypoxia-inducible factor 1 (HIF)-1α is a transcription factor that functions as a master regulator of mammalian oxygen homeostasis. HIF-1 is composed of two subunits: constitutively expressed HIF-1β and oxygen-regulated HIF-1α. Under normoxic conditions, HIF-1α is subjected to hydroxylation on proline residues. The modification is required for the binding of the von Hippel-Lindau (VHL) tumor suppressor protein, the recognition component of an E3 ubiquitin protein ligase that targets HIF-1α for proteasomal degradation. Under hypoxic conditions, hydroxylation is inhibited, and the VHL protein does not bind to HIF-1, eventually leading to stabilization of the alpha-subunit, heterodimerization, nuclear translocation, and transcription of HIF-dependent genes [reviewed in (207)]. Although the erythropoietin (EPO) gene has been identified as a primary target of HIF-1 transcription, binding of HIF-1 to the EPO enhancer promoter region results in the transcriptional induction of a number of genes that are involved in inflammation or infection processes (216). HIF-1α has also been identified as a key regulator of NF-κB (243), and a recent study suggests that NF-κB is a critical transcriptional activator of HIF-1α, and that basal NF-κB activity is required for HIF-1 protein accumulation under hypoxic conditions (199). RSV infection of respiratory cells leads to HIF-1α activation via nitric oxide (NO)-dependent protein stabilization (94). Activation is replication-dependent, as addition of UV-inactivated virus to epithelial cells is unable to increase HIF-1α protein levels. HIF-1α activation in viral-infected cells regulates the expression of several genes, including vascular endothelial growth factor (VEGF), CD73, and cyclo-oxygenase-2 (COX-2), whose induction is abolished in pulmonary epithelia after siRNA-mediated repression of HIF-1α. Hypoxia does not seem to be the major trigger of RSV-induced HIF-1α activation, as measurements of the partial pressure of oxygen in the supernatants of RSV-infected epithelia or controls revealed no apparent differences in oxygen content. Finally, in vivo studies of RSV infection have confirmed HIF-1α activation in the lung of experimentally infected mice (94).
III. ROS in RSV-Induced Cellular Signaling and Oxidative Stress
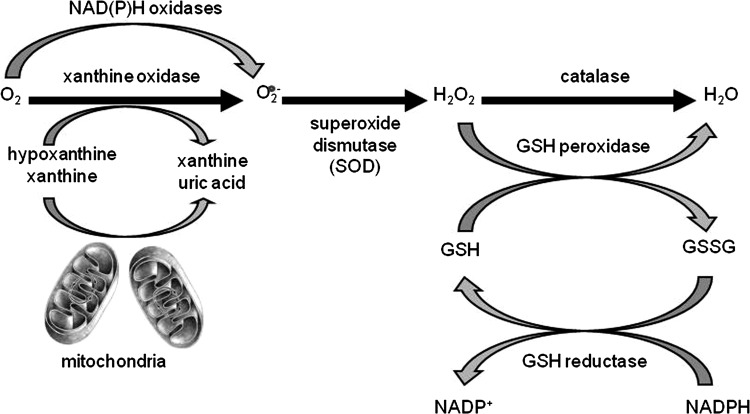
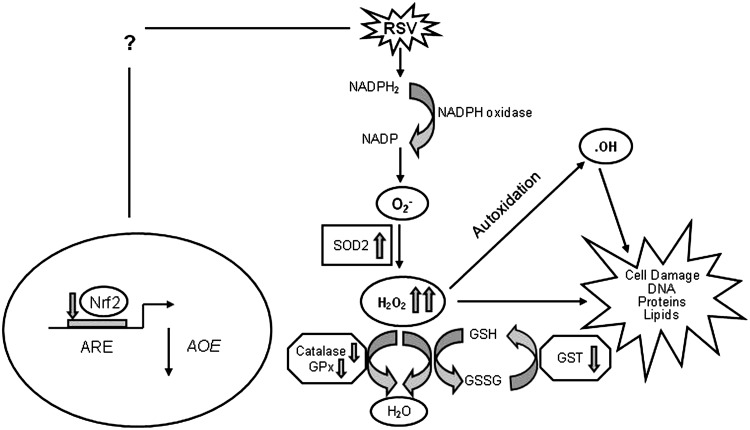
Reactive oxygen species (ROS) are ubiquitous, highly diffusible, and reactive molecules produced as a result of reduction of molecular oxygen, including species such as superoxide anion radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), and they have been implicated in damaging cellular components like lipids, proteins, and DNA. The toxicity of ROS depends on the presence of a Fenton catalyst, such as iron ions and peroxidases, which in the presence of O2− and H2O2 give rise to the extremely reactive •OH radical. ROS and in particular •OH can interact with a variety of molecules, such as membrane lipids, leading to lipid peroxidation, which impairs membrane functions, inactivates receptors, and increases tissue permeability [reviewed in (66)]. Aldehydes, generated during the process of lipid peroxidation, such as malonaldehyde (MDA) and 4-hydrynonenal (4-HNE), can diffuse within and escape from cells and attack targets far from the site of the original free-radical generation. ROS formation takes place constantly in every cell during metabolic processes. Cellular sites for ROS generation include the mitochondria, microsomes, and various enzymes like COX, lipoxygenase, xanthine oxidase, and membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Fig. 4). Excessive levels of ROS can be generated by increased stimulation of the otherwise tightly regulated NADPH oxidase (mitochondrial- and cell membrane-associated) or by other mechanisms that produce ROS accidentally, which is often due to mitochondrial dysfunction or increased activity of the above-mentioned enzymes. Endogenous antioxidants comprise a number of enzyme systems, such as superoxide dismutase (SOD) (three isoforms of SOD have been identified in mammals: the cytoplasmic Cu/ZnSOD or SOD1, the mitochondrial MnSOD or SOD2, and the extracellular ECSOD or SOD3), catalase, and glutathione peroxidase (GPx), as well as nonenzymatic molecules such as reduced glutathione (GSH) [reviewed in (66)] (Fig. 4). Depending on the source and type of ROS generation, each system plays a major role in limiting intracellular and extracellular oxidative stress. In the lungs, neutrophils, eosinophils, monocytes, and macrophages recruited at sites of infection/inflammation are a major source of supraphysiological levels of O2− production, most of which undergoes dismutation resulting in H2O2 formation. The presence of eosinophil- and neutrophil-derived peroxidases, EPO and myeloperoxidase (MPO), respectively, then greatly enhances the oxidizing potential of H2O2 by leading to the formation of •OH.
FIG. 4.
Pathways of ROS production and clearance. Major sources of intracellular superoxide production and the AOEs involved in its clearance (not inclusive of all enzymes with known scavenging activity for superoxide). AOE, antioxidant enzyme. ROS, reactive oxygen species.
In addition to being responsible for cellular oxidative stress, there has been increased recognition of the role of ROS as central regulators of cellular signaling [reviewed in (10, 66, 194)]. Modulation of gene expression in response to ROS formation is a complex phenomenon, as a variety of signal transduction molecules have been shown to be redox sensitive, from transcription factors to kinases and phosphatases, to chromatin-remodeling proteins. In the following sections, we will describe the suggested mechanisms of RSV-induced ROS production and their role in cellular signaling and oxidative cellular damage in response to RSV infection.
A. ROS generation in RSV infection
Several viruses, including human immunodeficiency virus (HIV), Hepatitis B and C, rhinovirus, and influenza, have been shown to induce ROS in a variety of cell types [reviewed in (189, 190)]. In the past few years, we have shown that infection of airway epithelial cells with RSV induces ROS formation, measured by oxidation of the cell membrane permeable compound 2′,7′ dichlorodihydrofluorescein diacetate (DCFDA), which is trapped intracellularly after cleavage by cellular esterases and becomes fluorescent once oxidized (144, 159). RSV-inducible ROS production was detectable as early as 2 h p.i., with a progressive increase observed up to 24 h p.i., resulting in several-fold induction in DCFDA fluorescence in infected cells compared to uninfected (37, 120). In addition to epithelial cells, RSV interaction with professional phagocytes, such as monocytes and polymorphonuclear cells, in particular neutrophils and eosinophils, has been shown to lead to superoxide production (70, 137). H2O2 generated in these two types of cells becomes the substrate of the eosinophil and neutrophil MPO, leading to the release of potent pro-oxidative mediators in the extracellular environment.
As mentioned before, cellular sites for ROS generation include the mitochondria, microsomes, and various enzymes such as COX, lipoxygenase, xanthine oxidase, and membrane-bound NADPH oxidase. The latter one is an important source of inducible intracellular ROS, generated in response to a variety of stimuli [reviewed in (13)]. Initially thought to be restricted to phagocytic cells and used to kill invading microorganisms, superoxide production by the NADPH oxidase system has been reported in a variety of nonphagocytic cells, including epithelial cells. This system consists of the catalytic subunit gp91phox (known as NOX2), together with the regulatory subunits p22phox, p47phox, p40phox, p67phox, and the small GTPase RAC. The enzyme activity of gp91phox is regulated by the assembly of these regulatory subunits with gp91phox to form an active complex. In addition to NOX2, six functional oxidase homologs, NOX1, NOX3, NOX4, NOX5, Duox1 and 2, and two homologs of the regulatory units, NOXO1 and NOXA1, have been identified in the past several years, with different tissue expression [reviewed in (147)]. Although the role of NADPH oxidases in innate responses to bacterial infection is well documented, little is known regarding their role in ROS generation and modulation of cellular responses after viral infections, in particular in cells other than phagocytes, such as airway epithelial cells, the primary target of RSV infection.
The first demonstration that RSV induces superoxide production and chemokine secretion through a NADPH oxidase-dependent pathway came from the observation that skin fibroblasts derived from a patient with congenital granulomatous disease, due to lack of p47phox expression, showed a significant reduction in RSV-induced ROS production and IL-8 secretion, compared to skin fibroblasts of normal donors (130). Using a variety of inhibitors, including diphenylene iodonium chloride (DPI), apocynin, and 4-(2-aminoethyl) benzene sulfonyl fluoride (AEBSF), we have shown that NADPH oxidases play an important role in modulating RSV-induced RANTES expression, as well as activation of the transcription factors IRF-3 and STAT1 and of the upstream kinase IKKɛ in airway epithelial cells (114, 154). Inhibition of NADPH oxidases also resulted in a modest decrease of viral replication (114). The importance of the NADPH oxidase system in regulating redox-dependent cellular signaling in response to RSV infection was confirmed by the inhibition of NF-κB serine phosphorylation and transcriptional activity, as well as RIG-I/MAVS-dependent IRF-3 activation in airway epithelial cells in which the expression of NOX2 was significantly downregulated by siRNA treatment (72, 218).
In regard to other respiratory viruses, rhinovirus infection of bronchial epithelial cells has been shown to induce ROS formation (24) through NOX1 (53), whereas NOX2 seems to play an important role in inflammatory cell superoxide production in response to influenza infection (240).
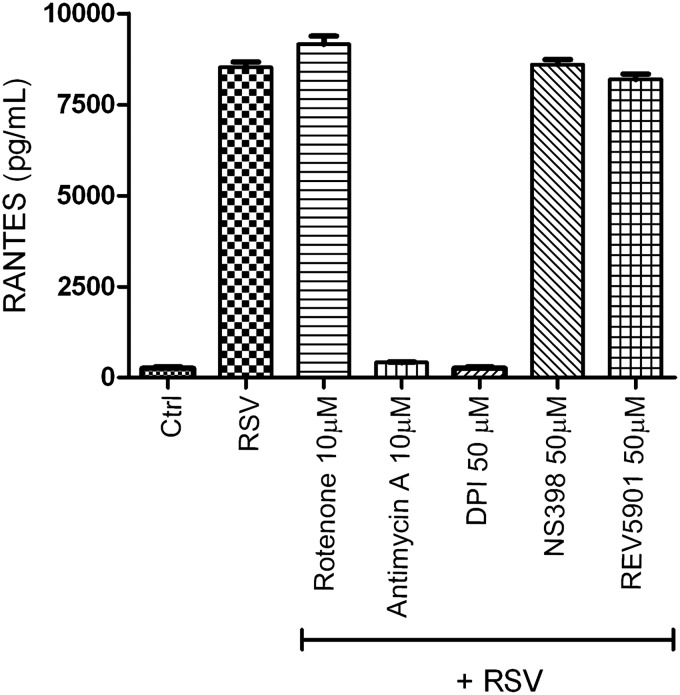
In an attempt to identify the other sources of RSV-induced ROS generation, we tested the effect of inhibiting different sources of intracellular ROS on RSV-induced chemokine secretion (Casola A, unpublished observation). We infected A549 cells with RSV, MOI of 3, in the presence or absence of DPI, rotenone (an inhibitor of mitochondrial complex 1), antimycin A (a mitochondrial complex 3 inhibitor), NS 398 (a COX inhibitor), and REV 5901 (a lipoxygenase inhibitor). Cell supernatants were harvested 24 h after infection, and the RANTES concentration was determined by ELISA. As shown in Figure 5, RSV-induced RANTES production was significantly inhibited by DPI and antimycin A treatment, suggesting that the mitochondria could represent also an important site of ROS production in viral-infected epithelial cells.
FIG. 5.
Effect of inhibition of various intracellular sources of ROS on RSV-induced chemokine secretion. A549 cells were infected with RSV in the presence or absence of various inhibitors of intracellular sources of ROS. Culture supernatants, from control and infected cells, were assayed 24 h later for RANTES production by ELISA. RANTES, regulated upon activation, normal T-cell expressed, and secreted.
B. ROS as mediators of cellular signaling in RSV infection
As described before, RSV infection of airway epithelial cells results in activation of a subset of transcription factors, including NF-κB, AP-1, IRF, and STAT proteins, which control the expression of a variety of proinflammatory/immunological mediators, such as cytokine, chemokines, and type I IFNs. While the role of ROS in activation of some of these transcription factors has been extensively investigated, as in the case of NF-κB, AP-1, and STAT proteins (35, 205, 213), little is known on the potential redox regulation of others, such as IRF proteins. We summarize the evidence in support of ROS-dependent regulation of RSV-induced cellular signaling in the following sections.
1. NF-κB/NF-IL6/AP-1 activation
The first evidence that changes in oxidant tone regulate RSV-induced cellular signaling came from a study of Mastronarde et al., in which RSV-infected epithelial cells treated with the antioxidant dimethyl sulfoxide (DMSO) showed a dose-dependent decrease in IL-8 secretion and IL-8 mRNA, without significant changes in viral replication (162). The same group demonstrated that DMSO, as well as the other antioxidants dimethyl pyrroline N-oxide and N-acetylcysteine (NAC), significantly reduced RSV-induced nuclear translocation and DNA-binding activity of AP-1 and NF-IL6 to their respective binding sites of the IL-8 promoter, whereas there was no effect on NF-κB binding (163). That study however did not examine the effect of antioxidants on NF-κB transcriptional activity, which is regulated by a variety of post-translational modifications, including phosphorylation and acetylation (42, 43). In particular, inducible phosphorylation on distinct serine residues, including Ser276 and Ser536, has been shown to regulate NF-κB transcriptional activity without modification of nuclear translocation or DNA-binding affinity (260). We have recently shown that treatment of airway epithelial cells with NAC or DMSO significantly reduced RSV-dependent NF-κB serine phosphorylation, resulting in the inhibition of RSV-induced expression of several NF-κB-dependent genes, without affecting nuclear translocation (120). Inhibition of RSV-induced NF-κB serine phosphorylation was also shown in epithelial cells in which the expression of NOX2 was significantly downregulated by siRNA treatment (72), as previously mentioned, indicating that in airway epithelial cells, ROS regulation of RSV-induced NF-κB activation occurs at a step subsequent to activation through the canonical pathway. Cellular treatment with either NAC or DMSO had no significant effect on RSV gene transcription, as N-protein mRNA expression was the same in treated versus untreated infected cells, although overall viral replication, assessed in terms of formation of a fully assembled virus, was not assessed. As mentioned earlier in this review, MSK1 is one of the upstream kinases regulating RSV-induced NF-κB serine phosphorylation (120), and its kinase activity has been reported to be redox sensitive (3). Airway epithelial cell exposure to either NAC or DMSO strongly inhibited viral-induced MSK1 activity to nearly that of uninfected cells, suggesting that this is an important mechanism of redox control of viral-induced NF-κB activation (120) (Fig. 6). The molecular basis of ROS-dependent activation of MSK1 in RSV-infected cells is currently unknown.
FIG. 6.
Schematic representation of the proposed role of ROS in viral-induced NF-κB activation. After initial activation of the IKK complex, leading to phosphorylation, ubiquitination, and degradation of IκBα, NF-κB is phosphorylated on multiple serine residues through different redox-sensitive pathways involving the MSK1 kinase as well as TLR3 activation. IκB, inhibitor of kappa B. MSK1, mitogen- and stress-activated protein kinase 1; TLR, Toll-like receptor.
In addition, RSV-induced NF-κB phosphorylation is also controlled through activation of TLR3 (90, 152), and TLR3 activation in airway epithelial cells has recently been shown to be enhanced by oxidative stress (140). Treatment of macrophage with melatonin, a pineal gland-secreted hormone with antioxidant properties, significantly reduced TLR3-dependent NF-κB activation and gene expression in RSV-infected cells (109), suggesting a possible additional ROS-dependent mechanism controlling NF-κB induction in response to RSV infection (Fig. 6).
A role of ROS in IL-8 and NF-κB activation has been demonstrated for other respiratory viruses as well, such as rhinovirus (24) and influenza (48, 139), suggesting a common regulatory mechanism of this pathway in response to viral infections of airway epithelial cells.
2. IRF/STAT activation
IRF transcription factors have been shown to play a fundamental role in the induction of several genes involved in the immune/inflammatory response to viral infections, including type I IFN, cytokines like IL-15, adhesion molecules, major histocompatibility complex-I (MHC-I) molecules, and inducible nitric oxide synthase (iNOS) [reviewed in (180)]. As mentioned before, IRF protein binding to the ISRE of the RANTES promoter is necessary for viral induction of RANTES transcription and gene expression (151). Pretreatment of RSV-infected airway epithelial cells with a panel of chemically unrelated antioxidants can effectively inhibit RANTES secretion, mRNA induction, and gene transcription, indicating the involvement of ROS in RANTES gene expression (37). Treatment of airway epithelial cells with the antioxidant butylated hydroxyanisole (BHA) blocks RSV-induced de novo IRF-1 and 7 gene expression and protein synthesis, and inhibits IRF-3 nuclear translocation and DNA binding to the RANTES ISRE (37), an event required for RSV-induced RANTES gene transcription. The mechanism by which BHA affects RSV-induced IRF-3 activation involves inhibiting its serine phosphorylation, a fundamental mechanism of IRF protein activation, necessary for nuclear translocation, dimerization, binding to DNA, and activation of transcription [reviewed in (208)].
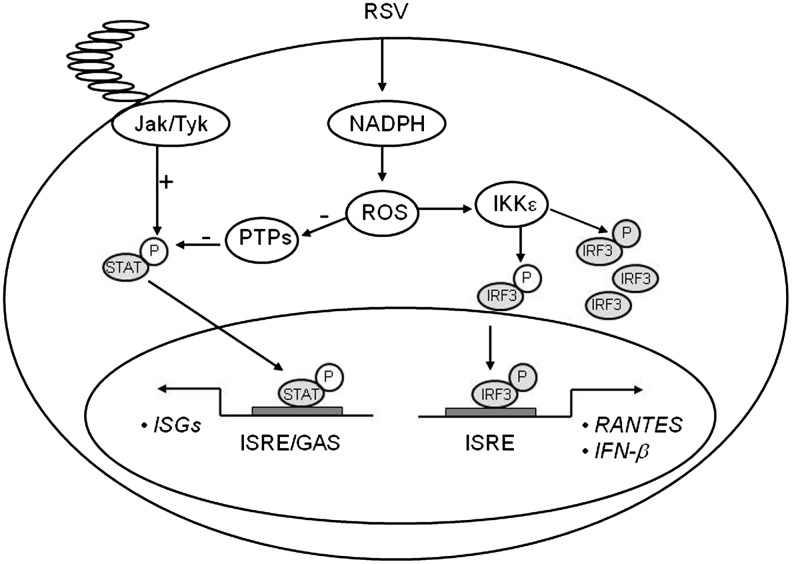
Treatment of airway epithelial cells with BHA or a panel of NADPH oxidase inhibitors, including DPI, apocyanin, and AEBSF, inhibited gene expression and protein synthesis of IKKɛ, a kinase that plays a major role in RSV-induced IRF-3 activation (114), indicating for the first time that ROS formation plays a major role in the signaling pathway leading to viral-induced IRF-3 activation. A confirmation of the redox sensitivity of this pathway, in the context of viral infections, was provided by the observation that interference with NOX2 expression inhibited RSV- and Sendai virus-induced activation of the mitochondrial-associated adaptor MAVS, as well as IRF-3 serine phosphorylation and dimerization, with subsequent inhibition of IRF-3-dependent gene expression (218) (Fig. 7).
FIG. 7.
Schematic representation of the proposed role of ROS in viral-induced IRF and STAT activation. Signaling pathways leading to IRF-3 and STAT activation in response to RSV infection. PTP, protein tyrosine phosphatases; ISRE, interferon-stimulated responsive element; GAS, gamma-interferon-activated sequence; ISG, interferon-stimulated genes; STAT, signal transducers and activators of transcription.
One additional study has shown that induction of IFN-stimulated genes (ISGs) by LPS, which occurs in an IRF-3-dependent manner, requires the generation of ROS by the NADPH-dependent oxidase system. In that study, activation of ASK1 kinase linked LPS-induced ROS production to the activation of MKK6 and p38, two kinases that had previously identified as components of the LPS-induced IRF-3 activation cascade (44).
As mentioned above, BHA treatment of airway epithelial cells blocks RSV-induced IRF-1 and 7 gene expression, and this inhibition occurs at the level of gene transcription. IRF-1 gene expression is induced by IFN-γ and cytokines through the activation of STAT and NF-κB transcription factors (99). Similarly, IFN-γ activates IRF-7 gene transcription through an ISRE site that binds members of the STAT family (157). RSV infection induces STAT protein phosphorylation, nuclear translocation, and binding to the IRF-1 and 7 promoters (155), an event necessary for IRF-inducible transcription, as seen with IFN stimulation (99, 157). Antioxidant treatment completely abolishes RSV-induced STAT activation, by blocking tyrosine phosphorylation, therefore preventing STAT translocation and DNA binding to both the IRF-1 and IRF-7 ISRE promoter sequences (155). Recent studies have shown that activation of the JAK-STAT pathway is redox-sensitive. Simon et al. demonstrated that STAT1 and STAT3 are activated in response to H2O2 in fibroblasts (215), and JAK-STAT activation after stimulation with angiotensinogen II and oxidized low-density lipoprotein is inhibited by antioxidant treatment (165, 166). Little is known about the role of ROS in viral-induced STAT activation. Very recently, Gong et al. have shown that the human hepatitis C virus NS5A protein alters intracellular calcium levels, triggering ROS formation and nuclear translocation of NF-κB and STAT3, which are completely inhibited by the use of antioxidants (89). Similarly, the hepatitis B virus X protein induces ROS formation, through association with an outer mitochondrial anion channel, an event that leads to NF-κB and STAT3 activation, which is sensitive to antioxidant treatment, as well as SOD2 overexpression (247). The mechanism involved in ROS formation and STAT activation, after RSV infection, is not fully understood. In airway epithelial cells, we found that RSV infection significantly inhibited protein tyrosine phosphatase (PTP) activity, which was restored by antioxidant treatment (155). Very little information is available regarding viral-induced regulation of PTPs activity and their role in signaling pathways activated by viral infections. Exogenous H2O2 treatment has been shown to induce an increase in the overall intracellular levels of protein tyrosine phosphorylation, triggering activation of multiple signaling molecules and transcription factors, ultimately leading to gene expression (100), and it has been suggested that one of the mechanisms mediating the biological effects of H2O2 treatment is inhibition of protein phosphatases (100). More specifically, two studies have shown that H2O2 inhibits specific PTPs, whereas antioxidants can increase PTPs activity (58, 168, 200). It is possible that viral-induced ROS activate STAT through an imbalance between cellular tyrosine kinases and phosphatases, resulting in increased net phosphorylation and therefore activation of this transcription factor (Fig. 7).
C. RSV and oxidative stress
Oxidative stress has been implicated in the pathogenesis of several acute and chronic airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD) [reviewed in (74)], as well as in influenza-induced lung disease (7, 226). Oxidant species have been shown to mimic in vivo of the pathophysiological features of airway diseases such as bronchoconstriction, airway hyper reactivity, mucous hypersecretion, enhanced arachidonic acid cascade, increased synthesis of chemoattractants, epithelial damage, and microvascular leakage [reviewed in (51)].
In the past few years, we have shown that RSV infection induces significant oxidative stress, defined as a disruption of the pro-oxidant–antioxidant balance in favor of the former, in vitro as well as in vivo, due to an impairment of the antioxidant defense system. Our findings of a progressive increase in lipid peroxidation products, such as 8-isoprostanes, MDA, and 4-HNE, and a progressive reduction of the GSH/glutathione disulfide (GSSG) ratio provide strong evidence of increased oxidative stress in RSV-infected airway epithelial cells (106). When lipid peroxidation products were measured in the BAL of RSV-infected mice, there was a significant increase of MDA and 4-HNE levels in BAL samples at all timepoints tested, when compared to uninfected mice, indicating that lung oxidative stress damage indeed occurs in RSV infection (40). In addition, we measured the levels of F2 8-isoprostane and MDA in NPS of children with RSV-proven infections of increasing clinical severity, that is, from milder upper respiratory tract infections (URTI), to bronchiolitis with or without hypoxia (the group of children with hypoxic bronchiolitis included also subjects who required intubation and ventilatory support [VS] because of respiratory failure) (105). Concentration of F2 8-isoprostane in NPS was slightly increased in subjects with mild (nonhypoxic) bronchiolitis compared to those with URTI, but the difference was not statistically significant. However, subjects with hypoxic bronchiolitis had significantly more F2 8-isoprostane in NPS than did subjects with URTI alone (p<0.01) or with nonhypoxic bronchiolitis (p<0.001). A similar trend was observed for MDA concentrations in a smaller number of NPS samples that were tested (105).
As mentioned before, cells are protected against oxidative damage by well-developed enzymatic and nonenzymatic antioxidant systems, including SOD, catalase, GSH-dependent enzymes, thioredoxin (TRX), and peroxiredoxins (Prdxs), which protect cells against ROS and cytotoxic products of lipid peroxidation. Antioxidant enzymes (AOEs) can either directly decompose ROS (e.g., SOD and catalase) or facilitate these antioxidant reactions (e.g., peroxidase using GSH as a reducing agent). In the case of RSV infection, the protective mechanism of upregulating antioxidant defenses after increased ROS formation occurred only at the very beginning of infection, with an increase in SOD1, SOD2, catalase, and glutathione S-transferase (GST) expression and GPx activity in airway epithelial cells at 6 h p.i. While SOD2 expression and total SOD activity continued to increase during the timecourse of RSV infection, there was a progressive decrease in the expression and activity of all the other tested AOEs. SOD enzymes convert superoxide anion to H2O2, and catalase and GPx convert H2O2 to water and oxygen (106). The increase in total SOD activity together with the progressive decrease in catalase, GPx, and GST expression and activity suggests that RSV infection likely results in enhanced intracellular H2O2 production, which is not detoxified by AOEs, leading to the generation of free radicals, such as the hydroxyl radical (•OH), which reacts with lipids, proteins, and DNA, causing structural cellular damage.
Using a proteomic approach to investigate changes in abundance/function of nuclear proteins in response to RSV infection, we also found that several family members of the Prdx family, such as Prdx-1, Prdx-3, and Prdx-4, were oxidized in response to RSV without changes in their total abundance (122). Cells lacking Prdx-1, Prdx-4, or both showed increased levels of ROS formation and a higher level of protein carbonylation in response to RSV infection. Using a saturation fluorescence labeling and 2-dimensional electrophoresis gel analysis, we showed that 15 unique proteins had enhanced oxidative modifications of at least >1.2-fold in the Prdx knockdowns in response to RSV, including annexin A2 and desmoplakin, suggesting that Prdx-1 and Prdx-4 are essential for preventing RSV-induced oxidative damage in a subset of nuclear intermediate filament and actin-binding proteins in epithelial cells. Prdxs are a family of AOEs that catalyze reduction of H2O2 and alkyl hydroperoxides in the presence of TRX, TRX reductase, and NADPH [reviewed in (193)]. There are six family members, all expressed in the lung, and they have been shown to play an important role not only in peroxide scavenging but also in peroxide-dependent cellular signaling (206). Enhanced TRX expression in a mouse model of influenza infection was protective against severe disease, with significant reduction in lung inflammation and pneumonia in TRX-expressing mice (177), indicating that this system plays an important role in regulating inflammatory process during host defense against respiratory viral infections.
Decreased expression/activity of antioxidant proteins was also observed in vivo, both in a mouse model of RSV infection, as well as in children with severe bronchiolitis (105). Using a proteomic approach to identify proteins whose expression in BAL changed during the course of RSV infection, we found that levels of several AOEs, from Prdx enzyme to catalase, SOD1, GPx 1, and various forms of GST, were significantly decreased in the lungs of infected animals compared to uninfected (Table 1 summarizes all antioxidant proteins whose expression in BAL changes in response to RSV infection). Most of the AOE levels were significantly reduced as early as day 1 p.i. (with the exception of Prdx-2), and they were significantly lower throughout the acute phase of RSV infection, compared to control mice, to return to basal or slightly above basal levels by day 25 p.i., indicating that RSV significantly diminishes antioxidant defenses in the lung. To confirm the data obtained in the mouse model, we measured levels of SOD1, 2, and 3, catalase, and GST-mu in NPS of infected children. For this analysis, infants on ventilator support (VS), that is, those with most severe illness, were analyzed as a separate group. SOD1 levels were lower in infants with bronchiolitis, hypoxic bronchiolitis, and on ventilatory support (VS), compared to those with URTI alone. The VS group also showed significantly lower levels of SOD3, catalase, and GST-mu compared to the other illness groups, suggesting a correlation between levels of AOE expression and severity of RSV infection.
Table 1.
Differential Expression of Antioxidant Proteins in Bronchoalveolar Lavage of Respiratory Syncytial Virus-Infected Mice
| |
Fold change in RSV BAL compared to control BAL |
||||
|---|---|---|---|---|---|
| Antioxidant enzymes | Day 1 | Day 3 | Day 5 | Day 9 | Day 25 |
| 1-Cys peroxiredoxin protein | −1.0 | −6.1 | − | −4.1 | 1 |
| Catalase | 1 | −2.5 | −2.1 | — | — |
| Cu/Zn Superoxide dismutase (SOD1) | −2.3 | −3.4 | −2.0 | −2.0 | — |
| Glutathione peroxidase 1 | −1.8 | −2.3 | — | 1.3 | — |
| Glutathione S-transferase | — | — | — | −6.0 | — |
| Glutathione S-transferase omega 1 | −6.8 | −3.6 | −2.3 | −2.0 | 1.3 |
| Glutathione S-transferase, alpha 4 | −2.2 | — | — | — | New spot |
| Glutathione S-transferase, mu 1 | — | −4.0 | −7.0 | −1.7 | 1.4 |
| Glutathione S-transferase, mu 2 | — | −4.3 | −3.4 | −1.3 | |
| Glutathione disulfide reductase | — | — | — | 3 | — |
| Nonselenium glutathione peroxidase | −2.6 | — | −4.2 | −1.3 | 1.2 |
| Peroxiredoxin 6 | New spot | −3.1 | −3.9 | −4.1 | 1.3 |
| Peroxiredoxin 2 | 2.7 | 2.4 | −2.1 | 1.7 | New spot |
| Thioredoxin 1 | — | 1.5 | — | New spot | 1.1 |
Shown are high probability antioxidant protein identifications and their expression (in terms of fold changes in RSV BAL compared to control mice) at different days of postinfection from peptide mass fingerprinting in MALDI-TOF/MS.
Reprinted with permission of the American Thoracic Society. Copyright © 2011 American Thoracic Society (81).
BAL, bronchoalveolar lavage; —, not determined.
In support of the concept that oxidative stress could be linked to the pathogenesis of paramyxovirus-induced lung diseases, we have recently shown that human metapneumovirus (hMPV), a pneumovirus belonging to the Paramyxoviridae family responsible for a significant portion of lower respiratory tract infections in children (31), significantly affects AOE expression in vitro and in vivo. hMPV infection of airway epithelial cells induces a progressive decrease of SOD3, catalase, GST, and Prdx gene expression and protein levels, with a concomitant increase in SOD2 and no change in SOD1 expression (17), similar to what we have observed with RSV. Similar findings were also observed in a mouse model of hMPV infection (105).
In regard to other respiratory viruses, rhinovirus infection of bronchial epithelial cells has been shown to induce ROS formation (24) and to increase SOD1 expression and total SOD activity at early timepoints of infection, with no changes in SOD2, catalase, and GPx (129). However, AOE expression/activity was investigated only at 6 h p.i., but not at later timepoints. Similar to RSV, influenza virus induces SOD2 gene expression in airway epithelial cells, with a concomitant decrease in catalase gene expression (116). Similar findings were reported in a mouse model of influenza infection, with increased expression of SOD2 (47); however, total lung SOD and catalase activity, as well as the ratio of GSH/GSSG, have been shown to be reduced in mice after influenza infection (146). Taken together, these data clearly suggest that airway oxidant–antioxidant imbalance occurring during RSV infection, and possibly viral respiratory infections in general, could play a very important role in the pathogenesis of RSV-induced lung disease.
D. Potential regulatory mechanisms of AOE gene expression in RSV infection
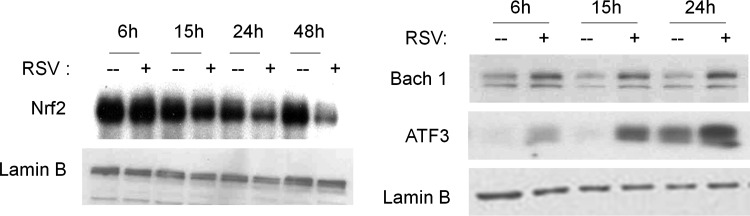
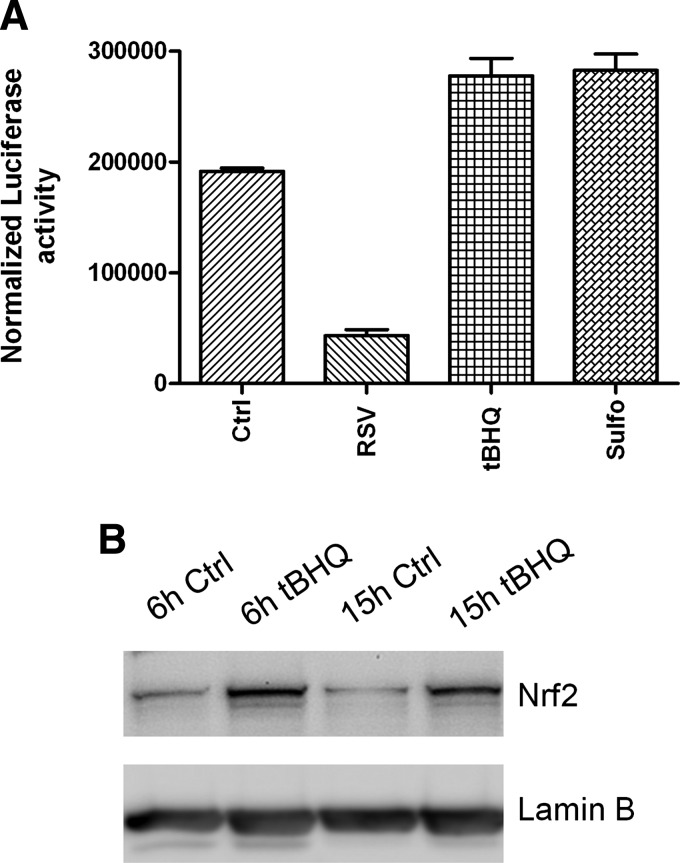
The only AOE gene that showed significant induction after RSV infection of airway epithelial cells is SOD2. The human SOD2 gene promoter contains binding sites for several transcription factors such as NF-κB and AP-1 [reviewed in (138)], with NF-κB being necessary for SOD2 gene expression, in response to cytokine stimulation (54, 55). RSV being a potent activator of NF-κB in airway epithelial cells (83), it is likely that its induction is responsible for the observed increase in the SOD2 expression and activity level in the course of RSV infection. The mechanism leading to decreased expression/activity of the majority of AOEs after RSV infection is currently unknown. SOD3 and catalase expression has been shown to be negatively regulated in response to cytokine stimulation such as IL-1, tumor necrosis factor-α, and IFN-γ (50). Transcription of many oxidative stress-inducible genes is regulated in part through cis-acting antioxidant-responsive element (ARE) sequences. This element has been identified in the regulatory regions of many genes encoding phase-2 detoxification enzymes and various other cytoprotective proteins such as NAD(P)H:quinone oxidoreductase (NQO1), which catalyzes the reduction of a variety of quinones and quinoid compounds and many AOEs, including SOD1, catalase, heme oxygenase 1 (HO-1), GST, and GSH-generating enzymes such as glutamate cysteine ligase [reviewed in (117)]. NF-E2-related factor 2 (Nrf2) is a protein that has been well established as an important redox-responsive protein that helps protect the cells from oxidative stress and injury [reviewed in (117)]. It is a basic leucine-zipper transcription factor that is normally bound in the cytosol to an inhibitor called Kelch-like-ECH-associated protein 1 (Keap1). This association normally renders Nrf2 inactive by shuttling it toward degradation by the ubiquitin–proteasome pathway. However, when ROS are generated, Keap1 undergoes conformational change and releases Nrf2, which associates with small MAF proteins and translocates to the nucleus to bind to ARE or MAF recognition elements (MARE) and promote gene transcription (117).
Several transcription factors can antagonize Nrf2-dependent gene transcription by (i) competing for binding to the ARE, (ii) inhibiting Nrf2 activation through direct physical association, or (iii) interfering with recruitment of coactivators (CBP) to the ARE site [reviewed in (242)]. Bach1 is also a basic leucine-zipper protein that, similar to Nrf2, binds to small MAF proteins and then to ARE/MARE, acting as a transcriptional repressor (60). Transcription factors belonging to the AP-1 family, such as the immediate early proteins c-Fos and FRA1, can also compete with Nrf2 for binding to the ARE and inhibit gene transcription (242). On the other hand, ATF-3, as well as retinoic acid receptor α, can form inhibitory complexes with Nrf2, leading to displacement of the coactivator CBP from AREs and inhibition of Nrf2-dependent gene transcription (242). Finally, activation of NF-κB can lead to decreased Nrf2-dependent gene transcription by decreasing the availability of coactivators (CBP) and promoting the recruitment of corepressors (histone deacetylases) (242).
A recent study has shown that the Nrf2-ARE pathway plays a protective role in the murine airways against RSV-induced injury and oxidative stress (46). More severe RSV disease, including higher viral titers, augmented inflammation, and enhanced mucus production, and epithelial injury were found in Nrf2−/− mice compared to Nrf2+/+ mice. Preliminary studies indicate that RSV infection indeed induces a time-dependent decrease in ARE-dependent gene transcription, investigated using luciferase reporter assays (Casola A, unpublished observation) (Fig. 8). RSV leads to a decrease in nuclear levels of Nrf2 in A549 and in SAECs (106), while increasing nuclear levels of known ARE transcriptional repressors such as Bach1 and ATF-3 (Casola A, unpublished observation) (Fig. 9), suggesting a potential mechanism for viral-induced downregulation of AOE gene expression. Reduced nuclear levels of Nrf2 can occur as a result of various mechanisms, including decreased expression, increased degradation, or through increased nuclear export (128). As Nrf2 positively regulates its own gene transcription, there are reduced Nrf2 mRNA levels in airway epithelial cells at a late timepoint of RSV infection, as we recently published (106). In Figure 10, we summarized possible mechanisms leading to RSV-induced oxidative stress in airway epithelial cells.
FIG. 8.
Effect of RSV infection on ARE-driven gene transcription. A549 cells were transiently transfected with a plasmid containing multiple copies of the NQO1 ARE site linked to the luciferase gene and then infected with RSV. Cells were harvested at different times p.i. to measure luciferase activity. Uninfected plates served as controls. For each plate, luciferase was normalized to the β-galactosidase reporter activity. Data are expressed as mean±standard deviation of normalized luciferase activity. ARE, antioxidant-responsive element; NQO1, quinone oxidoreductase.
FIG. 9.
RSV infection modulates nuclear levels of AOE transcriptional regulators. A549 cells were infected with RSV for various lengths of time and harvested to prepare nuclear extracts. Nuclear amounts of Nrf2, Bach1, and ATF-3 proteins were determined by Western blot. Membranes were stripped and reprobed for lamin B as an internal control for protein integrity and loading. ATF, activating transcription factor.
FIG. 10.
Schematic representation of the proposed mechanisms of oxidative cell damage in RSV infection. RSV infection of airway epithelial cells leads to increased superoxide formation and increased H2O2 production, due to upregulation of SOD2 expression and activity. RSV-induced inhibition of Nrf2 activation causes a progressive decrease in the expression of a variety of AOEs involved in H2O2 detoxification leading to accumulation of highly reactive radicals, such as hydroxyl radical, and subsequent cellular damage. H2O2, hydrogen peroxide.
IV. Role of Reactive Nitrogen Species in RSV Infection
Reactive nitrogen species (RNS) are a group of chemically reactive unstable metabolites generated by NO metabolism. NO, a gaseous nitrogen-centered inorganic radical, is an endogenously synthesized free radical first characterized as a noneicosanoid component of endothelial-derived relaxation factor (78). NO is produced by a variety of mammalian cells, including vascular endothelium, neurons, smooth muscle cells, macrophages, neutrophils, platelets, and pulmonary epithelium. NO is synthesized in biological tissues by the oxidative deamidation of l-arginine to citrulline by the family of three nitric oxide synthases, neuronal type (nNOS), inducible type (iNOS), and endothelial type (eNOS) [reviewed in (225)]. Out of these, iNOS expression is transcriptionally regulated by cytokines and redox-sensitive transcriptional factors, and viral infections, which often induce significant cytokine production, can lead to the induction of iNOS gene expression (6). NO has proven to be a ubiquitous signal transduction molecule and a mediator of tissue injury because of its chemical properties and is critically involved in innate immunological host defense [reviewed in (52, 172)]. The ability of NO to react with molecular oxygen and oxygen-derived free radicals provides tissues with a nonenzymatic method for modulating the local concentration of NO and leads to many of the immunoregulatory and toxic actions of NO. At high concentrations and under aerobic conditions, NO is rapidly oxidized to RNS [reviewed in (201)]. The most common RNS formed are nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), and dinitrogen tetroxide (N2O4), with N2O3 being the major oxidative product in biological systems (Fig. 11). Under conditions when the superoxide anion is formed at high concentrations, NO production leads to the generation of the highly reactive oxidant peroxynitrite anion (ONOO−) (Fig. 11). RNS are unstable and rapidly nitrosylate thiols or amines, or are hydrolyzed and excreted as nitrite (NO2−). The covalent addition of NO to thiol residues is termed nitrosylation, and can promote altered expression or function of enzymes and structural and signaling proteins (221). At higher concentrations, RNS induce a condition known as nitrosative stress by covalent modification of protein tyrosine residues through nitration, leading to structural alterations and often loss of function, and by inducing DNA damage and lipid peroxidation (201).
FIG. 11.
Formation of reactive nitrogen intermediates. NO is produced by one of the three known isoforms of NO synthase (NOS, eNOS, nNOS, and iNOS) that catalyze the oxidative deamination of l-arginine. NO reacts with superoxide (O2·−) to produce ONOO−, or with oxygen to form NO2. Both of these species will result in the formation of a variety of reactive oxygen–nitrogen intermediates. Under physiological conditions, NO may react with RSH to form either RSNO or RSOH. eNOS, endothelial-type NOS; iNOS, inducible NOS; NOS, nitric oxide synthase; nNOS, neuronal type NOS. NO, nitric oxide; NO2, nitrogen dioxide; RSH, thiol groups; RSNO, nitrosothiols; RSOH, sulfenic acid.
A variety of lung cells have been shown to produce NO. Human airway epithelial cells can express eNOS and iNOS, with the latter one highly induced after exposure to proinflammatory cytokines and oxidants (12). iNOS is also expressed in monocytes/macrophages and other types of antigen-presenting cells (52). In the respiratory system, NO derived from constitutive NOS (cNOS) has homeostatic effects, including dilation of the pulmonary blood vessels and relaxation of airway smooth muscle. In contrast, NO derived from iNOS, which usually produces larger amounts (100–1000 times more) of NO compared to cNOS enzymes, is involved in RNS formation and nitrosative stress, and has been implicated in the pathogenesis of a number of inflammatory lung diseases (22). In the following sections, we will briefly review the available information regarding the role of NO/NRS in RSV infection.
A. NO production and iNOS expression in RSV infection
NO production has been demonstrated in a variety of viral infection models (5, 6, 255), and iNOS induction is now being considered as a possible universal event in all viral infections. Induction of iNOS in response to RSV has been shown in A549 cells and primary SAECs (125, 235), Hep-2 cells (9), bronchial epithelial cells, and Clara cells (217). Tsutsumi et al. were the first to demonstrate induction of the iNOS gene by RSV infection in A549 cells (235). Although in several viral infections, including RSV, iNOS gene expression can be regulated through the secretion of cytokines such as IL-1β, TNF-α, and IFN-γ (6, 91), in case of RSV infection, initial iNOS induction appears to be independent of cytokine stimulation (235), similar to what has been reported with hepatitis B and polio virus (156, 158). Increase in iNOS protein, with a concomitant increase in nitrite levels, was also reported in SAECs after RSV infection (125). UV-inactivated RSV failed to increase either NO release or iNOS protein in these studies, suggesting again that iNOS expression in airway epithelial cells depends on viral replication. Pretreatment with IL-4 reduced the production of NO in response to RSV infection in A549 cells, without any effect on iNOS expression, suggesting that the Th1/Th2 balance affects the ability of epithelial cells to produce NO in response to RSV infection (125). In contrast to these observations in epithelial cells, no increase in NO production was reported in human monocytes infected with RSV (197).
Induction of iNOS occurs at the transcriptional level. The promoter of the iNOS gene has two NF-κB-binding sites and an ISRE site, to which IFN regulatory factors bind (230). Although the mechanism of RSV-induced iNOS expression has not been investigated, we and others have shown that RSV infection of airway epithelial cells strongly induces transcription factors belonging to both the NF-κB and IRF families (38, 83, 235).
RSV induction of NO production and iNOS expression has also been investigated in vivo. Significantly increased production of NO, NOS activity, and expression of iNOS mRNA has been demonstrated in the lung of RSV-infected mice (222). Immunohistochemical analysis identified iNOS in the respiratory epithelium as the major NOS enzyme expressed during acute RSV infection. In agreement to this study, mouse lung epithelial cell extracts from the lungs of RSV-infected mice show a significant induction of iNOS protein (Kolli D, unpublished data) (Fig. 12A). In addition, using a macrophage-depletion model, we found that the NO/NRS production in mice infected with RSV also depends upon resident lung macrophages (Kolli D, unpublished data) (Fig. 12B). A similar role of macrophages in RSV-induced NO production was recently confirmed in a lamb model of RSV infection (219). Compared to the full term, the lungs of preterm lambs showed decreased nitrite levels and a trend toward increased arginase activity, suggesting that macrophages are either immature or differentially activated in preterm animals infected with RSV.
FIG. 12.
Induction of iNOS in mouse lung following RSV infection. (A) Balb/c mice were infected with RSV (1×107 pfu), and lung epithelial cell extracts were collected at various timepoints p.i. Proteins were resolved on an SDS–polyacrylamide gel and immunoblotted with a murine monoclonal antibody to iNOS. RSV infection produced an increase in iNOS protein compared to uninfected mice. (B) Nitrite production in BALF of BALB/C mice: Nitrite concentration was determined in BALF recovered at 24 h after RSV infection from regular mice and mice treated with CL2MBP to deplete macrophages. Nitrites were significantly increased by RSV alone, and significant reduction of nitrite production in AM depleted mice was observed. **p<0.001 AM depleted RSV-infected mice compared RSV-infected only. BALF, bronchoalveolar lavage fluid; CL2MBP, clodronate liposomes.
In contrast to the in vitro and animal studies, adult volunteers experimentally infected with RSV (but not showing clinical signs of lower respiratory tract infection) did not demonstrate a change in nasal and oral NO production compared to control subjects (86). In addition, a reduction of exhaled NO has been reported in infants during the acute phase of RSV bronchiolitis, compared with healthy controls, which returned to normal levels during the convalescence phase (79). High levels of NO metabolites (nitrite/nitrate) have been reported in the spinal fluid of children infected with RSV with central nervous system symptoms (173).
B. Effect of NO on RSV replication, cellular signaling, and lung disease
NO production in the course of a viral infection has been shown to have multiple effects; it can inhibit viral replication, cause viral mutation, and play a role in disease pathology. Using a series of HEp-2 cell clones that express iNOS constitutively and generate varying amounts of NO, Ali-Ahmad et al. demonstrated that iNOS and NO production results in a reversible, dose-dependent inhibition of RSV replication (9). Furthermore, when the HEp-2 parent cell line was treated with increasing concentrations of the chemical NO donor, S-nitroso-N-acetyl-penicillamine (SNAP), no inhibition of viral replication was observed except at high concentration of SNAP, suggesting that the NO derived from endogenous iNOS expression was much more efficient at inhibiting RSV replication than was the addition of the exogenous NO via the chemical donor SNAP (9). In a model of persistently infected dendritic cells, endogenous NO production seemed to be important in controlling RSV replication, as shown by increased viral titers in cells treated with the NOS inhibitor nitro-l-arginine methyl ester (L-NAME) (103).
NO production also seems to be important in modulating RSV replication in vivo. Using a mouse model of infection, Stark et al. demonstrated that inhibition of iNOS results in increased RSV lung titers (222). Similarly, in a mouse model of hypereosinophilia, due to overexpression of IL-5, which shows increased viral clearance, increased iNOS expression in the lungs correlated with decreased RSV replication (192). Treatment of mice with iNOS inhibitors before and during RSV infection significantly delayed RSV clearance in wild-type mice, and completely reversed the advantage conferred by the hypereosinophilic status of the IL-5 transgenic mice, suggesting that the eosinophil-derived NO contributes to innate protection against RSV (192).
Various intracellular signaling molecules are regulated by NO, from kinases to transcription factors such as NF-κB and AP-1, as they contain critical cysteine residues that undergo nitrosylation (221). Airway epithelial cell treatment with the iNOS inhibitors L-NAME, L-NG-monomethyl arginine (L-NMMA), and aminoguanidine did not have a significant effect on RSV-induced IL-8 or RANTES production (37, 162), suggesting that NO does not affect the intracellular signaling pathways leading to RSV-induced NF-κB, AP-1, and IRF activation. However, treatment of a human bronchial epithelial cell line (BEAS-2B) with free 3-nitrotyrosine (NO2Tyr) induced both a decrease in viral replication and a reduction of chemokine secretion via formation of nitrated α-tubulin, which resulted in alteration of cellular microtubule properties (110).
An important effect of increased NO production in response to RSV infection is the modulation of ion channel activity (217). Viral-infected cells show a significant reduction in activity of amiloride-sensitive epithelial Na+ channels, the main pathways through which Na+ ions enter lung epithelial cells (41). This inhibition appears to be mediated by NO, through upregulation of iNOS as a result of activation of the NF-κB pathway, as incubation of cells with the specific iNOS inhibitor 1400W significantly reverses viral-induced Na+ channel inhibition (217). This could represent an important mechanism by which RSV decreases alveolar fluid clearance, as demonstrated in BALB/c infected mice, resulting in increased levels of lung water and hypoxemia (56).
In addition, RSV-induced NO production causes stabilization of the transcription factor HIF-1α in a time-dependent manner (135). VEGF is one of the many gene targets of HIF-1α (76). Treatment of human primary bronchial epithelial cells infected with RSV with a NO inhibitor (Carboxy-PTIO) significantly reduced viral-induced HIF-1α expression and VEGF secretion (135). As VEGF is a cytokine known to alter vascular permeability, increased NO production in vivo could play an important role in airway mucosal edema, which is a prominent feature of RSV bronchiolitis and pneumonia.
Although NO production is associated with significant antiviral activity in vitro and in vivo against RSV, excessive NO formation has also been linked to the pathophysiology of RSV lung disease in vivo. In a mouse model of infection, prevention of NO formation was associated with a significant decreased recruitment of inflammatory cells into the lungs and with reduced airway hyper responsiveness (AHR) to methacholine (222). In summary, NO possesses antiviral activity against RSV, but is also involved in the recruitment of inflammatory cells and viral-induced AHR (Fig. 13). Modulation of NO during the early events in the course of RSV lower respiratory tract infection that cause inflammation and AHR could potentially ameliorate the resulting lung disease in children.
FIG. 13.
Summary of sources and possible effects of NO production in the course of RSV infection. CSE, cigarette smoke extract.
V. Potential Therapeutic Approaches
As there is strong supportive evidence that RSV-induced intracellular ROS formation regulates the expression of proinflammatory mediators and that oxidative stress, resulting from an imbalance between ROS production and airway antioxidant defenses, occurs in vitro and in vivo in response to RSV infection, antioxidant intervention would represent a rational approach for treatment of RSV lung disease. Two complementary approaches could be used to affect the outcome of RSV-associated lower respiratory tract infections; the first would be to increase airway antioxidant defenses by modulation of AOE expression/activity, and the second would be by enhancing nonenzymatic defenses through pharmacological intervention with molecules able to scavenge/detoxify ROS. In the following section, we will review several small-molecular-weight molecules with known antioxidant activity, from thiol to polyphenols, to antioxidant mimetics that have either been shown to affect RSV replication, viral-induced ROS production, and/or oxidative stress. We will also review compounds that stimulate Nrf2-dependent gene expression, with the potential to increase endogenous AOE levels in the context of RSV infection.
A. Vitamin A
There have been a few studies reporting low serum levels of vitamin A in severe cases of RSV bronchiolitis (179, 196), similar to what occurs in cases of severe infections with measles, another paramyxovirus for which vitamin A supplementation is currently recommended (1, 34). In two randomized clinical trials done in the United States, vitamin A administration in RSV infection did not show any clinical benefits (30, 196); however, there was some beneficial effect in other two studies performed in Chile and in Japan (64, 132), which showed a faster resolution of some symptoms, such as tachypnea, retractions, and wheezing, as well as the duration of hospitalization, in children treated with vitamin A, suggesting that possible underlying deficiencies can be a risk factor for developing more severe bronchiolitis.
B. Vitamin D
Although vitamin D does not have a direct effect on ROS formation, in the past few years, there has been increased recognition of its role in modulating pulmonary diseases, from asthma, COPD, and infections to cancer [reviewed in (101)], by affecting responses in a variety of cell types, from epithelial cells to antigen-presenting cells, such as monocytes/macrophages and dendritic cells, to lymphocytes, therefore shaping both innate and adaptive immune responses. In relationship to respiratory tract infections, there have been several studies in adults showing an inverse relationship between the levels of vitamin D3 and the number and severity of respiratory infections (101). In in-vitro studies, treatment of airway epithelial cells with vitamin D decreases RSV-induced NF-κB activation and proinflammatory mediator production. In addition, an SNP in the vitamin D receptor (123, 145) as well as low levels of vitamin D in the cord blood of neonates (21) have been both associated with increased risk of developing lower respiratory tract infection in response to RSV, suggesting a potential benefit of vitamin D treatment in the treatment/prophylaxis of RSV-associated pulmonary disease.
C. Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is a secretory product of the pineal gland involved in synchronizing circadian and seasonal timing of several physiological and behavioral processes. It has also been shown to be a powerful free-radical scavenger with a broad-spectrum antioxidant capacity, as it interacts with both ROS and RNS, therefore providing protection against cellular oxidative damage [reviewed in (229)]. In addition, melatonin has been shown to play an important role in the immune function under both physiological and physiopathological conditions [reviewed in (36)]. Several immune functions appear to be modulated by melatonin, including the antitumor defenses of the host and cytotoxicity of natural killer cells, as well as antibody production. Melatonin has also been shown to have a protective effect in the pathophysiology of various viral infections [reviewed in (28)]. Melatonin was shown to prevent paralysis and death in mice infected with encephalomyocarditis virus, to reduce viremia and significantly postpone the onset of the disease and death in mice infected with the lethal Semliki Forest virus, and to attenuate noninvasive West Nile virus-induced disease. A similar protective effect was demonstrated in mice infected with Venezuelan equine encephalomyelitis virus, where melatonin treatment delayed the onset of the disease and reduced mortality. There is one report on the administration of melatonin in RSV infection in vivo. Huang et al. reported that mice intranasally inoculated with RSV showed oxidative stress changes demonstrated by increased NO, MDA, and •OH levels and decreasing GSH and SOD activities, whereas administration of melatonin significantly reversed all these effects. Furthermore, melatonin inhibited RSV-induced production of proinflammatory cytokines such as TNF-α (108). These results suggest that melatonin ameliorates RSV-induced lung inflammatory injury in mice via inhibition of oxidative stress and proinflammatory cytokine production.
D. Thiols
NAC is a thiol-containing compound that is used to reduce viscosity and elasticity of mucus. Moreover, it is able to scavenge H2O2, hydroxyl radicals, and hypochlorous acid (11). Pretreatment of human alveolar and bronchial epithelial cells with NAC protects both cell types against injurious effects of H2O2 (174). It has also been shown that NAC protects against hypochlorous acid-induced contraction of guinea pig tracheal smooth muscles (19) and inhibits LPS-induced leukocyte accumulation in rat lungs. Furthermore, NAC can be deacetylated to cysteine, an important precursor of cellular GSH synthesis, and thus stimulating the cellular GSH system (87). NAC has been used in clinical practice for the treatment of chronic bronchitis, cystic fibrosis, pulmonary oxygen toxicity, and ARDS (87), with mixed results, although a recent meta-analysis of clinical studies using NAC has shown positive results in reducing exacerbation rates in COPD (223). Administration of NAC significantly decreases mortality in mice infected with influenza virus, and two placebo-controlled trials of NAC supplementation suggest that it could be effective in improving influenza-induced severity of respiratory symptoms in humans [reviewed in (236)]. Treatment of airway epithelial cells with NAC greatly diminishes RSV-induced NF-κB activation, by affecting its serine phosphorylation, and NF-κB-dependent gene expression and protein secretion, including IL-8, RANTES, IL-6, and TNF-α (37, 120, 162, 164), as well as it reduces RSV-induced mucin synthesis (164). In one of the studies, but not the others, NAC inhibits viral replication in A549 cells (164).
E. Polyphenols
Natural and synthetic polyphenols are molecules known for their antioxidant properties. Flavonoids are a ubiquitous group of polyphenolic substances present in seeds, fruit skin or peel, and flowers of most plants. Among them, quercetin and catechins, such as epigallocatechin 3-gallate (EGCG), are the ones best investigated for their antioxidant properties. Quercetin and the related molecule isoquercetin have been shown in a recent study to inhibit the replication of both influenza A and B viruses [reviewed in (236)]. In a double treatment of isoquercetin and amantadine, synergistic effects were observed on the reduction of viral replication in vitro. The serial passages of virus in the presence of isoquercetin did not lead to the emergence of resistant virus, and the addition of isoquercetin to amantadine or oseltamivir treatment suppressed the emergence of amantadine- or oseltamivir-resistant virus. In a mouse model of influenza virus infection, isoquercetin administered intraperitoneally to mice inoculated with human influenza A virus significantly decreased the viral titers and pathological changes in the lung, suggesting that isoquercetin may have the potential to be developed as a therapeutic agent for the treatment of influenza virus infection and for the suppression of resistance in combination therapy with existing drugs. Similarly, EGCG and related compounds from green tea have been shown to reduce influenza replication in vitro and to possibly affect influenza-induced respiratory symptoms in the elderly [reviewed in (236)]. There is only one study investigating the effect of dietary flavonoids, including quercetin and catechins, on the infectivity and replication of RSV. In this study, preincubation of HEp2 cells with quercetin did not affect the ability of the virus to infect the cells or replicate, whereas catechins inhibited the infectivity, but not the replication of the virus (131).
Resveratrol, a nonflavonoid polyphenolic compound contained in grapes, has been shown to have important antioxidant and anti-inflammatory properties. It has been shown to improve outcomes in a variety of chronic diseases such as cardiovascular disease and diabetes, as well as cancer. There is experimental evidence that resveratrol could also be useful in the treatment of acute and chronic respiratory diseases, such as asthma, COPD, and possibly viral infections [reviewed in (252)]. A study of influenza virus has shown that resveratrol reduces influenza virus replication in MDCK cells by inhibiting nuclear–cytoplasmic translocation of viral ribonucleoproteins, therefore reducing expression of late viral proteins, and by inhibiting protein kinase C activity and its dependent pathways (186). The same study also shows that resveratrol treatment significantly improves survival and decreases pulmonary viral titers in a mouse model of influenza infection. There are only two reports on the use of resveratrol in models of RSV infection. The first in vitro study investigated its antiviral activity against RSV, showing that resveratrol is effective in inhibiting RSV replication in HEp2 cells at a concentration of 2 mg/ml (65). In the second study, administration of resveratrol in a mouse model of RSV infection reduced viral lung titers, viral-induced AHR, and lung inflammation (256), suggesting a potential benefit in treating RSV-associated pulmonary disease.
BHA and the related compound butylated hydroxytoluene (BHT) are phenolic compounds that are often added to foods to preserve fats. Oxygen reacts preferentially with BHA or BHT rather than oxidizing fats or oils. BHA can undergo several metabolic pathways in the body, such as dimerization, conjugation, and O-demethylation (238). In the context of RSV infection, treatment of airway epithelial cells with BHA greatly reduces RSV-induced chemokine production and modulates signaling pathways leading to IRF and STAT activation, with a slight reduction in viral replication (37, 114, 154). In vivo, treatment with BHA significantly decreases the content of MDA and 4-HNE in BAL of RSV-infected mice and ameliorates both body weight loss and clinical illness, indicating that modulation of oxidative stress in the context of RSV infection can lead to improvement in lung disease (40). These findings were reproduced by the use of another antioxidant agent, DMSO. Oral administration of DMSO at the time of infection significantly reduces RSV-induced body weight loss and clinical disease. Although it is difficult to determine the precise mechanisms by which BHA provides protection in the context of RSV infection, attenuation of inflammation is likely a key one. BHA treatment significantly reduces pulmonary cytokine and chemokine production, after RSV infection, resulting in inhibition of lung recruitment of inflammatory cells, especially neutrophils, which are the major cell type responsible for oxidative burst in response to infectious stimuli. BHA treatment is effective if given before or at the moment of RSV infection; however, it does not result in significant improvement of clinical disease administered 1 day after infection, when clinical disease and lung inflammation are already present. This finding suggests that early therapeutic intervention may be necessary to improve the clinical outcome in RSV infection. In fact, treatment of children with RSV-induced lower respiratory tract infection with anti-inflammatory drugs, such as steroids, has been shown to be mostly ineffective in improving clinical disease, as they are usually administered when children are hospitalized, days after manifestation of initial symptoms [reviewed in (32)].
In addition to reducing body weight loss and pulmonary inflammation, BHA treatment also attenuates RSV-induced AHR, possibly due to a reduction in cysteinyl-leukotriene (LT) production. LTs are potent bronchoconstrictors, as well as proinflammatory mediators, and their role in the pathogenesis of airway inflammation and obstruction has been recently recognized as a new target for therapeutic intervention [reviewed in (124)]. They have been shown to increase in the respiratory secretion of children infected with RSV (62, 84) and are associated with RSV-induced wheezing (237). In a mouse model of RSV infection, increased levels of cysteinyl-LTs correlate with increased airway responsiveness (249), and either inhibition of LT production (249) or treatment with LT receptor antagonist (77) inhibits AHR. In children, treatment with a cysteinyl-LT receptor antagonist decreases exacerbations of reactive airway disease after RSV bronchiolitis (25). BHA administration significantly decreases leukotriene C4 (LTC4) secretion, with parallel reduction of AHR after a methacholine challenge, suggesting a casual relationship of the two phenomena, although we cannot exclude that inhibition of other important mediators could be responsible for AHR reduction after BHA treatment (40).
In terms of viral replication, BHA administration slightly increases RSV replication in the lung, likely due to the observed reduction in inflammatory cells recruited to the lungs of infected mice (40). A similar finding is observed after inhibition of NO production in a mouse model of RSV infection, in which iNOS inhibition results in decreased lung inflammation, but in increased viral titers (222).
F. SOD and SOD mimetics
The use of recombinant SOD and SOD mimetics has been explored as therapeutics in a variety of disease models either in vitro or in vivo. A number of SOD mimetics based around organomanganese complexes have been developed. They include metalloporphyrin-based compounds, such as AEOL10113 and 10150, cyclic polyamine-based molecules, such as M40403 and 40419, and the Salen compounds, such as EUK8, 134, and 189, the latter ones possessing also significant catalase and peroxidase activity [reviewed in (20)].
Administration of SOD1 and 2 in a mouse model of influenza infection has been shown to be protective against lethal disease, and SOD3 overexpression in a similar model of infection significantly decreases influenza-induced lung injury [reviewed in (236)]. Both SOD1 and 2 have been tested for antiviral activity in a cotton rat model of RSV infection, and they both significantly reduce pulmonary viral titers when administered either parenterally or intranasally (253), although the authors of the study did not investigate their effect on disease or lung inflammation.
Although EUKs have been used in a variety of disease models, there is no reported literature about their use in models of viral infections. In recent in vitro studies, we have shown that treatment of A549 cells with EUK-134 significantly inhibits RSV-induced chemokine secretion (106). In addition, we also found that EUK8 and EUK189 are also effective in reducing RSV-induced cytokine and chemokine production in A549 cells (Casola A, unpublished observation). EUK8 and EUK189 treatments of A549 cells can effectively scavenge RSV-induced intracellular ROS generation, measured by DCFDA oxidation, and reduce lipid peroxidation markers, such as MDA and 4-HNE, in infected cells (Casola A, unpublished observation).
G. Nrf2-inducing agents
A variety of other compounds that stimulate ARE-driven transcription have been identified from natural and dietary sources, metabolites, and synthetic agents. These compounds have been extensively reviewed in a recent review article (111), and we classify them in our brief summary based on this review. Although none of them have been tested in the context of RSV infection, either in vitro or in vivo, they hold great potential for modulating RSV-induced oxidative stress.
1. Triterpenoids
Extensive reviews on the state of the art of triterpenoids have been published, and we mainly refer to these review articles for an incomplete description of these compounds (150, 220). More than 20,000 triterpenoids exist in nature, representing one of the largest groups of plant products. Triterpenoids are synthesized in plants by cyclization of squalene and are included among others squalenes, lanostanes, fusidanes, dammaranes, euphanes, lupanes, oleananes, ursanes, hopanes, and tetranortriterpenoids. Two of these natural compounds, oleanolic acid and ursolic acid, which have weak anti-inflammatory and antitumorogenic activity in vivo, represent the base for the initial screen and subsequent synthetic modifications of over 300 derivatives that have been conducted at the Dartmouth College (104). These agents have been tested for their anti-inflammatory activity, mainly by measuring their ability to block synthesis of iNOS in IFN-γ-stimulated macrophages. Two potent synthetic oleanane triterpenoids, 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid (CDDO) and its methyl ester (CDDO-Me), are currently in phase I clinical trials for the treatment of leukemia and solid tumors. Further derivatization of CDDO to yield imidazolides (CDDO-Im), amides (methyl amide, CDDO-MA; ethyl amide, CDDO-EA), or a dinitrile (Di-CDDO) significantly increases biological activity (150). In addition to suppressing inflammation, these CDDO can activate cellular pathways that are associated with cytoprotection. This is the result of the induction of the coordinated response of Nrf2-induced, ARE-dependent cytoprotective proteins that include the enzymes of GSH synthesis and transfer, NQO, TRX, catalase, superoxide dismutase, and HO-1. The carbonyl groups on rings A and C of CDDO are critical functional groups to enable Michael addition with a nucleophilic target, such as Keap1 (a protein with multiple nucleophilic −SH residues), resulting in the release of Nrf2. The anti-inflammatory properties of CDDO also involve binding of CDDO to other protein targets with Michael addition on active cysteine residues. Among these targets, there are known signaling proteins such as IKKs, JAK and PTEN, and proteins associated with actin cytoskeleton [reviewed in (220)].
This class of agents has been tested in numerous experimental animal models for a variety of diseases, mainly those with important inflammatory and oxidative components (220). Relevant to airway diseases, these agents have been used in animal models of cystic fibrosis and emphysema induced by cigarette smoke (CS). In one study, mice carrying the R117H Cftr mutation of cystic fibrosis showed significantly reduced airway inflammatory responses to both LPS and flagellin when treated with CDDO before inflammatory challenge (181). Anti-inflammatory effects observed include reduced airway neutrophilia, reduced concentrations of proinflammatory cytokines and chemokines, and reduced weight loss. Mode of action of CDDO appeared to be due to an Nrf2-mediated increase in expression of a number of proteins with antioxidant properties. Parallel experiments performed with primary human bronchial cells after CDDO exposure and stimulation with TNF-α were consistent with changes observed in the mouse model. Using a mouse model of chronic exposure to CS, another study evaluated whether the strategy of activation of Nrf2 and its downstream network of cytoprotective genes with a small molecule would attenuate smoke-induced oxidative stress and emphysema (228). Nrf2+/+ and Nrf2−/− mice were fed a diet containing CDDO-Im, while being exposed to CS for 6 months. CDDO-Im significantly reduced lung oxidative stress, alveolar cell apoptosis, alveolar destruction, and pulmonary hypertension in Nrf2+/+ mice caused by chronic exposure to CS. This protection from CS-induced emphysema depended on Nrf2, as Nrf2−/− mice failed to show significant reduction in alveolar cell apoptosis and alveolar destruction after treatment with CDDO-Im. Similar protective effects of CDDO have been reported in studies of hyperoxia-mediated acute lung injury (198).
Although direct studies on the effect of CDDO in RSV infection have not been conducted so far, a randomized single-blind trial of the Chinese herbs Shuang Huang Lian was evaluated for the treatment of acute bronchiolitis (143). Shuang Huang Lian consists mainly of the three Chinese herbs, shuanghua, huangqin, and lianqiao, which are derived from plants and is composed of at least 29 known ingredients. Interestingly, the lianqiao herb contains oleanolic acid, ursolic acid, and triterpenoid saponin, the three major precursors of CDDO. In that study, children with acute bronchiolitis were randomized into three treatment groups: herbs, herbs with antibiotics, and antibiotics alone. The herbs were administered daily by intravenous infusion for 7 days. Main outcomes, assessed blindly, were symptomatic improvement in cough, fever, wheezing, chest signs, and duration of stay in hospital. The mean duration of symptoms from the beginning of treatment was 2.4 days shorter in the two groups treated with herbs compared to the group treated with antibiotics alone, suggesting a beneficial effect of one or more of the compounds contained in these specific Chinese herbs on RSV disease.
2. Sulforaphane and other isothiocyanates
Isothiocyanates are organosulfur compounds found in cruciferous vegetables (broccoli and cabbage), and a diet rich in such vegetables has been shown to reduce the risk of developing certain types of cancer. Sulforaphane is the best-known ARE inducer in this class, and as such, it modifies a number of Keap1 cysteine residues through formation of carbamodithioate (111). Recent reports have shown that supplementation with sulforaphane affects respiratory viral infections, both in vitro and in vivo. In one study, nasal epithelial cells treated with sulforaphane before infection with influenza virus show significant inhibition of viral replication, demonstrated by marked decreased hemagglutinin gene expression, and increase protein levels of Nrf2 and HO-1 (133). In a mouse model of RSV infection, sulforaphane treatment significantly decreases the RSV gene expression level and reduces numbers of BAL neutrophils and eosinophils (46).
3. Polyphenols
Polyphenols, in addition to their ability to scavenge ROS, can also react with thiols in Keap1 and elicit ARE-dependent responses. Among them are the flavonoids quercetin and EGCG, the nonflavonoids curcumin and resveratrol, and the phenolic acid and diterpens rosmarinic and carnosic (carnasol) acids. Curcumin has been tested in clinical trials for certain cancers and in degenerative neurological disease (18). Curcumin has been shown to induce Nrf2- and ARE-dependent genes in human cell lines and in mice fed with a diet containing curcumin (80). EGCG, as well as other catechins contained in green tea, has been shown to modulate Nrf2-dependent gene expression [reviewed in (227)]. Carnasol, a catechol-type antioxidant from Rosmarinus officinalis, increases levels of Nrf2 and the target gene HO-1 in cells, and transgenic mice expressing an ARE-driven luciferase reporter gene, treated by oral gavage with this compound, showed robust ARE activation in different organs (15). Quercetin is capable of activating Nrf2-dependent phase II enzymes (NQO1) in cell lines by both inhibiting ubiquitination of Nrf2 and increasing Nrf2 mRNA [reviewed in (67)]. A 2-week regimen of dietary quercetin has been shown to suppress colorectal carcinogenesis in F344 rats (61). Several studies have suggested that resveratrol activates Nrf2-dependent antioxidant gene expression in a number of cell types, including hepatocytes, epithelial cells, and endothelial cells, by activating Nrf2 (67, 227). In a study using A549 cells, resveratrol-attenuated CS extracts induced ROS and restored GSH level by upregulating GCL via Nrf2 (141).
As mentioned earlier in this review, BHA and BHT are phenolic compounds with known ROS-scavenging properties. One of the major metabolites of BHA is the demethylated product tert-butylhydroquinone (tBHQ), which exhibits its chemopreventive effects in a similar manner to those by BHA, including the modulation of the enzyme systems responsible for metabolic activation or deactivation of chemical carcinogens. Different studies have shown that BHA and tBHQ have the capacity to induce some phase II enzymes via Nrf2. For example, a study has shown that BHA and tBHQ treatments increased HO-1, NQO1, and Nrf2 proteins in both primary-cultured rat and human hepatocytes (134). BHA- and tBHQ-mediated induction of HO-1 and NQO1 proteins occurred through transcriptional activation in primary-cultured rat hepatocytes and involved activation of ERK1/2 and JNK1/2. In preliminary experiments, treatment of airway epithelial cells with tBHQ significantly increases ARE-dependent gene transcription and Nrf2 protein expression (Casola A, unpublished observation) (Fig. 14), suggesting the possibility that the beneficial effect we observed for BHA treatment on RSV-induced lung inflammation and lung diseases could be in part ascribed to the ability of these phenolic compounds to modulate Nrf2-dependent gene expression, in addition to directly scavenging ROS formed in response to the viral infection.
FIG. 14.
Effect of ARE inducers on Nrf2 activation in airway epithelial cells. A549 cells were transiently transfected with a plasmid containing multiple copies of the NQO1 ARE site linked to the luciferase gene and either infected with RSV or treated with 25 μM tBHQ or 10 μM sulforaphane (sulfo). Cells were harvested at different times p.i. to measure luciferase activity. Uninfected plates served as controls. For each plate, luciferase was normalized to the β-galactosidase reporter activity. Data are expressed as mean±standard deviation of normalized luciferase activity (A). SAE cells were treated with 25 μM tBHQ for 6 and 15 h and harvested to prepare nuclear extracts. Nuclear amounts of Nrf2 were determined by Western blot. Membranes were stripped and reprobed for lamin B as an internal control for protein integrity and loading (B). tBHQ, tert-butylhydroquinone.
4. Other classes of Nrf2 inducers
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is a prostaglandin metabolite produced by the action of COX-2, whose activity, including its anti-inflammatory activity, is mediated through its covalent reaction with the cysteine residues in target proteins. 15d-PGJ2 has been shown to increase the levels of HO-1 and GSH in various cell lines. In mouse peritoneal macrophages, 15d-PGJ2 activated Nrf2 by forming adducts with Keap1, resulting in an Nrf2-dependent induction of HO-1 and Prdx I (Prx1) gene expression (115). Administration of the COX-2 inhibitor NS-398 to mice with carrageenan-induced pleurisy caused persistence of neutrophil recruitment and, in macrophages, attenuated the 15d-PGJ2 accumulation and PrxI expression (115). Administration of 15d-PGJ2 into the pleural space of NS-398-treated wild-type mice largely counteracted both the decrease in PrxI and the persistence of neutrophil recruitment. In contrast, these changes did not occur in the Nrf2−/− mice, suggesting that Nrf2 regulates the inflammation process downstream of 15d-PGJ2 (115).
As an example of ARE inducers identified using high-throughput screening of unbiased small-molecule library described by Hur et al., AI-1 is a quinolinone–scaffold compound that exhibits a single-digit micromolar EC50 for inducing ARE luciferase reporter gene activity and Nrf2 stabilization in cells (112). AI-1 has attractive features such as a potent Nrf2 activation, low cytotoxicity, and a versatile chemistry for derivatization. A biotin-modified AI-1 was used to reveal that AI-1 alkylates C151 of Keap1 both in vitro and in cells, weakening the interaction between Cul3 and Keap1, thereby inhibiting Nrf2 ubiquitination and activating ARE-mediated gene transcription.
VI. Conclusions
RSV-induced respiratory disease is associated with increased ROS/RNS generation and oxidative stress that are likely to play a key role in initiating and amplifying lung injury and inflammation. In animal models, modulation of ROS/RNS production has been shown to be beneficial in ameliorating viral-induced lung inflammation and clinical disease, although intervention is associated with some increase in viral replication. Approaches that combine scavenging ROS/RNS together with the inhibition of viral replication would be likely the most effective in modulating severe lung disease associated with RSV infection. This could be achieved by administration of antioxidant compounds that possess antiviral activity (resveratrol, for example, has been shown to reduce RSV replication in vitro and in vivo), in addition to ROS-scavenging properties, or by combining old/novel antivirals for RSV with compounds able to increase lung antioxidant defenses, such as AOE mimetics or Nrf2 inducers. Antioxidant supplementation would be successful only if available at the site of infection/inflammation; therefore, route of administration, bioavailability, tissue distribution are all important parameters that will need to be taken into consideration when planning future therapeutic interventions. The incomplete understanding of the pathophysiology of RSV bronchiolitis and its genetic components remains a barrier to the development of targeted immunomodulatory, anti-inflammatory, or antioxidant therapeutics. As an example of our incomplete understanding of the disease process and its clinical progression, there are studies showing that a robust inflammatory response driven by certain cytokines and chemokines might be beneficial in the early symptomatic phases of RSV infection, yet detrimental at later times of disease (23). Therefore, potential anti-inflammatory interventions in RSV infections may be either detrimental or insufficient to prevent progression to the lower respiratory tract depending on the stage of infection. Ideally, the decision process for anti-inflammatory or antioxidant treatment for RSV infections will have to be guided by the integration of a number of clinical and laboratory criteria, including early at point-of-care viral diagnostics, assessment of risk factors, and identification of biomarkers of disease progression and genetic susceptibility. The latter may also involve regulation of the antioxidant response, since functional polymorphisms in the Nrf2 gene promoter, leading to reduced gene transcription, have been shown, for example, to associate with severity of certain airway diseases, including COPD and lung injury after trauma (107, 160).
As we explore new treatment and prophylactic regimens for viral bronchiolitis that target the oxidant response, other important aspects will have to be considered, including whether RSV is indeed unique compared to other respiratory viral pathogens in its ability to alter the expression of AOEs, to trigger the generation of ROS and the magnitude and type of oxidative damage in the airways, including the presence of other lipid peroxidation products or protein modifications, which characterize the biological targets of ROS formation. The role of possible co-infections in altering the pro-oxidative/antioxidative balance remains unexplored. Also, the known developmental process of the AOE system that starts during fetal life and that is characterized by a certain degree of immaturity in the neonatal period and early infancy may contribute to the severity of RSV infections that occur during this vulnerable period of life (57). All these factors are likely to contribute to the oxidative-mediated pathogenesis of viral bronchiolitis and perhaps to the relative greater effect of RSV compared to other pathogens. Therefore, in these earlier phases of postnatal life, the well-known antioxidant capacity of human milk in combination with the specific anti-RSV antibodies that are present in human milk may play a critical role in preventing or reducing the risk of severe RSV infections, as suggested by multiple studies of breastfeeding and bronchiolitis (214). Although the complete list of antioxidants present in human milk is not known, it is has been shown to contain a number of AOEs such as those that were found to be affected by RSV infection (including SOD and catalase) (88). Overall, human milk demonstrates an increased total antioxidant capacity compared to formula, and even preterm infants, which are at a higher risk for severe RSV infections, show evidence of lower oxidative damage when fed exclusively mother's milk compared with formula-fed infants (148).
With even broader implications, generation of such an oxidative stress environment in the airways, along with the impaired antioxidant response as result of RSV infection, may induce critical chemical modifications of bystander antigens and their immunogenicity. Such possibility has been suggested by studies of aldehyde-mediated carbonylation of protein allergens resulting in enhanced Th2 responses, perhaps via enhanced immune priming (171). Thus, treatment or prophylactic regimens aimed to reduce the RSV-induced pro-oxidative may have even longer protective effects by potentially reducing the risk of the development of allergic diseases and asthma.
Last but not least, therapeutic strategies aimed to increase the antioxidant airway capacity by increasing Nrf2 activity should probably be used for short periods of time with an appropriate therapeutic dose, avoiding continuous Nrf2 activation. In fact, although Nrf2 exhibits protective effects against several types of chronic airway injury, its overexpression might be harmful. There is increasing concern about the potential detrimental effects of Nrf2 overexpression in cancer and high doses of cancer chemopreventive phenolic antioxidants, such as BHA, which activates Nrf2, and have been reported to promote tumors.
Abbreviations Used
- 4-HNE
4-hydrynonenal
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- AEBSF
4-(2-aminoethyl) benzene sulfonyl fluoride
- AHR
airway hyper-responsiveness
- ALRI
acute lower respiratory tract infection
- AOE
antioxidant enzyme
- AP-1
activator protein-1
- ARE
antioxidant-responsive element
- ATF-3
activating transcription factor 3
- BAL
bronchoalveolar lavage
- BHA
butylated hydroxyanisole
- BHT
butylated hydroxytoluene
- Brd4
bromodomain-4
- C3
third component of complement
- CARD
caspase activation and recruitment domains
- CBP
CREB-binding protein
- CCAAT
(cytidine-cytidine-adenosine-adenosine-thymidine) enhancer-binding protein
- CCL5
chemokine (C-C motif) ligand 5
- CD73
cluster of differentiation 73
- CDDO
2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid
- CDK9
cyclin-dependent kinase 9
- C/EPBβ
CCAAT/enhancer-binding protein beta
- C/EPBδ
CCAAT/enhancer-binding protein delta
- CL2MBP
clodronate liposomes
- cNOS
constitutive NOS
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- COX-2
cyclo-oxygenase-2
- CS
cigarette smoke
- DBD
DNA-binding domain
- DCFDA
dichlorodihydrofluorescein diacetate
- DMSO
dimethyl sulfoxide
- DN
dominant negative
- DPI
diphenylene iodonium chloride
- dsRNA
double-stranded ribonucleic acid
- ECSOD
extracellular superoxide dismutase
- EGCG
epigallocatechin 3-gallate
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- eNOS
endothelial type NOS
- EPO
erythropoietin
- ERK1/2
extracellular-signal-regulated kinases 1/2
- GAS
gamma-interferon-activated sequence
- GPx
glutathione peroxidase
- Gro-β
growth-regulated protein beta
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- HIF
hypoxia-inducible factor
- HIV
human immunodeficiency virus
- hMPV
human metapneumovirus
- HO-1
heme oxygenase 1
- H2O2
hydrogen peroxide
- IAD
IRF-association domain
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- IHC
immunohistochemical
- IκB
inhibitor of kappa B
- IKK
IκB kinase
- IL-4
interleukin-4
- iNOS
inducible NOS
- IP-10
interferon-gamma-induced protein 10
- IRF
interferon regulatory factor
- ISG
interferon sensitive gene
- ISRE
interferon-stimulated responsive element
- JAK
Janus-activated kinases
- Keap1
Kelch-like-ECH-associated protein 1
- L-NAME
nitro-l-arginine methyl ester
- L-NMMA
L-NG-monomethyl arginine
- LPS
lipopolysaccharide
- LT
leukotrienes
- LTC4
leukotriene C4
- LT-β
lymphotoxin-beta
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- MARE
MAF recognition elements
- MAVS
mitochondrial antiviral-signaling protein
- MCP-1
monocyte chemotactic protein 1
- MDA
malonaldehyde
- MDC
macrophage-derived chemokine
- MEKK1
mitogen-activated protein kinase kinase kinase
- MHC-I
major histocompatibility complex-I
- MIP
macrophage inflammatory protein
- MOI
multiplicity of infection
- MPO
myeloperoxidase
- MSK1
mitogen- and stress-activated protein kinase 1
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NEMO
NF-κB essential modulator
- NF-IL6
nuclear factor IL-6
- NF-κB
nuclear factor kappa B
- NIK
NF-κB-inducing kinase
- nNOS
neuronal type NOS
- NO
nitric oxide
- NO2
nitrogen dioxide
- N2O3
dinitrogen trioxide
- N2O4
dinitrogen tetroxide
- NO2Tyr
3-nitrotyrosine
- NOS
nitric oxide synthase
- NPS
nasopharyngeal secretions
- NQO1
quinone oxidoreductase
- Nrf2
NF-E2-related factor 2
- O2−
superoxide anion radical
- •OH
hydroxyl radical
- p.i.
postinfection
- PKA
protein kinase A
- Prdx
peroxiredoxin
- PTP
protein tyrosine phosphatase
- RANTES
regulated upon activation, normal T-cell expressed, and secreted
- RHD
Rel homology domain
- RIG-I
retinoic acid-inducible gene I
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSH
thiol groups
- RSNO
nitrosothiols
- RSOH
sulfenic acid
- RSV
respiratory syncytial virus
- RSVRE
RSV-response element
- SAEC
small-airway epithelial cells
- siRNA
short interfering RNA
- SNAP
S-nitroso-N-acetyl-penicillamine
- SNP
single-nucleotide polymorphism
- SOD
superoxide dismutase
- SPA
surfactant protein A
- STAT
signal transducers and activators of transcription
- TAK1
transforming growth factor-beta-activated kinase 1
- TARC
thymus and activation regulated chemokine
- tBHQ
tert-butylhydroquinone
- TLR
Toll-like receptor
- TNF
tumor nuclear factor
- TRAF6
TNF receptor associated factor 6
- TRX
thioredoxin
- URTI
upper respiratory tract infection
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau
- VS
ventilatory support
Acknowledgments
Supported by NIH grants AI062885, AI30039, and HV28184, and by Flight Attendant Medical Research Institute (FAMRI) Clinical Innovator Awards to AC (# 072147) and RPG (# 42253). The authors would like to thank Mrs. Cynthia Tribble for her assistance in manuscript submission.
References
- 1.American Academy of Pediatrics Committee on Infectious Diseases. Vitamin A treatment of measles. Pediatrics. 1993;91:1014–1015. [PubMed] [Google Scholar]
- 2.Adli M. Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–26984. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- 3.Aggeli IK. Gaitanaki C. Beis I. Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal. 2006;18:1801–1812. doi: 10.1016/j.cellsig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Aherne WT. Bird T. Court SDB. Gardner PS. McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Path. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev Med Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akaike T. Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaike T. Noguchi Y. Ijiri S. Setoguchi K. Suga M. Zheng YM. Dietzschold B. Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci U S A. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S. Isshiki H. Sugita T. Tanabe O. Kinoshita S. Nishio Y. Nakajima T. Hirano T. Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali-Ahmad D. Bonville CA. Rosenberg HF. Domachowske JB. Replication of respiratory syncytial virus is inhibited in target cells generating nitric oxide in situ. Front Biosci. 2003;8:a48–a53. doi: 10.2741/986. [DOI] [PubMed] [Google Scholar]
- 10.Allen RG. Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 11.Aruoma OI. Halliwell B. Hoey BM. Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 12.Asano K. Chee CB. Gaston B. Lilly CM. Gerard C. Drazen JM. Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 14.Baggiolini M. Dewald B. Moser B. Human chemokines: an update. Ann Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 15.Balstad TR. Carlsen H. Myhrstad MC. Kolberg M. Reiersen H. Gilen L. Ebihara K. Paur I. Blomhoff R. Coffee, broccoli and spices are strong inducers of electrophile response element-dependent transcription in vitro and in vivo - studies in electrophile response element transgenic mice. Mol Nutr Food Res. 2011;55:185–197. doi: 10.1002/mnfr.201000204. [DOI] [PubMed] [Google Scholar]
- 16.Bao X. Indukuri H. Liu T. Liao SL. Tian B. Brasier AR. Garofalo RP. Casola A. IKKepsilon modulates RSV-induced NF-kappaB-dependent gene transcription. Virology. 2010;408:224–231. doi: 10.1016/j.virol.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao X. Sinha M. Liu T. Hong C. Luxon BA. Garofalo RP. Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology. 2008;374:114–127. doi: 10.1016/j.virol.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basnet P. Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bast A. Haenen GR. Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 20.Batinic-Haberle I. Reboucas JS. Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belderbos ME. Houben ML. Wilbrink B. Lentjes E. Bloemen EM. Kimpen JL. Rovers M. Bont L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 22.Belvisi M. Barnes PJ. Larkin S. Yacoub M. Tadjkarimi S. Williams TJ. Mitchell JA. Nitric oxide synthase activity is elevated in inflammatory lung disease in humans. Eur J Pharmacol. 1995;283:255–258. doi: 10.1016/0014-2999(95)00421-g. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BL. Garofalo RP. Cron SG. Hosakote YM. Atmar RL. Macias CG. Piedra PA. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 24.Biagioli MC. Kaul P. Singh I. Turner RB. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med. 1999;26:454–462. doi: 10.1016/s0891-5849(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 25.Bisgaard H. Montelukast in RSV-bronchiolitis. Am J Respir Crit Care Med. 2004;169:542–543. doi: 10.1164/ajrccm.169.4.952. [DOI] [PubMed] [Google Scholar]
- 26.Biswas S. Friedland JS. Remick DG. Davies EG. Sharland Elevated plasma interleukin 8 in respiratory syncytial virus bronchiolitis [letter] Pediatr Infect Dis J. 1995;14:919. [PubMed] [Google Scholar]
- 27.Bitko V. Velazquez A. Yank L. Yang Y-C. Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kB and is inhibited by sodium salicylate and aspirin. Virology. 1997;232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- 28.Bonilla E. Valero N. Chacin-Bonilla L. Medina-Leendertz S. Melatonin and viral infections. J Pineal Res. 2004;36:73–79. doi: 10.1046/j.1600-079X.2003.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brasier AR. The nuclear factor-kB signaling network: insights from system approaches. In: Allan RB, editor; Adolfo G, editor; Stanley ML, editor. Cellular Signaling and Innate Responses to RNA Virus Infections. Washington, DC: ASM Press; 2009. pp. 119–135. [Google Scholar]
- 30.Bresee JS. Fischer M. Dowell SF. Johnston BD. Biggs VM. Levine RS. Lingappa JR. Keyserling HL. Petersen KM. Bak JR. Gary HE., Jr. Sowell AL. Rubens CE. Anderson LJ. Vitamin A therapy for children with respiratory syncytial virus infection: a multicenter trial in the United States. Pediatr Infect Dis J. 1996;15:777–782. doi: 10.1097/00006454-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Broor S. Bharaj P. Chahar HS. Human metapneumovirus: a new respiratory pathogen. J Biosci. 2008;33:483–493. doi: 10.1007/s12038-008-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broughton S. Greenough A. Drugs for the management of respiratory syncytial virus infection. Curr Opin Investig Drugs. 2004;5:862–865. [PubMed] [Google Scholar]
- 33.Buss H. Dorrie A. Schmitz ML. Hoffmann E. Resch K. Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 34.Butler JC. Havens PL. Sowell AL. Huff DL. Peterson DE. Day SE. Chusid MJ. Bennin RA. Circo R. Davis JP. Measles severity and serum retinol (vitamin A) concentration among children in the United States. Pediatrics. 1993;91:1176–1181. [PubMed] [Google Scholar]
- 35.Carballo M. Conde M. El Bekay R. Martin-Nieto J. Camacho MJ. Monteseirin J. Conde J. Bedoya FJ. Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 36.Carrillo-Vico A. Guerrero JM. Lardone PJ. Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 37.Casola A. Burger N. Liu T. Jamaluddin M. Brasier AR. Garofal RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 38.Casola A. Garofalo RP. Haeberle H. Elliott TF. Lin A. Jamaluddin M. Brasier AR. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J Virol. 2001;75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casola A. Garofalo RP. Jamaluddin M. Vlahopoulos S. Brasier AR. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. J Immunol. 2000;164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- 40.Castro SM. Guerrero-Plata A. Suarez-Real G. Adegboyega PA. Colasurdo GN. Khan AM. Garofalo RP. Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L. Song W. Davis IC. Shrestha K. Schwiebert E. Sullender WM. Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2009;40:588–600. doi: 10.1165/rcmb.2008-0034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen LF. Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 43.Chen LF. Williams SA. Mu Y. Nakano H. Duerr JM. Buckbinder L. Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang E. Dang O. Anderson K. Matsuzawa A. Ichijo H. David M. Cutting edge: apoptosis-regulating signal kinase 1 is required for reactive oxygen species-mediated activation of IFN regulatory factor 3 by lipopolysaccharide. J Immunol. 2006;176:5720–5724. doi: 10.4049/jimmunol.176.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chini BA. Fiedler MA. Milligan L. Hopkins T. Stark JM. Essential roles of NF-kappaB and C/EBP in the regulation of intercellular adhesion molecule-1 after respiratory syncytial virus infection of human respiratory epithelial cell cultures. J Virol. 1998;72:1623–1626. doi: 10.1128/jvi.72.2.1623-1626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho HY. Imani F. Miller-Degraff L. Walters D. Melendi GA. Yamamoto M. Polack FP. Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus (RSV) disease. Am J Respir Crit Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi AM. Knobil K. Otterbein SL. Eastman DA. Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am J Physiol. 1996;271:L383–L391. doi: 10.1152/ajplung.1996.271.3.L383. [DOI] [PubMed] [Google Scholar]
- 48.This reference has been deleted
- 49.Choudhary S. Boldogh S. Garofalo R. Jamaluddin M. Brasier AR. Respiratory syncytial virus influences NF-kappaB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-kappaB2. J Virol. 2005;79:8948–8959. doi: 10.1128/JVI.79.14.8948-8959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung-man HJ. Zheng S. Comhair SA. Farver C. Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61:8578–8585. [PubMed] [Google Scholar]
- 51.Ciencewicki J. Trivedi S. Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 53.Comstock AT. Ganesan S. Chattoraj A. Faris AN. Margolis BL. Hershenson MB. Sajjan US. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol. 2011;85:6795–6808. doi: 10.1128/JVI.02074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das KC. Lewis-Molock Y. White CW. Activation of NF-kappaB and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma. Lung Cell Mol Physiol. 1995;13:L588–L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- 55.Das KC. Lewis-Molock Y. White CW. Thiol modulation of TNF alpha and IL-1 induced MnSOD gene expression and activation of NF-kappa B. Mol Cell Biochem. 1995;148:45–57. doi: 10.1007/BF00929502. [DOI] [PubMed] [Google Scholar]
- 56.Davis IC. Sullender WM. Hickman-Davis JM. Lindsey JR. Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L112–L120. doi: 10.1152/ajplung.00218.2003. [DOI] [PubMed] [Google Scholar]
- 57.Davis JM. Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15:191–195. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Denu JM. Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 59.Dey N. Liu T. Garofalo RP. Casola A. TAK1 regulates NF-KappaB and AP-1 activation in airway epithelial cells following RSV infection. Virology. 2011;418:93–101. doi: 10.1016/j.virol.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhakshinamoorthy S. Jain AK. Bloom DA. Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 61.Dihal AA. de BV. van der WH. Tilburgs C. Bruijntjes JP. Alink GM. Rietjens IM. Woutersen RA. Stierum RH. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J Nutr. 2006;136:2862–2867. doi: 10.1093/jn/136.11.2862. [DOI] [PubMed] [Google Scholar]
- 62.Dimova-Yaneva D. Russell D. Main M. Brooker RJ. Helms PJ. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:555–558. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- 63.Dong C. Davis RJ. Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 64.Dowell SF. Papic Z. Bresee JS. Larranaga C. Mendez M. Sowell AL. Gary HE., Jr. Anderson LJ. Avendano LF. Treatment of respiratory syncytial virus infection with vitamin A: a randomized, placebo-controlled trial in Santiago, Chile. Pediatr Infect Dis J. 1996;15:782–786. doi: 10.1097/00006454-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Drago L. Nicola L. Ossola F. De VE. In vitro antiviral activity of resveratrol against respiratory viruses. J Chemother. 2008;20:393–394. doi: 10.1179/joc.2008.20.3.393. [DOI] [PubMed] [Google Scholar]
- 66.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 67.Eggler AL. Gay KA. Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52(Suppl 1):S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 68.El Saleeby CM. Bush AJ. Harrison LM. Aitken JA. Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Everard ML. Swarbrick A. Wrightham M. McIntyre J. Dunkley C. James PD. Sewell HF. Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faden H. Kaul TN. Ogra PL. Activation of oxidative and arachidonic acid metabolism in neutrophils by respiratory syncytial virus antibody complexes: possible role in disease. J Infect Dis. 1983;148:110–116. doi: 10.1093/infdis/148.1.110. [DOI] [PubMed] [Google Scholar]
- 71.Falsey AR. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28:171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- 72.Fink K. Duval A. Martel A. Soucy-Faulkner A. Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and Sendai virus-induced activation of NF-{kappa}B in airway epithelial cells. J Immunol. 2008;180:6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 73.Fitzgerald KA. McWhirter SM. Faia KL. Rowe DC. Latz E. Golenbock DT. Coyle AJ. Liao SM. Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 74.Folkerts G. Kloek J. Muijsers RB. Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 75.Fonceca AM. Flanagan BF. Trinick R. Smyth RL. McNamara PS. Primary airway epithelial cultures from children are highly permissive to respiratory syncytial virus infection. Thorax. 2012;67:43–48. doi: 10.1136/thoraxjnl-2011-200131. [DOI] [PubMed] [Google Scholar]
- 76.Forsythe JA. Jiang BH. Iyer NV. Agani F. Leung SW. Koos RD. Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fullmer JJ. Khan AM. Elidemir O. Chiappetta C. Stark JM. Colasurdo GN. Role of cysteinyl leukotrienes in airway inflammation and responsiveness following RSV infection in BALB/c mice. Pediatr Allergy Immunol. 2005;16:593–601. doi: 10.1111/j.1399-3038.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 78.Furchgott RF. Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 79.Gadish T. Soferman R. Merimovitch T. Fireman E. Sivan Y. Exhaled nitric oxide in acute respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med. 2010;164:727–731. doi: 10.1001/archpediatrics.2010.128. [DOI] [PubMed] [Google Scholar]
- 80.Garg R. Gupta S. Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 81.Garofalo RP. Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol. 2000;23:581–585. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 82.Garofalo RP. Patti J. Hintz KA. Hill V. Ogra PL. Welliver RC. Macrophage inflammatory protein 1-alpha, and not T-helper type 2 cytokines, is associated with severe forms of bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 83.Garofalo RP. Sabry M. Jamaluddin M. Yu RK. Casola A. Ogra PL. Brasier AR. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garofalo RP. Welliver RC. Ogra PL. Concentrations of LTB4, LTC4, LTD4 and LTE4 in bronchiolitis due to respiratory syncytial virus. Pediatr Allergy Immunol. 1991;2:30–37. [Google Scholar]
- 85.Garoufalis E. Kwan I. Lin R. Mustafa A. Pepin N. Roulston A. Lacoste J. Hiscott J. Viral induction of the human beta interferon promoter: modulation of transcription by NF-kB/rel proteins and interferon regulatory factors. J Virol. 1994;68:4707–4715. doi: 10.1128/jvi.68.8.4707-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gentile DA. Doyle WJ. Belenky S. Ranck H. Angelini B. Skoner DP. Nasal and oral nitric oxide levels during experimental respiratory syncytial virus infection of adults. Acta Otolaryngol. 2002;122:61–66. doi: 10.1080/00016480252775751. [DOI] [PubMed] [Google Scholar]
- 87.Gillissen A. Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92:609–623. doi: 10.1016/s0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- 88.Goldman AS. Chheda S. Garofalo R. Evolution of immunologic functions of the mammary gland and the postnatal development of immunity. Pediatr Res. 1998;43:155–162. doi: 10.1203/00006450-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 89.Gong G. Waris G. Tanveer R. Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Groskreutz DJ. Monick MM. Powers LS. Yarovinsky TO. Look DC. Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 91.Hacking D. Rockett K. Hull J. Kwiatkowski D. Synergistic action of cytokines and purified respiratory syncytial virus in nitric oxide induction. J Leukoc Biol. 2002;71:729–730. [PubMed] [Google Scholar]
- 92.Haeberle H. Takizawa R. Casola A. Brasier AR. Dieterich H-J. van Rooijen N. Gatalica Z. Garofalo RP. Respiratory syncytial virus-induced activation of NF-kB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 93.Haeberle HA. Casola A. Gatalica Z. Petronella S. Dieterich HJ. Ernst PB. Brasier AR. Garofalo RP. IkappaB kinase is a critical regulator of chemokine expression and lung inflammation in respiratory syncytial virus infection. J Virol. 2004;78:2232–2241. doi: 10.1128/JVI.78.5.2232-2241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haeberle HA. Durrstein C. Rosenberger P. Hosakote YM. Kuhlicke J. Kempf VA. Garofalo RP. Eltzschig HK. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE. 2008;3:e3352. doi: 10.1371/journal.pone.0003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haeberle HA. Kuziel WA. Dieterich HJ. Casola A. Gatalica Z. Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall CB. Douglas RG., Jr. Schnabel KC. Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hall CB. Weinberg GA. Iwane MK. Blumkin AK. Edwards KM. Staat MA. Auinger P. Griffin MR. Poehling KA. Erdman D. Grijalva CG. Zhu Y. Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han LL. Alexander JP. Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 99.Harada H. Takahashi E. Itoh S. Harada K. Hori TA. Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol Cell Biol. 1994;14:1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heffetz D. Bushkin I. Dror R. Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990;265:2896–2902. [PubMed] [Google Scholar]
- 101.Herr C. Greulich T. Koczulla RA. Meyer S. Zakharkina T. Branscheidt M. Eschmann R. Bals R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 103.Hobson L. Everard ML. Persistent of respiratory syncytial virus in human dendritic cells and influence of nitric oxide. Clin Exp Immunol. 2008;151:359–366. doi: 10.1111/j.1365-2249.2007.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Honda T. Rounds BV. Bore L. Finlay HJ. Favaloro FG., Jr. Suh N. Wang Y. Sporn MB. Gribble GW. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000;43:4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 105.Hosakote YM. Jantzi P. Schiblisky D. Esham A. Casola RP. Garofalo Viral inhibition of antioxidant enzymes contributes to the pathogenesis of severe RSV bronchiolitis. Amer J Resp Critic Care Med. 2011;183:1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosakote YM. Liu T. Castro SM. Garofalo RP. Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hua CC. Chang LC. Tseng JC. Chu CM. Liu YC. Shieh WB. Functional haplotypes in the promoter region of transcription factor Nrf2 in chronic obstructive pulmonary disease. Dis Markers. 2010;28:185–193. doi: 10.3233/DMA-2010-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang SH. Cao XJ. Liu W. Shi XY. Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010;48:109–116. doi: 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 109.Huang SH. Cao XJ. Wei W. Melatonin decreases TLR3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected RAW264.7 macrophages. J Pineal Res. 2008;45:93–100. doi: 10.1111/j.1600-079X.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- 110.Huang YC. Li Z. Brighton LE. Carson JL. Becker S. Soukup JM. 3-nitrotyrosine attenuates respiratory syncytial virus infection in human bronchial epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2005;288:L988–L996. doi: 10.1152/ajplung.00378.2004. [DOI] [PubMed] [Google Scholar]
- 111.Hur W. Gray NS. Small molecule modulators of antioxidant response pathway. Curr Opin Chem Biol. 2011;15:162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 112.Hur W. Sun Z. Jiang T. Mason DE. Peters EC. Zhang DD. Luesch H. Schultz PG. Gray NS. A small-molecule inducer of the antioxidant response element. Chem Biol. 2010;17:537–547. doi: 10.1016/j.chembiol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 113.Imada K. Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 114.Indukuri H. Castro SM. Liao SM. Feeney LA. Dorsch M. Coyle AJ. Garofalo RP. Brasier AR. Casola A. Ikkepsilon regulates viral-induced interferon regulatory factor-3 activation via a redox-sensitive pathway. Virology. 2006;353:155–165. doi: 10.1016/j.virol.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 115.Itoh K. Mochizuki M. Ishii Y. Ishii T. Shibata T. Kawamoto Y. Kelly V. Sekizawa K. Uchida K. Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacoby DB. Choi AM. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med. 1994;16:821–824. doi: 10.1016/0891-5849(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 117.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 118.Jamaluddin M. Casola A. Garofalo RP. Han Y. Elliott T. Ogra PL. Brasier AR. The major component of IkBa proteolysis occurs independently of the proteasome pathway in respiratory syncytial virus-infected pulmonary epithelial cells. J Virol. 1998;72:4849–4857. doi: 10.1128/jvi.72.6.4849-4857.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jamaluddin M. Garofalo RP. Ogra PL. Brasier AR. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jamaluddin M. Tian B. Boldogh I. Garofalo RP. Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jamaluddin M. Wang S. Boldogh I. Tian B. Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 122.Jamaluddin M. Wiktorowicz JE. Soman KV. Boldogh I. Forbus JD. Spratt H. Garofalo RP. Brasier AR. Role of peroxiredoxin 1 and peroxiredoxin 4 in protection of respiratory syncytial virus-induced cysteinyl oxidation of nuclear cytoskeletal proteins. J Virol. 2010;84:9533–9545. doi: 10.1128/JVI.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Janssen R. Bont L. Siezen CL. Hodemaekers HM. Ermers MJ. Doornbos G. van ‘t SR. Wijmenga C. Goeman JJ. Kimpen JL. van Houwelingen HC. Kimman TG. Hoebee B. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 124.Kanaoka Y. Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 125.Kao YJ. Piedra PA. Larsen GL. Colasurdo GN. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am J Respir Crit Care Med. 2001;163:532–539. doi: 10.1164/ajrccm.163.2.9912068. [DOI] [PubMed] [Google Scholar]
- 126.Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4(Suppl 1):S92–S99. [PubMed] [Google Scholar]
- 127.Karin M. Liu Z. Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 128.Kaspar JW. Niture SK. Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaul P. Singh I. Turner RB. Effect of rhinovirus challenge on antioxidant enzemes in respiratory epithelial cells. Free Rad Res. 2002;36:1085–1089. doi: 10.1080/1071576021000028316. [DOI] [PubMed] [Google Scholar]
- 130.Kaul P. Biagioli MC. Singh I. Turner RB. Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J Infect Dis. 2000;181:1885–1890. doi: 10.1086/315504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaul TN. Middleton E., Jr. Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 132.Kawasaki Y. Hosoya M. Katayose M. Suzuki H. The efficacy of oral vitamin A supplementation for measles and respiratory syncytial virus (RSV) infection. Kansenshogaku Zasshi. 1999;73:104–109. doi: 10.11150/kansenshogakuzasshi1970.73.104. [DOI] [PubMed] [Google Scholar]
- 133.Kesic MJ. Simmons SO. Bauer R. Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keum YS. Han YH. Liew C. Kim JH. Xu C. Yuan X. Shakarjian MP. Chong S. Kong AN. Induction of heme oxygenase-1 (HO-1) and NAD[P]H: quinone oxidoreductase 1 (NQO1) by a phenolic antioxidant, butylated hydroxyanisole (BHA) and its metabolite, tert-butylhydroquinone (tBHQ) in primary-cultured human and rat hepatocytes. Pharm Res. 2006;23:2586–2594. doi: 10.1007/s11095-006-9094-2. [DOI] [PubMed] [Google Scholar]
- 135.Kilani MM. Mohammed KA. Nasreen N. Hardwick JA. Kaplan MH. Tepper RS. Antony VB. Respiratory syncytial virus causes increased bronchial epithelial permeability. Chest. 2004;126:186–191. doi: 10.1378/chest.126.1.186. [DOI] [PubMed] [Google Scholar]
- 136.Kim HW. Canchola JG. Brandt CD. Respiratory syncytial virus disease infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 137.Kimpen JL. Garofalo RP. Welliver RC. Ogra PL. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Kinnula VL. Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 139.Knobil K. Choi AM. Weigand GW. Jacoby DB. Role of oxidants in influenza virus-induced gene expression. Am J Physiol. 1998;274:L134–L142. doi: 10.1152/ajplung.1998.274.1.L134. [DOI] [PubMed] [Google Scholar]
- 140.Koarai A. Sugiura H. Yanagisawa S. Ichikawa T. Minakata Y. Matsunaga K. Hirano T. Akamatsu K. Ichinose M. Oxidative stress enhances toll-like receptor 3 response to double-stranded RNA in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:651–660. doi: 10.1165/rcmb.2008-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kode A. Rajendrasozhan S. Caito S. Yang SR. Megson IL. Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 142.Kong X. San JH. Kumar M. Behera AK. Mohapatra A. Hellermann GR. Mane S. Lockey RF. Mohapatra SS. Respiratory syncytial virus infection activates STAT signaling in human epithelial cells. Biochem Biophys Res Commun. 2003;306:616–622. doi: 10.1016/s0006-291x(03)01008-8. [DOI] [PubMed] [Google Scholar]
- 143.Kong XT. Fang HT. Jiang GQ. Zhai SZ. O'Connell DL. Brewster DR. Treatment of acute bronchiolitis with Chinese herbs. Arch Dis Child. 1993;68:468–471. doi: 10.1136/adc.68.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kooy NW. Royall JA. Ischiropoulos H. Oxidation of 2',7'-dichlorofluorescin by peroxynitrite. Free Rad Res. 1997;27:245–254. doi: 10.3109/10715769709065763. [DOI] [PubMed] [Google Scholar]
- 145.Kresfelder TL. Janssen R. Bont L. Venter M. Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. J Med Virol. 2011;83:1834–1840. doi: 10.1002/jmv.22179. [DOI] [PubMed] [Google Scholar]
- 146.Kumar P. Khanna M. Srivastava V. Tyagi YK. Raj HG. Ravi K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res. 2005;31:449–459. doi: 10.1080/019021490927088. [DOI] [PubMed] [Google Scholar]
- 147.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 148.Ledo A. Arduini A. Asensi MA. Sastre J. Escrig R. Brugada M. Aguar M. Saenz P. Vento M. Human milk enhances antioxidant defenses against hydroxyl radical aggression in preterm infants. Am J Clin Nutr. 2009;89:210–215. doi: 10.3945/ajcn.2008.26845. [DOI] [PubMed] [Google Scholar]
- 149.Leonard WJ. O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 150.Liby KT. Yore MM. Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 151.Lin R. Heylbroeck C. Genin P. Pitha PM. Hiscott J. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Molec Cell Biol. 1999;19:959–966. doi: 10.1128/mcb.19.2.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu P. Jamaluddin M. Li K. Garofalo RP. Casola A. Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu P. Li K. Garofalo RP. Brasier AR. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I·NF- kappa B-inducing kinase signaling pathway. J Biol Chem. 2008;283:23169–23178. doi: 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu T. Castro S. Brasier AR. Jamaluddin M. Garofalo RP. Casola A. ROS mediate viral-induced stat activation: role of tyrosine phosphatases. J Biol Chem. 2003;279:2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 155.Liu T. Castro S. Brasier AR. Jamaluddin M. Garofalo RP. Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem. 2004;279:2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 156.Lopez-Guerrero JA. Carrasco L. Effect of nitric oxide on poliovirus infection of two human cell lines. J Virol. 1998;72:2538–2540. doi: 10.1128/jvi.72.3.2538-2540.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu R. Au WC. Yeow WS. Hageman N. Pitha PM. Regulation of the promoter activity of interferon regulatory factor-7 gene: activation by interferon and silencing by hypermethylation. J Biol Chem. 2000;275:31805–31812. doi: 10.1074/jbc.M005288200. [DOI] [PubMed] [Google Scholar]
- 158.Majano PL. Garcia-Monzon C. Lopez-Cabrera M. Lara-Pezzi E. Fernandez-Ruiz E. Garcia-Iglesias C. Borque MJ. Moreno-Otero R. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a virus-induced gene upregulation. J Clin Invest. 1998;101:1343–1352. doi: 10.1172/JCI774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marchesi E. Rota C. Fann YC. Chignell CF. Mason RP. Photoreduction of the fluorescent dye 2'-7'-dichlorofluorescein: a spin trapping and direct elecgron spin resonance study with implications for oxidative stress measurements. Free Radic Biol Med. 1999;26:148–161. doi: 10.1016/s0891-5849(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 160.Marzec JM. Christie JD. Reddy SP. Jedlicka AE. Vuong H. Lanken PN. Aplenc R. Yamamoto T. Yamamoto M. Cho HY. Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 161.Mastronarde JG. He B. Monick MM. Mukaida N. Matsushima K. Hunninghake GW. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-kB and NF-IL-6. J Infect Dis. 1996;174:262–267. doi: 10.1093/infdis/174.2.262. [DOI] [PubMed] [Google Scholar]
- 162.Mastronarde JG. Monick MM. Hunninghake GW. Oxidant tone regulates IL-8 production in epithelium infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:237–244. doi: 10.1165/ajrcmb.13.2.7626291. [DOI] [PubMed] [Google Scholar]
- 163.Mastronarde JG. Monick MM. Mukaida N. Matsushima K. Hunninghake GW. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J Infect Dis. 1998;177:1275–1281. doi: 10.1086/515279. [DOI] [PubMed] [Google Scholar]
- 164.Mata M. Morcillo E. Gimeno C. Cortijo J. N-acetyl-l-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV) Biochem Pharmacol. 2011;82:548–555. doi: 10.1016/j.bcp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 165.Maziere C. Alimardani G. Dantin F. Dubois F. Conte MA. Maziere JC. Oxidized LDL activates STAT1 and STAT3 transcription factors: possible involvement of reactive oxygen species. FEBS Lett. 1999;448:49–52. doi: 10.1016/s0014-5793(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 166.Maziere C. Conte MA. Maziere JC. Activation of JAK2 by the oxidative stress generated with oxidized low- density lipoprotein. Free Radic Biol Med. 2001;31:1334–1340. doi: 10.1016/s0891-5849(01)00649-9. [DOI] [PubMed] [Google Scholar]
- 167.McNamara PS. Flanagan BF. Hart CA. Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191:1225–1232. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 168.Meng TC. Fukada T. Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 169.Miller AL. Strieter RM. Gruber AD. Ho SB. Lukacs NW. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 170.Miyairi I. Devincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moghaddam A. Olszewska W. Wang B. Tregoning JS. Helson R. Sattentau QJ. Openshaw PJ. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 172.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morichi S. Kawashima H. Ioi H. Ushio M. Yamanaka G. Kashiwagi Y. Takekuma K. Hoshika A. Watanabe Y. Cerebrospinal fluid NOx (nitrite/nitrate) in RSV-infected children with CNS symptoms. J Infect. 2009;59:299–301. doi: 10.1016/j.jinf.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 174.Mulier B. Rahman I. Watchorn T. Donaldson K. MacNee W. Jeffery PK. Hydrogen peroxide-induced epithelial injury: the protective role of intracellular nonprotein thiols (NPSH) Eur Respir J. 1998;11:384–391. doi: 10.1183/09031936.98.11020384. [DOI] [PubMed] [Google Scholar]
- 175.Mullooly JP. Bridges CB. Thompson WW. Chen J. Weintraub E. Jackson LA. Black S. Shay DK. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 176.Nair H. Nokes DJ. Gessner BD. Dherani M. Madhi SA. Singleton RJ. O'Brien KL. Roca A. Wright PF. Bruce N. Chandran A. Theodoratou E. Sutanto A. Sedyaningsih ER. Ngama M. Munywoki PK. Kartasasmita C. Simoes EA. Rudan I. Weber MW. Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nakamura H. Tamura S. Watanabe I. Iwasaki T. Yodoi J. Enhanced resistancy of thioredoxin-transgenic mice against influenza virus-induced pneumonia. Immunol Lett. 2002;82:165–170. doi: 10.1016/s0165-2478(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 178.Neilson KA. Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- 179.Neuzil KM. Gruber WC. Chytil F. Stahlman MT. Engelhardt B. Graham BS. Serum vitamin A levels in respiratory syncytial virus infection. J Pediatr. 1994;124:433–436. doi: 10.1016/s0022-3476(94)70369-8. [DOI] [PubMed] [Google Scholar]
- 180.Nguyen H. Hiscott J. Pithas PM. The growing family of interferon regulatory factors. Cytokine Growth Ed Rev. 1997;4:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 181.Nichols DP. Ziady AG. Shank SL. Eastman JF. Davis PB. The triterpenoid CDDO limits inflammation in preclinical models of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2009;297:L828–L836. doi: 10.1152/ajplung.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ninomiya-Tsuji J. Kishimoto K. Hiyama A. Inoue J. Cao Z. Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 183.Noah TL. Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 184.Noah TL. Henderson FW. Wortman IA. Devlin RB. Handy J. Koren HS. Becker S. Nasal cytokine production in virus acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 185.Olszewska-Pazdrak B. Casola A. Saito T. Alam R. Crowe SE. Mei F. Ogra PL. Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Palamara AT. Nencioni L. Aquilano K. De CG. Hernandez L. Cozzolino F. Ciriolo MR. Garaci E. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 187.Peebles RS., Jr. Moore ML. A mechanistic advance in understanding RSV pathogenesis, but still a long way from therapy. Am J Respir Cell Mol Biol. 2007;37:375–377. doi: 10.1165/rcmb.2007-0003ED. [DOI] [PubMed] [Google Scholar]
- 188.Pelletier AJ. Mansbach JM. Camargo CA., Jr Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 189.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr. 1997;127:962S–965S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 190.Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res. 1997;56:107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- 191.Peters RT. Liao SM. Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 192.Phipps S. Lam CE. Mahalingam S. Newhouse M. Ramirez R. Rosenberg HF. Foster PS. Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 193.Poole LB. Hall A. Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol Chapter. 2011;7 doi: 10.1002/0471140856.tx0709s49. Unit 7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Prasad Gabbita S. Robinson KA. Stewart CA. Floyd RA. Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–136. doi: 10.1006/abbi.1999.1685. [DOI] [PubMed] [Google Scholar]
- 195.Prince GA. An update on respiratory syncytial virus antiviral agents. Expert Opin Investig Drugs. 2001;10:297–308. doi: 10.1517/13543784.10.2.297. [DOI] [PubMed] [Google Scholar]
- 196.Quinlan KP. Hayani KC. Vitamin A and respiratory syncytial virus infection. Serum levels and supplementation trial. Arch Pediatr Adolesc Med. 1996;150:25–30. doi: 10.1001/archpedi.1996.02170260029004. [DOI] [PubMed] [Google Scholar]
- 197.Raza MW. Essery SD. Weir DM. Ogilvie MM. Elton RA. Blackwell CC. Infection with respiratory syncytial virus and water-soluble components of cigarette smoke alter production of tumour necrosis factor alpha and nitric oxide by human blood monocytes. FEMS Immunol Med Microbiol. 1999;24:387–394. doi: 10.1111/j.1574-695X.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 198.Reddy NM. Suryanaraya V. Yates MS. Kleeberger SR. Hassoun PM. Yamamoto M. Liby KT. Sporn MB. Kensler TW. Reddy SP. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:867–874. doi: 10.1164/rccm.200905-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rius J. Guma M. Schachtrup C. Akassoglou K. Zinkernagel AS. Nizet V. Johnson RS. Haddad GG. Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Robinson KA. Stewart CA. Pye Q. Floyd RA. Hensley K. Basal protein phosphorylation is decreased and phosphatase activity increased by an antioxidant and a free radical trap in primary rat glia. Arch Biochem Biophys. 1999;365:211–215. doi: 10.1006/abbi.1999.1178. [DOI] [PubMed] [Google Scholar]
- 201.Rubbo H. Darley-Usmar V. Freeman BA. Nitric oxide regulation of tissue free radical injury. Chem Res Toxicol. 1996;9:809–820. doi: 10.1021/tx960037q. [DOI] [PubMed] [Google Scholar]
- 202.Rudd BD. Burstein E. Duckett CS. Li X. Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saito T. Deskin RW. Casola A. Haeberle H. Olszewska B. Ernst PB. Alam R. Ogra PL. Garofalo R. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 204.Sakurai H. Chiba H. Miyoshi H. Sugita T. Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 205.Schieffer B. Luchtefeld M. Braun S. Hilfiker A. Hilfiker-Kleiner D. Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–1201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 206.Schremmer B. Manevich Y. Feinstein SI. Fisher AB. Peroxiredoxins in the lung with emphasis on peroxiredoxin VI. Subcell Biochem. 2007;44:317–344. doi: 10.1007/978-1-4020-6051-9_15. [DOI] [PubMed] [Google Scholar]
- 207.Semenza GL. O2 sensing: only skin deep? Cell. 2008;133:206–208. doi: 10.1016/j.cell.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 208.Servant MJ. ten Oever B. LePage C. Conti L. Gessani S. Julkunen I. Lin R. Hiscott J. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J Biol Chem. 2001;276:355–363. doi: 10.1074/jbc.M007790200. [DOI] [PubMed] [Google Scholar]
- 209.Sharma S. ten Oever BR. Grandvaux N. Zhou GP. Lin R. Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 210.Shay DK. Holman RC. Newman RD. Liu LL. Stout JW. Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 211.Sheeran P. Jafri H. Carubelli C. Saavedra J. Johnson C. Krisher K. Sanchez PJ. Ramilio MO. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 212.Shimada T. Kawai T. Takeda K. Matsumoto M. Inoue J. Tatsumi Y. Kanamaru A. Akira S. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 213.Shlomai J. Redox control of protein-DNA interactions: from molecular mechanisms to significance in signal transduction, gene expression, and DNA replication. Antioxid Redox Signal. 2010;13:1429–1476. doi: 10.1089/ars.2009.3029. [DOI] [PubMed] [Google Scholar]
- 214.Simoes EA. RSV disease in the pediatric population: epidemiology, seasonal variability, and long-term outcomes. Manag Care. 2008;17:3–6. discussion. [PubMed] [Google Scholar]
- 215.Simon AR. Rai U. Fanburg BL. Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 216.Sitkovsky MV. Lukashev D. Apasov S. Kojima H. Koshiba M. Caldwell C. Ohta A. Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 217.Song W. Liu G. Bosworth CA. Walker JR. Megaw GA. Lazrak A. Abraham E. Sullender WM. Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem. 2009;284:7294–7306. doi: 10.1074/jbc.M806816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Soucy-Faulkner A. Mukawera E. Fink K. Martel A. Jouan L. Nzengue Y. Lamarre D. Vande VC. Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sow FB. Gallup JM. Krishnan S. Patera AC. Suzich J. Ackermann MR. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro-inflammatory response in the lungs of preterm lambs. Respir Res. 2011;12:106. doi: 10.1186/1465-9921-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sporn MB. Liby KT. Yore MM. Fu L. Lopchuk JM. Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74:537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stamler JS. Lamas S. Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 222.Stark JM. Khan AM. Chiappetta CL. Xue H. Alcorn JL. Colasurdo GN. Immune and functional role of nitric oxide in a mouse model of respiratory syncytial virus infection. J Infect Dis. 2005;191:387–395. doi: 10.1086/427241. [DOI] [PubMed] [Google Scholar]
- 223.Stey C. Steurer J. Bachmann S. Medici TC. Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J. 2000;16:253–262. doi: 10.1034/j.1399-3003.2000.16b12.x. [DOI] [PubMed] [Google Scholar]
- 224.Stokes KL. Chi MH. Sakamoto K. Newcomb DC. Currier MG. Huckabee MM. Lee S. Goleniewska K. Pretto C. Williams JV. Hotard A. Sherrill TP. Peebles RS., Jr. Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Stuehr DJ. Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- 226.Suliman HB. Ryan LK. Bishop L. Folz RJ. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L69–L78. doi: 10.1152/ajplung.2001.280.1.L69. [DOI] [PubMed] [Google Scholar]
- 227.Surh YJ. NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr. 2008;17(Suppl 1):269–272. [PubMed] [Google Scholar]
- 228.Sussan TE. Rangasamy T. Blake DJ. Malhotra D. El-Haddad H. Bedja D. Yates MS. Kombairaju P. Yamamoto M. Liby KT. Sporn MB. Gabrielson KL. Champion HC. Tuder RM. Kensler TW. Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tan DX. Manchester LC. Terron MP. Flores LJ. Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 230.Taylor BS. Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock. 2000;13:413–424. doi: 10.1097/00024382-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 231.Tekkanat KK. Maassab H. Miller A. Berlin AA. Kunkel SL. Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur J Immunol. 2002;32:3276–3284. doi: 10.1002/1521-4141(200211)32:11<3276::AID-IMMU3276>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 232.Teran LM. Seminario MC. Shute JK. Papi A. Compton SJ. Low JL. Gleich GJ. Johnston SL. RANTES, macrophage-inhibitory protein 1alpha, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J Infect Dis. 1999;179:677–681. doi: 10.1086/314618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tian B. Zhang Y. Luxon B. Garofalo RP. Casola A. Sinha M. Brasier AR. Identification of NF-kB dependent gene networks in respiratory syncytial virus-infected cells. J Virol. 2002;76:6800–6814. doi: 10.1128/JVI.76.13.6800-6814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Torrence PF. Powell LD. The quest for an efficacious antiviral for respiratory syncytial virus. Antivir Chem Chemother. 2002;13:325–344. doi: 10.1177/095632020201300601. [DOI] [PubMed] [Google Scholar]
- 235.Tsutsumi H. Takeuchi R. Ohsaki M. Seki K. Chiba S. Respiratory syncytial virus infection of human respiratory epithelial cells enhances inducible nitric oxide synthase gene expression. J Leukoc Biol. 1999;66:99–104. [PubMed] [Google Scholar]
- 236.Uchide N. Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules. 2011;16:2032–2052. doi: 10.3390/molecules23100000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.van Schaik SM. Tristram DA. Nagpal IS. Hintz KM. Welliver RC., 2nd Welliver RC. Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630–636. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 238.Verhagen H. Schilderman PA. Kleinjans JC. Butylated hydroxyanisole in perspective. Chem Biol Interact. 1991;80:109–134. doi: 10.1016/0009-2797(91)90019-4. [DOI] [PubMed] [Google Scholar]
- 239.Vermeulen L. De Wilde G. Van Damme P. Vanden Berghe W. Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vlahos R. Stambas J. Bozinovski S. Broughton BR. Drummond GR. Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wada N. Matsumura M. Ohba Y. Kobayashi N. Takizawa T. Nakanishi N. Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 242.Wakabayashi N. Slocum SL. Skoko JJ. Shin S. Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Walmsley SR. Print C. Farahi N. Peyssonnaux C. Johnson RS. Cramer T. Sobolewski A. Condliffe AM. Cowburn AS. Johnson N. Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang D. Baldwin AS., Jr Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 245.Wang D. Westerheide SD. Hanson JL. Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 246.Wang EE. Law BJ. Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995;126:212–219. doi: 10.1016/s0022-3476(95)70547-3. [DOI] [PubMed] [Google Scholar]
- 247.Waris G. Huh KW. Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Welliver RC. Pharmacotherapy of respiratory syncytial virus infection. Curr Opin Pharmacol. 2010;10:289–293. doi: 10.1016/j.coph.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 249.Welliver RC. Hintz KH. Glori M. Welliver RC., Sr Zileuton reduces respiratory illness and lung inflammation, during respiratory syncytial virus infection, in mice. J Infect Dis. 2003;187:1773–1779. doi: 10.1086/375277. [DOI] [PubMed] [Google Scholar]
- 250.Welliver TP. Garofalo RP. Hosakote Y. Hintz KH. Avendano L. Sanchez K. Velozo L. Jafri H. Chavez-Bueno S. Ogra PL. McKinney L. Reed JL. Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wietek C. Cleaver CS. Ludbrook V. Wilde J. White J. Bell DJ. Lee M. Dickson M. Ray KP. O'Neill LA. IkappaB kinase epsilon interacts with p52 and promotes transactivation via p65. J Biol Chem. 2006;281:34973–34981. doi: 10.1074/jbc.M607018200. [DOI] [PubMed] [Google Scholar]
- 252.Wood LG. Wark PA. Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010;13:1535–1548. doi: 10.1089/ars.2009.3064. [DOI] [PubMed] [Google Scholar]
- 253.Wyde PR. Moore DK. Pimentel DM. Gilbert BE. Nimrod R. Panet A. Recombinant superoxide dismutase (SOD) administered by aerosol inhibits respiratory syncytial virus infection in cotton rats. Antiviral Res. 1996;31:173–184. doi: 10.1016/0166-3542(95)06967-4. [DOI] [PubMed] [Google Scholar]
- 254.Yoboua F. Martel A. Duval A. Mukawera E. Grandvaux N. Respiratory syncytial virus-mediated NF-kappaB p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6 and IKKbeta. J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zaki MH. Akuta T. Akaike T. Nitric oxide-induced nitrative stress involved in microbial pathogenesis. J Pharmacol Sci. 2005;98:117–129. doi: 10.1254/jphs.crj05004x. [DOI] [PubMed] [Google Scholar]
- 256.Zang N. Xie X. Deng Y. Wu S. Wang L. Peng C. Li S. Ni K. Luo Y. Liu E. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J Virol. 2011;85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang Y. Luxon B. Casola A. Garofalo RP. Jamaluddin M. Brasier AR. Expression of RSV-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang L. Peeples ME. Boucher RC. Collins PL. Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhong H. Suyang H. Erdjument-Bromage H. Tempst P. Ghosh The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 260.Zhong H. Voll RE. Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Molecular Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]