Abstract
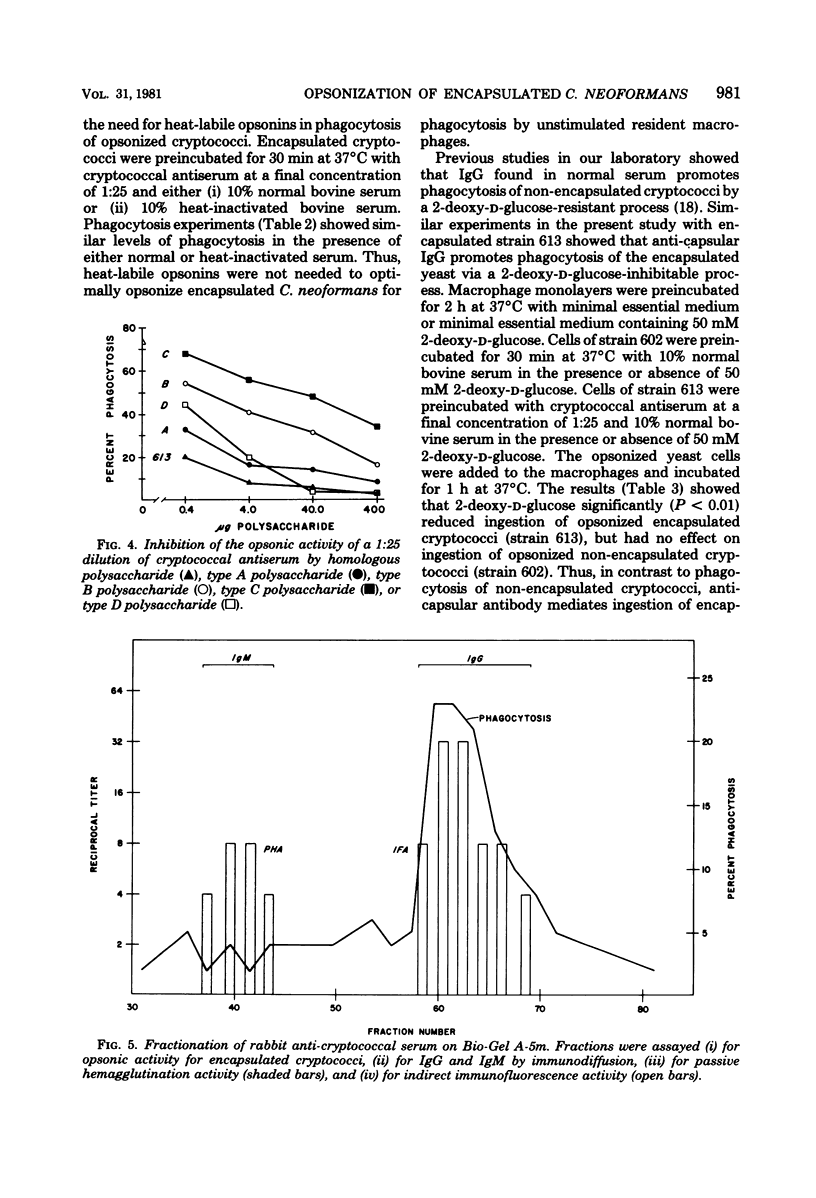
Antisera prepared in rabbits against either whole encapsulated cells of Cryptococcus neoformans or purified cryptococcal polysaccharide were opsonic for the encapsulated yeast. The opsonic activity was removed by absorption with whole cryptococci and was inhibited by free polysaccharide. As little as 0.13 microgram of cryptococcal polysaccharide produced a 50% inhibition of opsonization. Various degrees of neutralization by polysaccharides from the four cryptococcal serotypes suggested that the opsonins were type specific. Fractionation of antiserum on Bio-Gel A-5m (Bio-Rad Laboratories) and diethylaminoethyl cellulose showed that the opsonins were antibodies of the immunoglobulin G class. These opsonizing antibodies did not require heat-labile serum components for optimal phagocytosis of the yeast. Inhibition studies using 2-deoxy-D-glucose demonstrated that ingestion of encapsulated cryptococci opsonized with anticapsular antibody was a 2-deoxy-D-glucose-inhibitable process. This result differed from similar studies with non-encapsulated cryptococci which showed that ingestion of non-encapsulated cryptococci opsonized with normal serum was not inhibited by 2-deoxy-D-glucose.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Immunological and biochemical studies of meningococcal C polysaccharides isolated by diethylaminoethyl chromatography. Infect Immun. 1976 Jul;14(1):106–113. doi: 10.1128/iai.14.1.106-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT J. E., HASENCLEVER H. F. CRYPTOCOCCUS NEOFORMANS POLYSACCHARIDE: STUDIES OF SEROLOGIC PROPERTIES AND ROLE IN INFECTION. J Immunol. 1965 Jun;94:916–920. [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Bindschadler D. D., Bennett J. E. Serology of human cryptococcosis. Ann Intern Med. 1968 Jul;69(1):45–52. doi: 10.7326/0003-4819-69-1-45. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Diamond R. D. Effects of stimulation and suppression of cell-mediated immunity on experimental cryptococcosis. Infect Immun. 1977 Jul;17(1):187–194. doi: 10.1128/iai.17.1.187-194.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Goren M. B., Middlebrook G. M. Protein conjugates of polysaccharide from Cryptococcus neoforman s. J Immunol. 1967 May;98(5):901–913. [PubMed] [Google Scholar]
- Goren M. B., Warren J. Immunofluorescence studies of reactions at the Cryptococcal capsule. J Infect Dis. 1968 Apr;118(2):215–229. doi: 10.1093/infdis/118.2.215. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide. I. Serological assay for antigen and antibody. Infect Immun. 1972 Jan;5(1):35–41. doi: 10.1128/iai.5.1.35-41.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga D. P., Kumar R., Mohapatra L. N., Malaviya A. N. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect Immun. 1979 Oct;26(1):1–3. doi: 10.1128/iai.26.1.1-3.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson-Porat S., Shahar A., Aronson M. Formation of histiocyte rings in response to Cryptococcus neoformans infection. J Reticuloendothel Soc. 1965 Sep;2(3):249–255. [PubMed] [Google Scholar]
- Swenson F. J., Kozel T. R. Phagocytosis of Cryptococcus neoformans by normal and thioglycolate-activated macrophages. Infect Immun. 1978 Sep;21(3):714–720. doi: 10.1128/iai.21.3.714-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J. E., Jones R. D. Serodiagnosis of clinical cryptococcosis. Am Rev Respir Dis. 1968 Feb;97(2):275–282. doi: 10.1164/arrd.1968.97.2.275. [DOI] [PubMed] [Google Scholar]


