Abstract
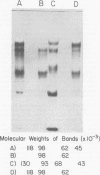
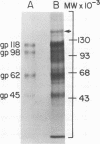
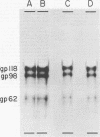
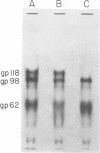
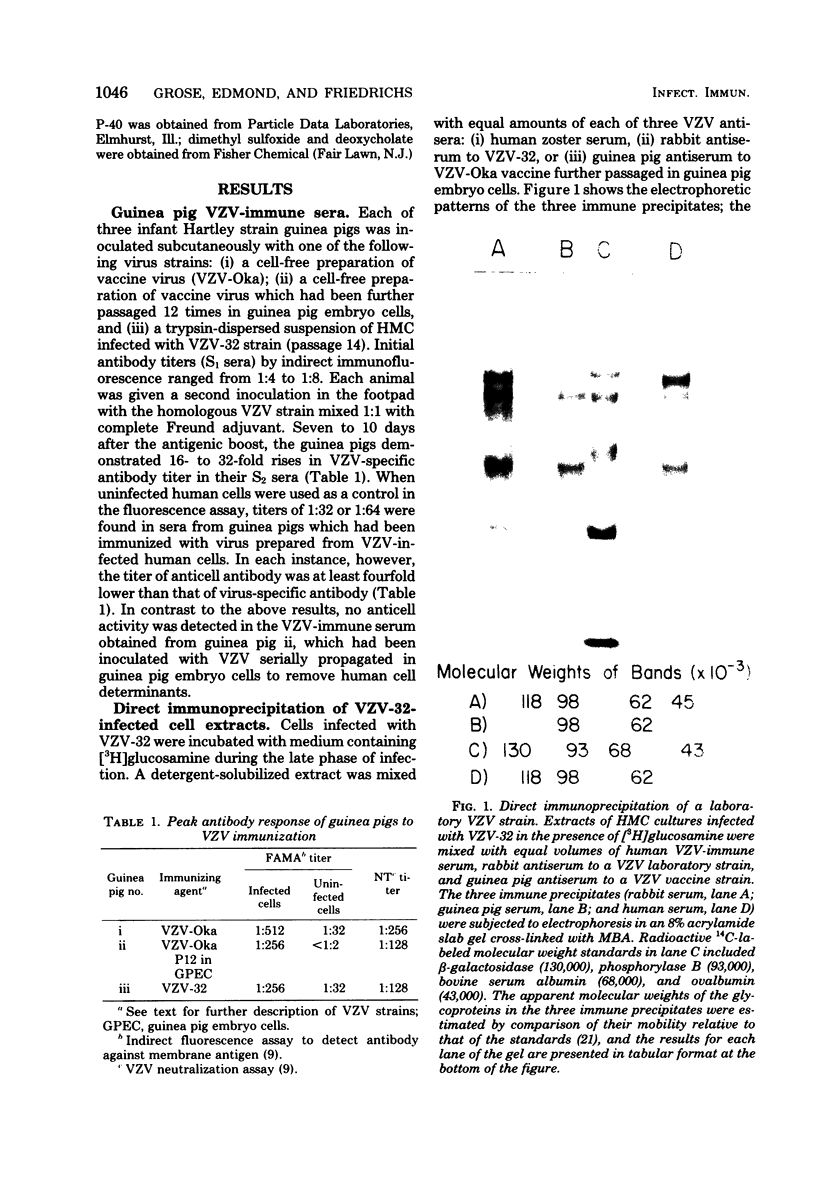
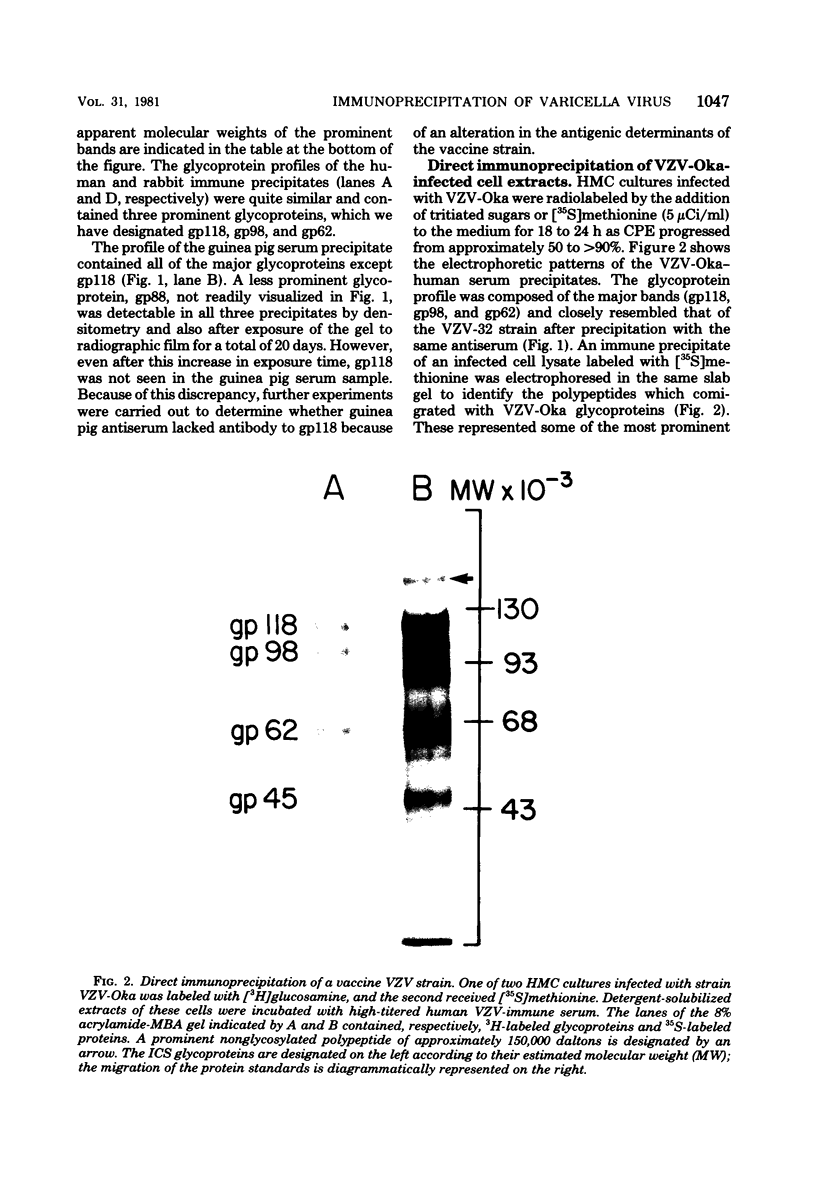
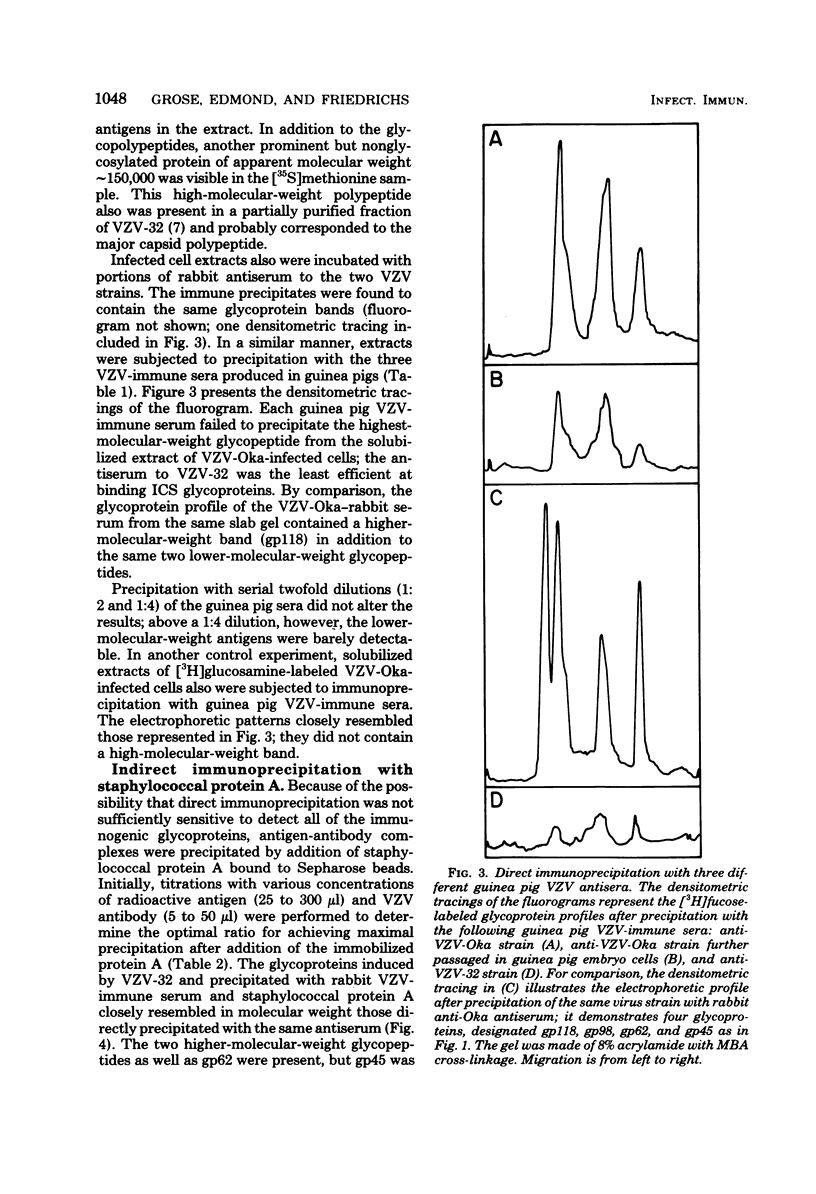
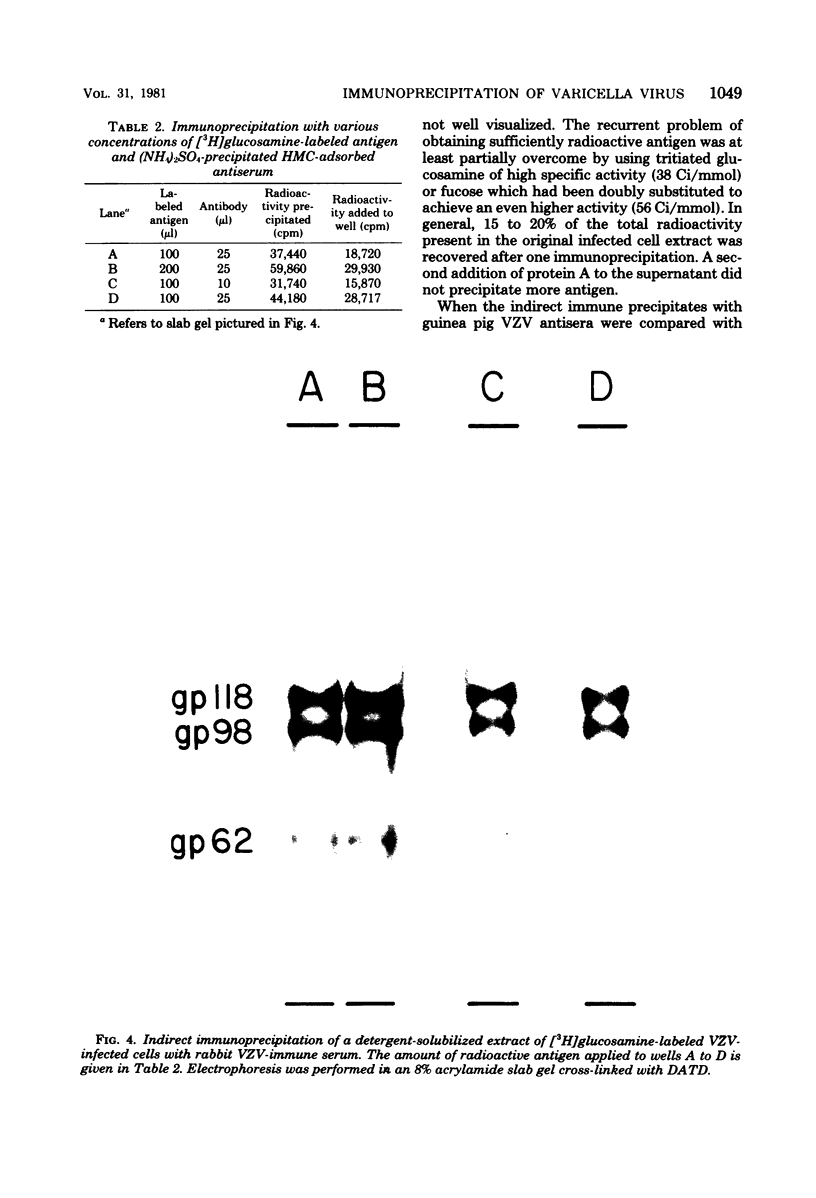
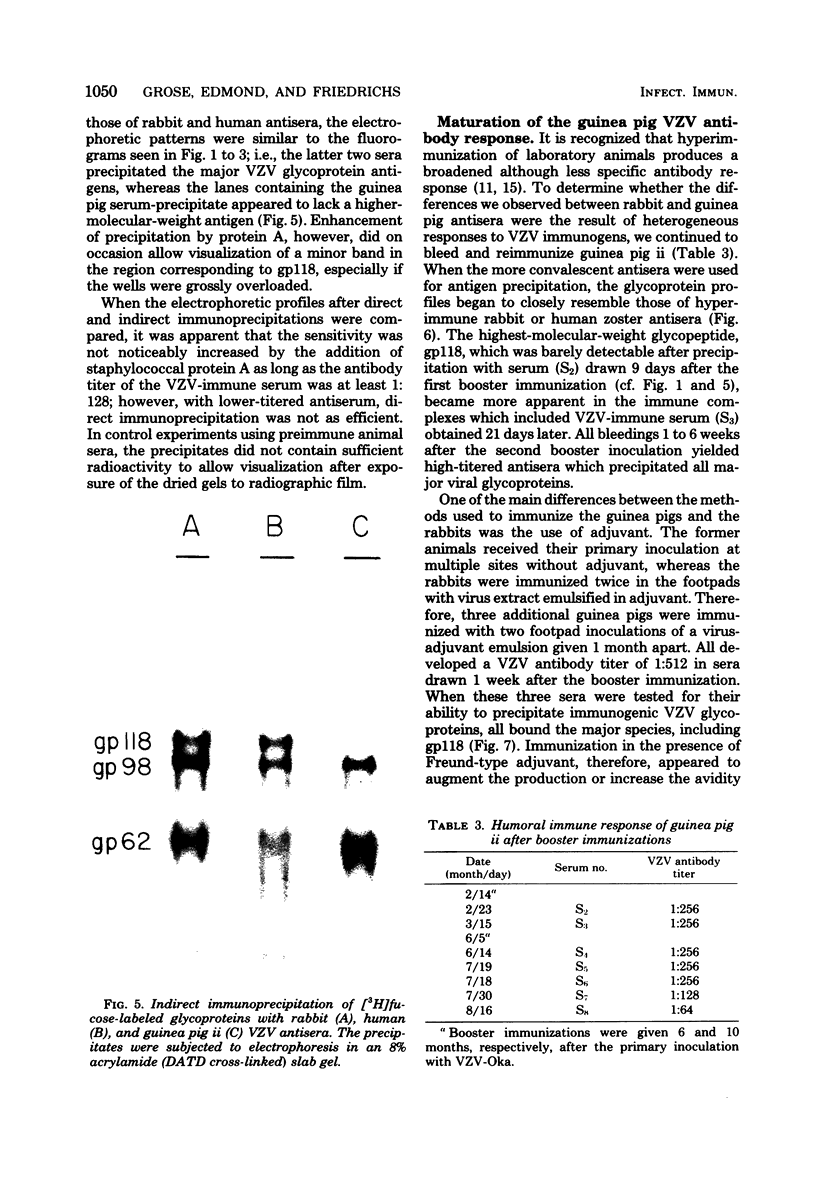
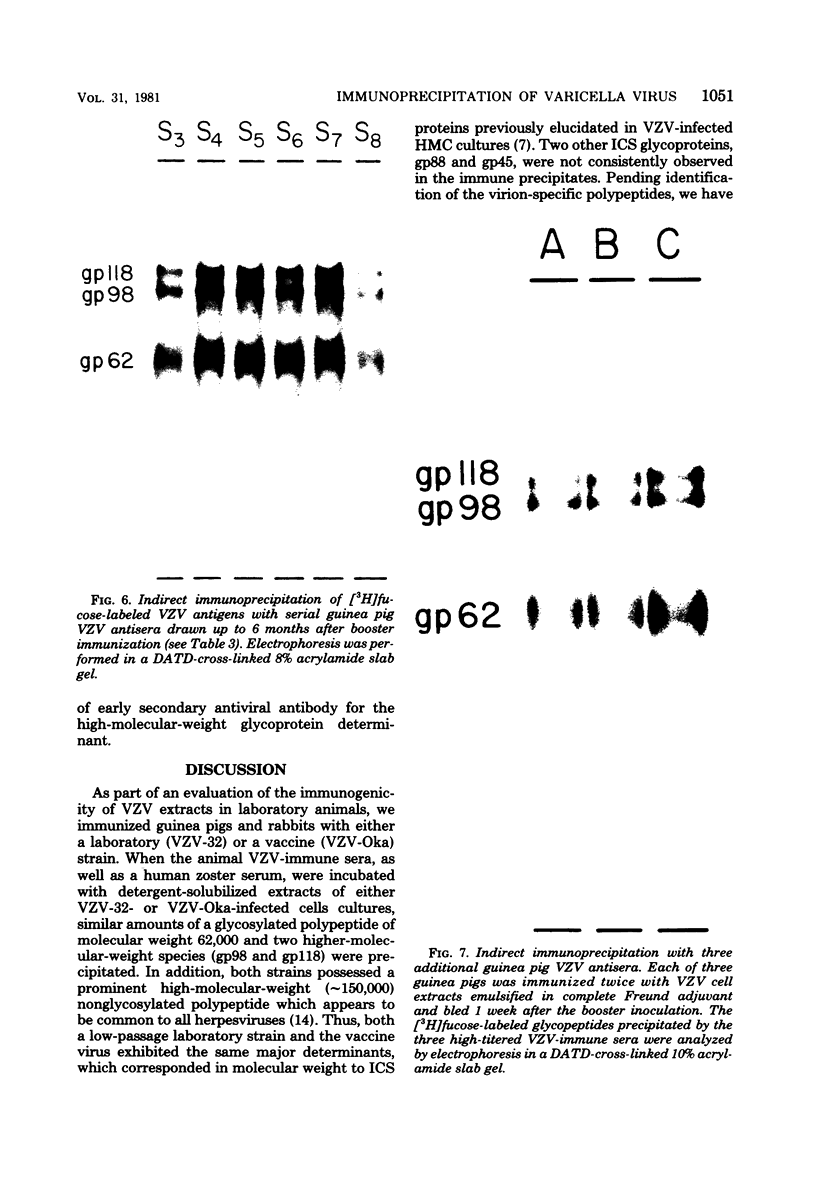
High-titered antisera were prepared in guinea pigs and rabbits against two strains of varicella-zoster virus (VZV): VZV-32, a low-passage laboratory strain, and VZV-Oka, a vaccine strain attenuated by passage in both human and guinea pig embryo cells. When the animal VZV-immune sera, as well as a human zoster serum, were used to precipitate radiolabeled glycoproteins from VZV-infected cells and the immune precipitates were analyzed by polyacrylamide gel electrophoresis and fluorography, it was observed that cell cultures infected with either strain had similar electrophoretic profiles containing major glycoproteins of approximate molecular weights 62,000, 98,000, and 118,000. A prominent high-molecular-weight (approximately 150,000) nonglycosylated polypeptide was identified in both strains also. These determinants were demonstrable by both indirect (staphylococcal protein A-antibody adsorbent) and direct immunoprecipitation, as long as VZV-immune sera with an antibody titer greater than or equal to 1:128 were used. Further analysis of individual caviid VZV antisera demonstrated some heterogeneity which appeared to be related to the method of immunization rather than the level of virus-specific antibody. VZV extracts emulsified with complete Freund adjuvant elicited an antibody response to all major immunogenic viral glycoproteins, whereas guinea pigs inoculated with virus alone during the primary immunization initially produced VZV antibody which failed to precipitate the highest-molecular-weight glycoprotein (gp118). Thus, Freund-type adjuvants promoted the maturation of the humoral immune response after VZV immunization in outbred guinea pigs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Asano Y., Takahashi M. Clinical and serologic testing of a live varicella vaccine and two-year follow-up for immunity of the vaccinated children. Pediatrics. 1977 Dec;60(6):810–814. [PubMed] [Google Scholar]
- Asano Y., Takahashi M. Studies on the polypeptides of varicella-zoster (V-Z) virus. 1. Detection of varicella-zoster virus polypeptides in infected cells. Biken J. 1979 Sep;22(3):81–89. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Grose C., Brunel P. A. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect Immun. 1978 Jan;19(1):199–203. doi: 10.1128/iai.19.1.199-203.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edmond B. J., Brunell P. A. Complement-enhanced neutralizing antibody response to varicella-zoster virus. J Infect Dis. 1979 Apr;139(4):432–437. doi: 10.1093/infdis/139.4.432. [DOI] [PubMed] [Google Scholar]
- Grose C., Perrotta D. M., Brunell P. A., Smith G. C. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol. 1979 Apr;43(1):15–27. doi: 10.1099/0022-1317-43-1-15. [DOI] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Just M., Berger-Hernández R., Buergin-Wolff A. Initial experience with varicella immunization. Monogr Paediatr. 1979;11:54–57. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Killington R. A., Yeo J., Honess R., Watson D. H., Duncan B. E., Halliburton I. W., Mumford J. Comparative analyses of the proteins and antigens of five herpesviruses. J Gen Virol. 1977 Nov;37(2):297–310. doi: 10.1099/0022-1317-37-2-297. [DOI] [PubMed] [Google Scholar]
- LEONE C. A. Effect of multiple injections of antigen upon the specificity of antisera. J Immunol. 1952 Sep;69(3):285–295. [PubMed] [Google Scholar]
- Myers M. G., Duer H. L., Hausler C. K. Experimental infection of guinea pigs with varicella-zoster virus. J Infect Dis. 1980 Sep;142(3):414–420. doi: 10.1093/infdis/142.3.414. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Buchan A., Sim C., Watson D. H. Type-specific protein in herpes simplex virus envelope reacts with neutralising antibody. Nature. 1974 May 24;249(455):360–361. doi: 10.1038/249360a0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Okuno Y., Otsuka T., Osame J., Takamizawa A. Development of a live attenuated varicella vaccine. Biken J. 1975 Mar;18(1):25–33. [PubMed] [Google Scholar]
- WARD R., RADER D., LIPTON M. M., FREUND J. Formation of neutralizing antibody in monkeys injected with poliomyelitis virus and adjuvants. Proc Soc Exp Biol Med. 1950 Jul;74(3):536–539. doi: 10.3181/00379727-74-17964. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Matsunaga Y., Otsuka T., Takahashi M. Immune response of guinea pigs to varicella vaccine strain (OKA) and wild strains. Biken J. 1980 Mar;23(1):53–55. [PubMed] [Google Scholar]