Abstract
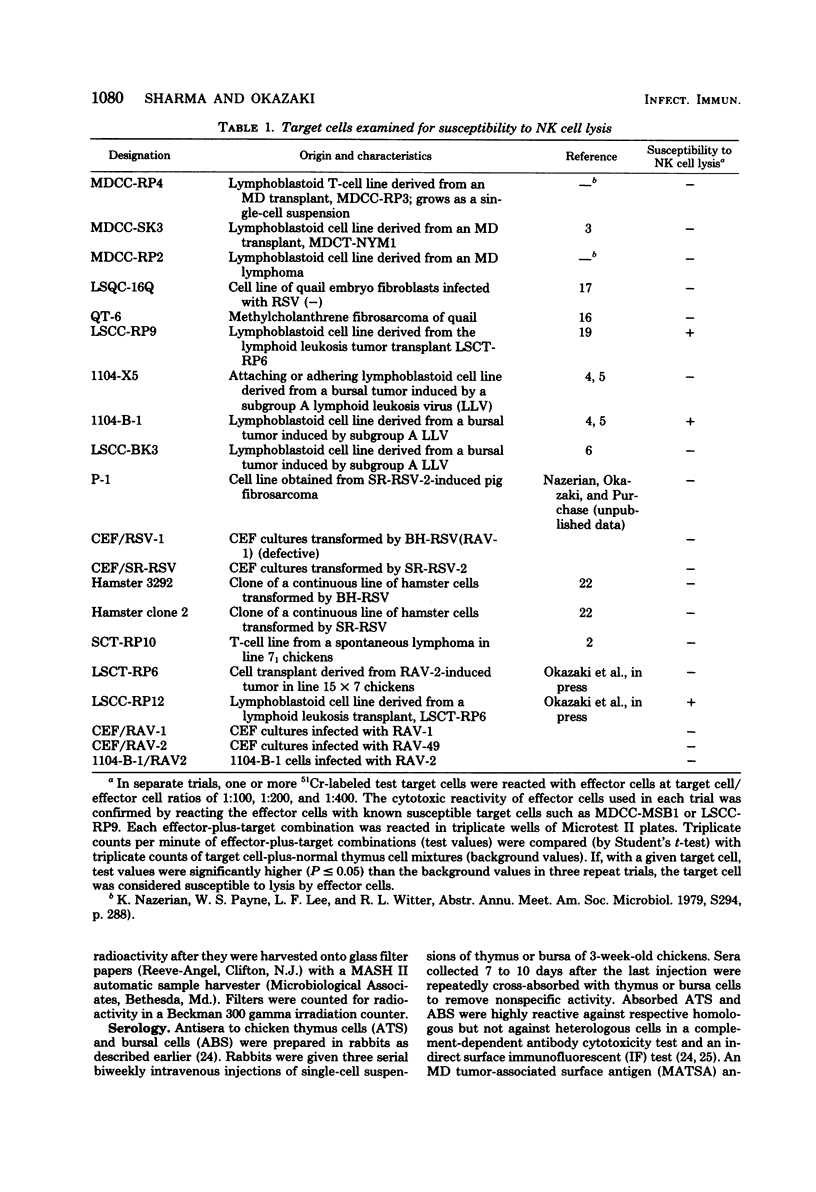
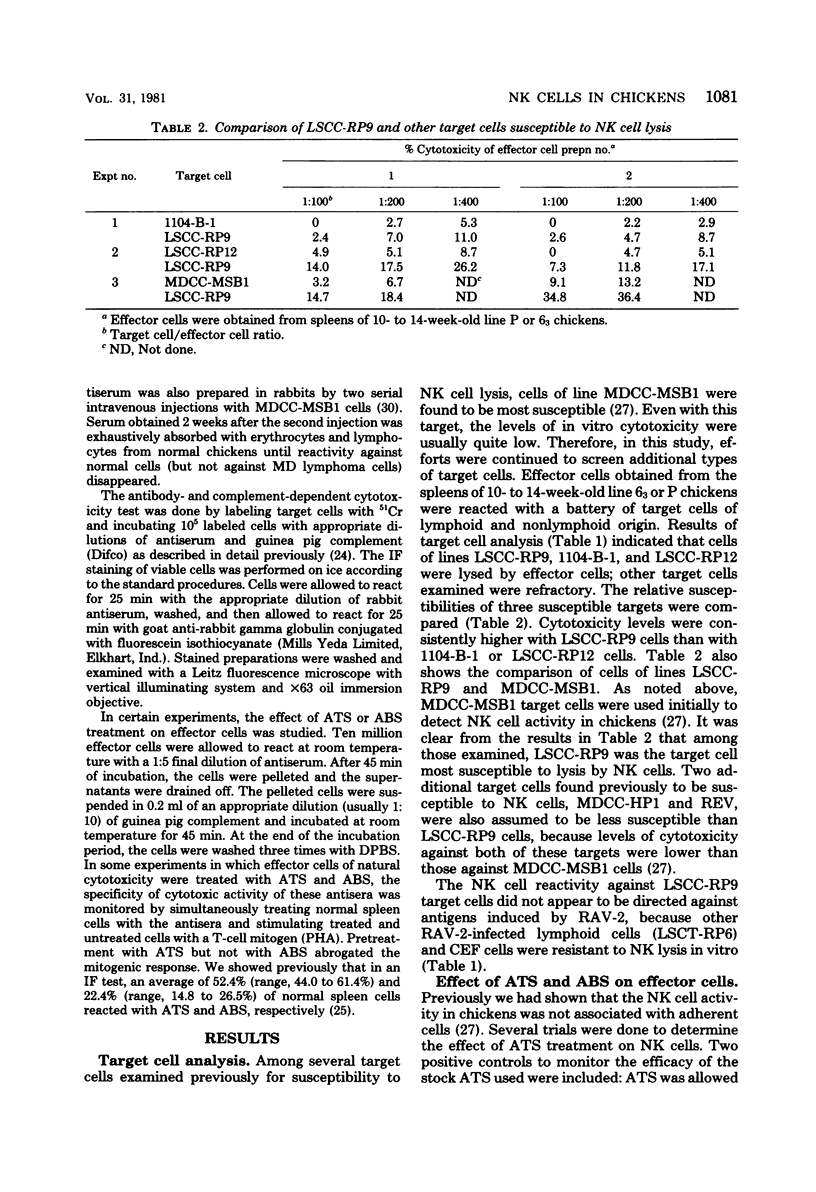
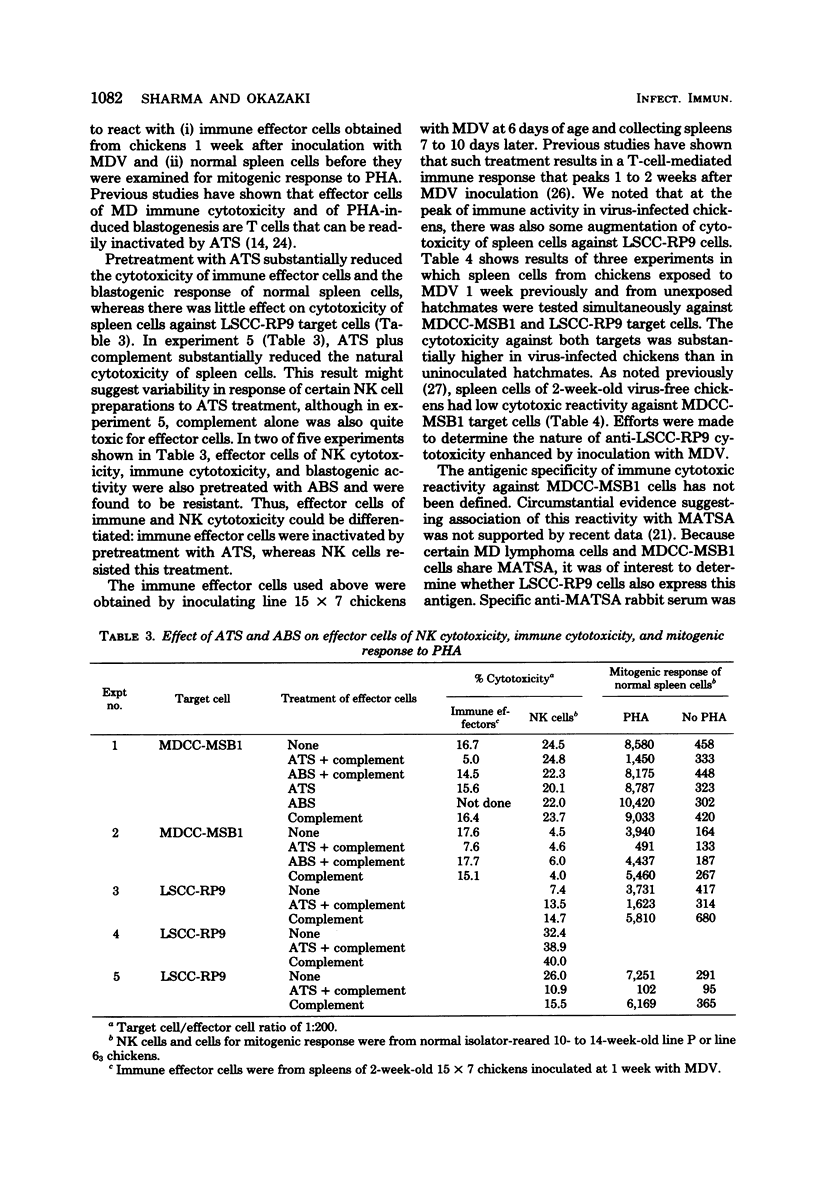
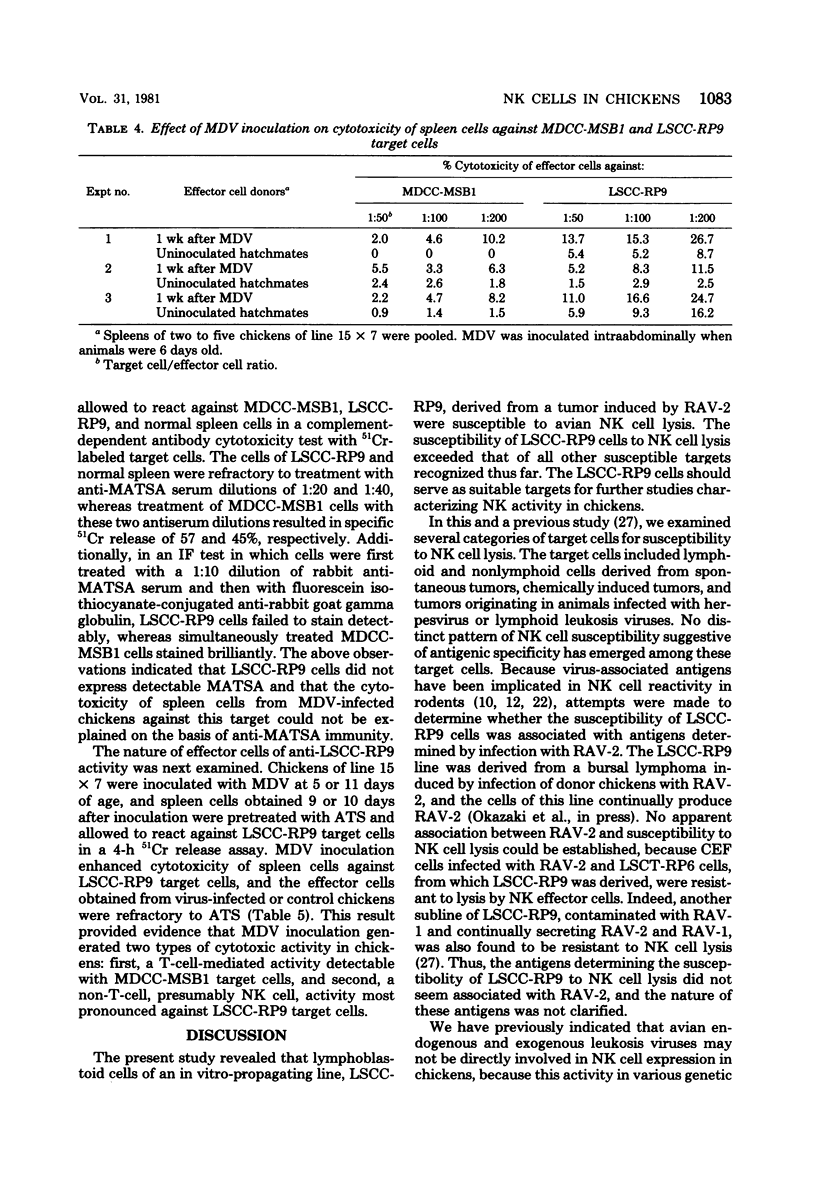
A battery of lymphoid and nonlymphoid cells was examined for susceptibility to lysis by natural killer cells of chickens. Several susceptible targets were recognized, and most susceptible among these were cells of line LSCC-RP9, derived from a lymphoid tumor induced by Rous-associated virus 2. The natural killer reactivity against LSCC-RP9 target cells did not appear to be directed against an antigen(s) induced by Rous-associated virus 2 because other lymphoid and nonlymphoid cells infected with this were resistant to lysis in vitro by natural killer cells. The effector cells of natural killer reactivity in chickens were refractory to treatment with potent anti-T-cell and anti-B-cell sera. Inoculation of Marek's disease virus in line 15 X 7 chickens resulted in enhanced natural killer cell activity, and the effector cells of this enhanced activity, and the effector cells of this enhanced activity were also resistant to anti-T-cell serum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Crittenden L. B., Witter R. L., Okazaki W., Neiman P. E. Lymphoid neoplasms in chicken flocks free of infection with exogenous avian tumor viruses. J Natl Cancer Inst. 1979 Jul;63(1):191–200. [PubMed] [Google Scholar]
- Hahn E. C., Jakowski R. M., Ramos L., Kenyon A. J. Lymphoproliferative diseases of fowl: characterization of transplantable G-B1 Marek's disease tumor cells in culture. Avian Dis. 1978 Jul-Sep;22(3):409–421. [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hihara H., Shimizu T., Yamamoto H. Establishment of tumor cell lines cultured from chickens with avian lymphoid leukosis. Natl Inst Anim Health Q (Tokyo) 1974 Winter;14(4):163–173. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Lam K. M., Linna T. J. Transfer of natural resistance to Marek's disease (JMV) with non-immune spleen cells. I. Studies of cell population transferring resistance. Int J Cancer. 1979 Nov 15;24(5):662–667. doi: 10.1002/ijc.2910240521. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Sharma J. M., Nazerian K., Witter R. L. Suppression of mitogen-induced proliferation of normal spleen cells by macrophages from chickens inoculated with Marek's disease virus. J Immunol. 1978 May;120(5):1554–1559. [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Vass W., Huebner R. J. Evidence for the in vitro transfer of defective Rous sarcoma virus genome from hamster tumor cells to chick cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1435–1442. doi: 10.1073/pnas.55.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K. A., Murthy K. K. In vitro cytotoxicity against Marek's disease lymphoblastoid cell lines after enzymatic removal of Marek's disease tumor-associated surface antigen. J Virol. 1980 Apr;34(1):130–135. doi: 10.1128/jvi.34.1.130-135.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendo F., Aoki T., Boyse E. A., Buafo C. K. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975 Sep;55(3):603–609. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- Sharma J. M. Cell-mediated immunity to tumor antigen in Marek's disease: susceptibility of effector cells to antithymocyte serum and enhancement of cytotoxic activity by Vibrio cholerae neuraminidase. Infect Immun. 1977 Oct;18(1):46–51. doi: 10.1128/iai.18.1.46-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. M., Coulson B. D. Cell-mediated cytotoxic response to cells bearing Marek's disease tumor-associated surface antigen in chickens infected with Marek's disease virus. J Natl Cancer Inst. 1977 Jun;58(6):1647–1651. doi: 10.1093/jnci/58.6.1647. [DOI] [PubMed] [Google Scholar]
- Sharma J. M., Coulson B. D. Presence of natural killer cells in specific-pathogen-free chickens. J Natl Cancer Inst. 1979 Aug;63(2):527–531. [PubMed] [Google Scholar]
- Sharma J. M. In vitro suppression of T-cell mitogenic response and tumor cell proliferation by spleen macrophages from normal chickens. Infect Immun. 1980 Jun;28(3):914–922. doi: 10.1128/iai.28.3.914-922.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M., Hallenbeck L. A. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. II. "Specificities" of the natural killer cells. J Immunol. 1979 Feb;122(2):475–481. [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Witter R. L., Stephens E. A., Sharma J. M., Nazerian K. Demonstration of a tumor-associated surface antigen in Marek's disease. J Immunol. 1975 Jul;115(1):177–183. [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. Introduction of "natural" killer' cells by BCG. Nature. 1976 Aug 12;262(5569):584–586. doi: 10.1038/262584a0. [DOI] [PubMed] [Google Scholar]


