Abstract
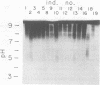
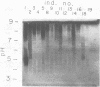
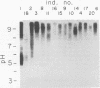
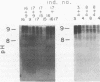
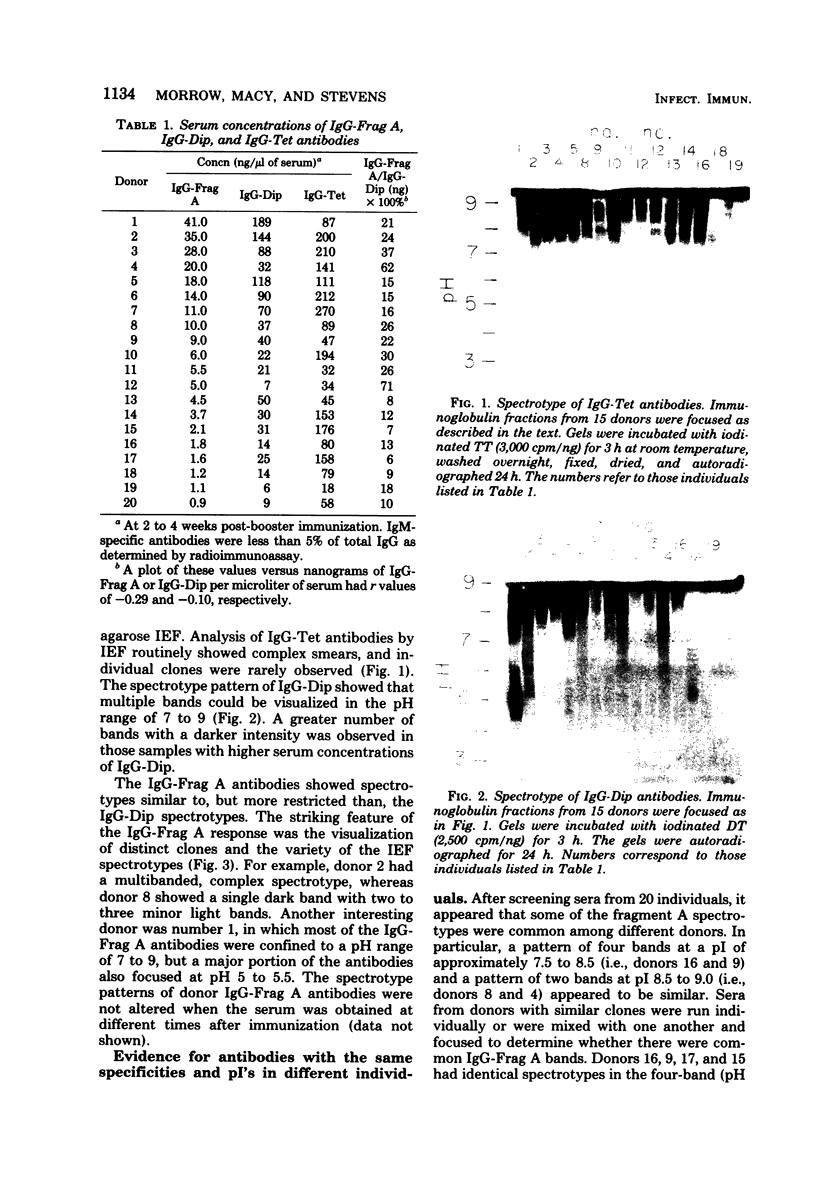
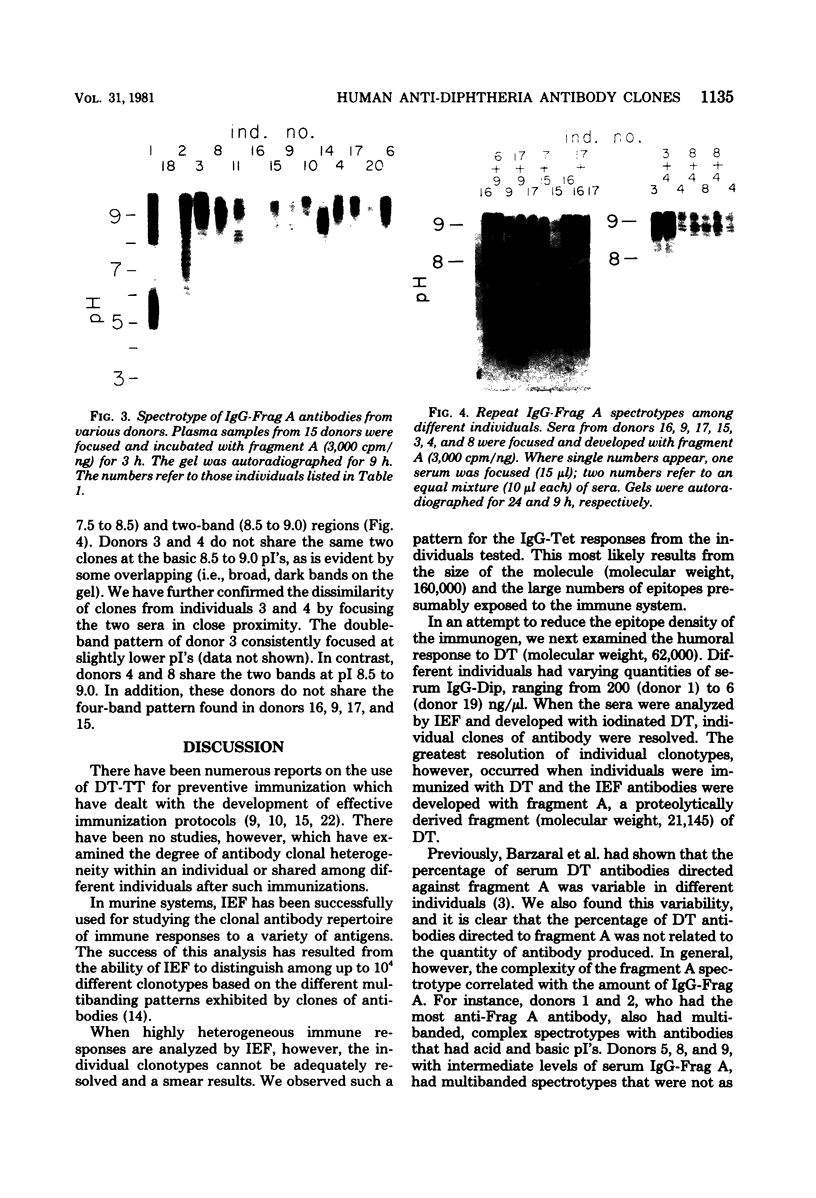
The in vivo human humoral response to diphtheria toxoid-tetanus toxoid booster immunization was studied by isoelectric focusing analysis of sera obtained after immunization. The anti-diphtheria toxoid (immunoglobulin G [IgG]-Dip), anti-fragment A (IgG-Frag A), and anti-tetanus toxoid antibodies from 20 donors post-booster immunization were focused by using agarose isoelectric focusing and visualized by development with radiolabeled antigens. The quantities of the IgG-Dip and IgG-Frag A antibodies correlated with the number of bands seen on the isoelectric focusing pattern in that more bands were found in the spectrotypes of donors with high serum levels of antibody. No difference was apparent in the antibody spectrotypes obtained from sera of donors at successive times post-booster immunization. Individual heterogeneity of the different donors' spectrotypes was often found for IgG-Frag A antibodies, but a close comparison of several different donors revealed antibodies with the same spectrotype patterns. Thus, individual clones of antibody were revealed in humans after in vivo immunization, particularly when antibodies against antigens of restricted epitope size were analyzed. Additionally, the sharing of certain antibody spectrotypes among several individuals raised the possibility that certain antibody clones may be preferentially expressed in the human population.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Harvey M., Sercarz E. E. The fine specificity of regulatory T cells. IV. Idiotypic complementarity and antigen-bridging interactions in the anti-lysozyme response. Eur J Immunol. 1979 Nov;9(11):906–909. doi: 10.1002/eji.1830091113. [DOI] [PubMed] [Google Scholar]
- Bandilla K. K., McDuffie F. C., Gleich G. J. Immunoglobulin classes of antibodies produced in the primary and secondary responses in man. Clin Exp Immunol. 1969 Dec;5(6):627–641. [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Goscienski P. J., Hamburger R. N. Characteristics of human antibody to diphtheria toxin. Infect Immun. 1973 Feb;7(2):130–136. doi: 10.1128/iai.7.2.130-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Davie J. M. Clonal nature of the immune response. II. The effect of immunization on clonal commitment. J Exp Med. 1980 Jul 1;152(1):151–160. doi: 10.1084/jem.152.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cancro M. P., Sigal N. H., Klinman N. R. Differential expression of an equivalent clonotype among BALB/c and C57BL/6 mice. J Exp Med. 1978 Jan 1;147(1):1–12. doi: 10.1084/jem.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M., Harris J. E., McBride C., Freireich E. J. The human primary immune response to keyhole limpet haemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin Exp Immunol. 1970 Apr;6(4):473–491. [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDSALL G., ALTMAN J. S., GASPAR A. J. Combined tetanus-diphtheria immunization of adults: use of small doses of diphtheria toxoid. Am J Public Health Nations Health. 1954 Dec;44(12):1537–1545. doi: 10.2105/ajph.44.12.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDSALL G., BANTON H. J., WHEELER R. E. The antigenicity of single, graded doses of purified diphtheria toxoid in man. Am J Hyg. 1951 May;53(3):283–295. doi: 10.1093/oxfordjournals.aje.a119454. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Hansburg D., Perlmutter R. M., Briles D. E., Davie J. M. Analysis of the diversity of murine antibodies to dextran B1355. III. Idiotypic and spectrotypic correlations. Eur J Immunol. 1978 May;8(5):352–359. doi: 10.1002/eji.1830080512. [DOI] [PubMed] [Google Scholar]
- LEVINE L., IPSEN J., Jr, MCCOMB J. A. Adult immunization. Preparation and evaluation of combined fluid tetanus and diphtheria toxoids for adult use. Am J Hyg. 1961 Jan;73:20–35. [PubMed] [Google Scholar]
- Nicolotti R. A., Briles D. E., Schroer J. A., Davie J. M. Isoelectric focusing of immunoglobulins: improved methodology. J Immunol Methods. 1980;33(2):101–115. doi: 10.1016/s0022-1759(80)80001-9. [DOI] [PubMed] [Google Scholar]
- Rosén A., Ek K., Aman P. Agarose isoelectric focusing of native human immunoglobulin M and alpha 2-macroglobulin. J Immunol Methods. 1979;28(1-2):1–11. doi: 10.1016/0022-1759(79)90322-3. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969 Oct;5(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Differential synthesis of IgM and IgA anti-tetanus toxoid antibody in vitro following in vivo booster immunization of humans. Cell Immunol. 1979 Jun;45(1):142–150. doi: 10.1016/0008-8749(79)90369-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK V. K., GOTTSHALL R. Y., ANDERSON H. D., TOP F. H., BUNNEY W. E., SERFLING R. E. Antigenic response to booster dose of diphtheria and tetanus toxoids. Seven to thirteen years after primary inoculation of nonistitutionalized children. Public Health Rep. 1962 Mar;77:185–194. [PMC free article] [PubMed] [Google Scholar]
- Wells J. V., Fudenberg H. H., MacKay I. R. RElation of the human antibody response to flagellin to GM genotype. J Immunol. 1971 Dec;107(6):1505–1511. [PubMed] [Google Scholar]