Abstract
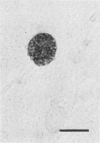
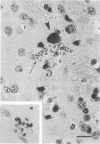
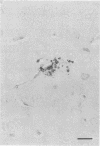
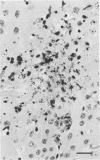
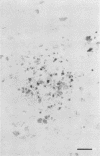
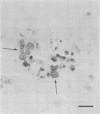
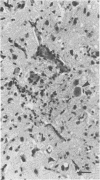
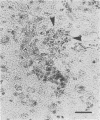
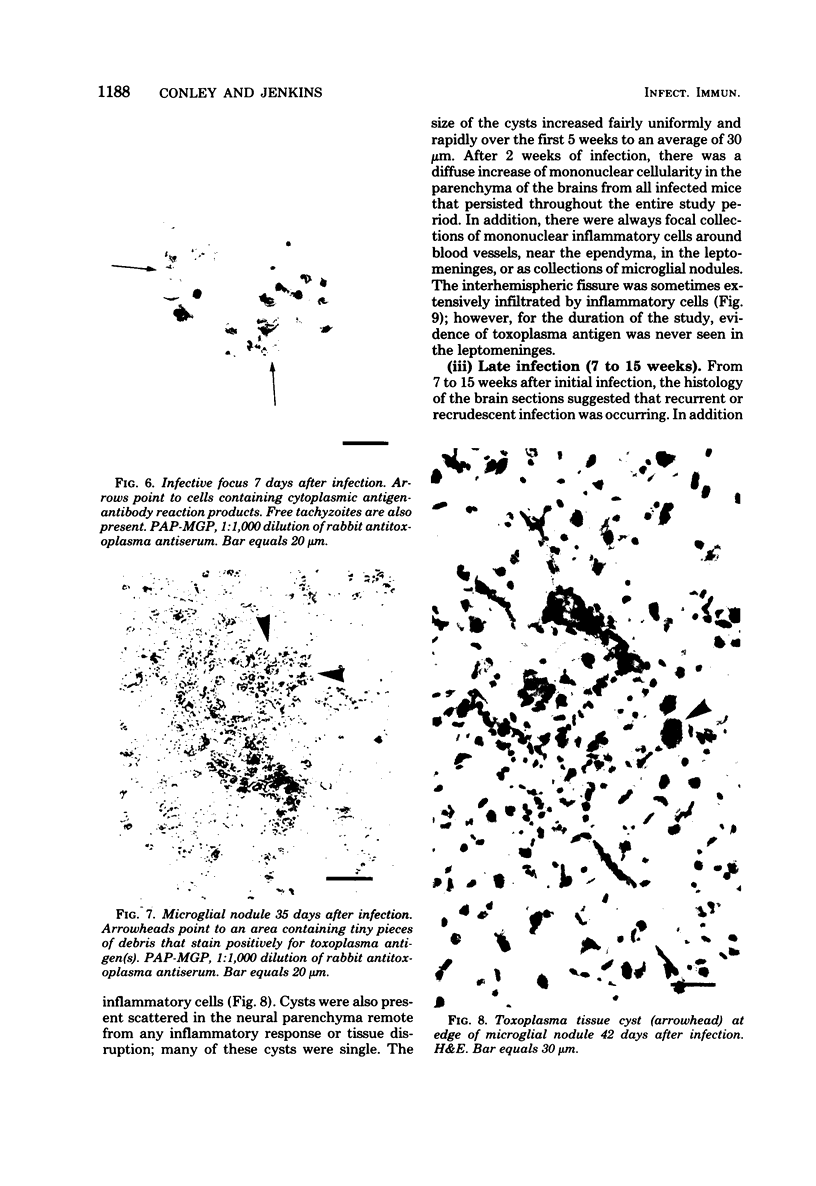
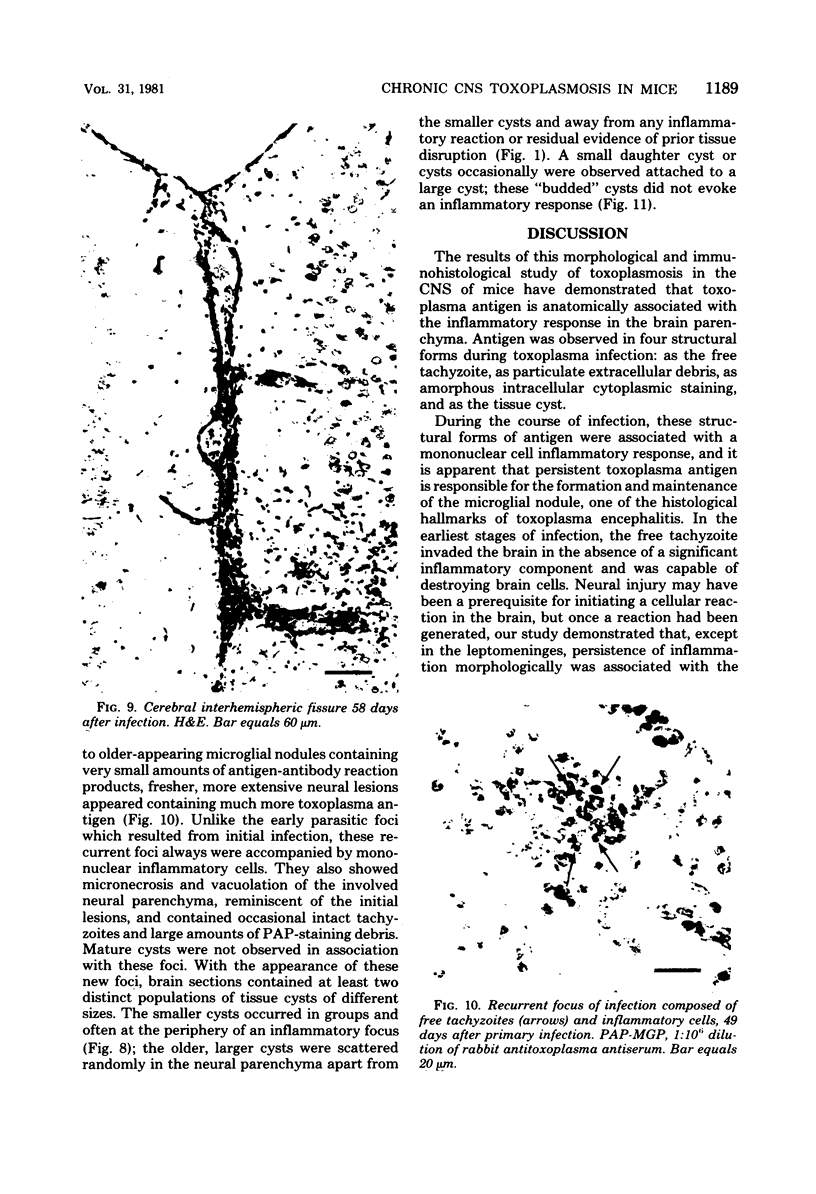
The relationship of toxoplasma antigen(s) to the origin and long-term persistence of the mononuclear cell inflammatory infiltrate that is present in the brains of mice chronically infected with Toxoplasma gondii was studied by using the peroxidase-antiperoxidase immunohistochemical staining technique. C3H/Km mice were infected with the avirulent C37 strain of T. gondii and sequentially sacrificed over the ensuing 107 days. Comparable sections of each brain were prepared for routine light microscopy. Antisera to toxoplasma made in rabbits were used for immunohistological staining, and adjacent slides were also stained with conventional histological stains. The peroxidase-antiperoxidase stain demonstrated toxoplasma tissue cysts, tachyzoites, and intra- and extracellular antigen-antibody reaction products. Early infection was characterized by small tight clusters of free tachyzoites gaining access to brain substance in the absence of an inflammatory response. Once there was disruption of neural parenchyma, a mononuclear cellular infiltrate rapidly ensued. After the first days of infection, mononuclear cells were always present in all infected brains and were anatomically associated with some component of toxoplasma antigen(s). The histological picture of late infection suggested that recurrent episodes of hematogenous dissemination of tachyzoites occurred in infected mice and that such episodes were at least partially responsible for persistence of an antigenic stimulus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARVER R. K., GOLDMAN M. Staining Toxoplasma gondii with fluorescein-labeled antibody. III. The reaction in frozen and paraffin sections. Am J Clin Pathol. 1959 Aug;32(2):159–164. doi: 10.1093/ajcp/32.2.159. [DOI] [PubMed] [Google Scholar]
- Conley F. K. Effect of Corynebacterium parvum and chronic Toxoplasma infection on metastatic brain tumors in mice. J Natl Cancer Inst. 1979 Nov;63(5):1237–1244. [PubMed] [Google Scholar]
- Conley F. K., Remington J. S. Nonspecific inhibition of tumor growth in the central nervous system: observations of intracerebral ependymoblastoma in mice with chronic Toxoplasma infection. J Natl Cancer Inst. 1977 Sep;59(3):963–973. doi: 10.1093/jnci/59.3.963. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K. Adoptive immunity to intracellular infection. J Immunol. 1967 Jun;98(6):1309–1319. [PubMed] [Google Scholar]
- Frenkel J. K., Nelson B. M., Arias-Stella J. Immunosuppression and toxoplasmic encephalitis: clinical and experimental aspects. Hum Pathol. 1975 Jan;6(1):97–111. doi: 10.1016/s0046-8177(75)80111-0. [DOI] [PubMed] [Google Scholar]
- Ghatak N. R., Sawyer D. R. A morphologic study of opportunistic cerebral toxoplasmosis. Acta Neuropathol. 1978 Jun 30;42(3):217–221. doi: 10.1007/BF00690360. [DOI] [PubMed] [Google Scholar]
- Ghatak N. R., Zimmerman H. M. Fine structure of Toxoplasma in the human brain. Arch Pathol. 1973 Apr;95(4):276–283. [PubMed] [Google Scholar]
- HULDT G. EXPERIMENTAL TOXOPLASMOSIS. PARASITEMIA IN GUINEA-PIGS. Acta Pathol Microbiol Scand. 1963;58:457–470. [PubMed] [Google Scholar]
- Huldt G. Experimental toxoplasmosis. Studies of the multiplication and spread of toxoplasma in experimentally infected rabbits. Acta Pathol Microbiol Scand. 1966;67(3):401–423. doi: 10.1111/apm.1966.67.3.401. [DOI] [PubMed] [Google Scholar]
- Huldt G. Studies on experimental toxoplasmosis. Ann N Y Acad Sci. 1971 Jun 21;177:146–155. doi: 10.1111/j.1749-6632.1971.tb35041.x. [DOI] [PubMed] [Google Scholar]
- Lycke E., Carlberg K., Norrby R. Interactions between Toxoplasma gondii and its host cells: function of the penetration-enhancing factor of toxoplasma. Infect Immun. 1975 Apr;11(4):853–861. doi: 10.1128/iai.11.4.853-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUBAYASHI H., AKAO S. MORPHOLOGICAL STUDIES ON THE DEVELOPMENT OF THE TOXOPLASMA CYST. Am J Trop Med Hyg. 1963 May;12:321–333. doi: 10.4269/ajtmh.1963.12.321. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H., Akao S. Immuno-electron microscopic studies on toxoplasma gondii. Am J Trop Med Hyg. 1966 Jul;15(4):486–491. doi: 10.4269/ajtmh.1966.15.486. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Balentine J. D. Macrophages migration through the brain parenchyma to the perivascular space following particle ingestion. Am J Pathol. 1978 Oct;93(1):153–164. [PMC free article] [PubMed] [Google Scholar]
- Powell H. C., Gibbs C. J., Jr, Lorenzo A. M., Lampert P. W., Gajdusek D. C. Toxoplasmosis of the central nervous system in the adult. Electron microscopic observations. Acta Neuropathol. 1978 Mar 15;41(3):211–216. doi: 10.1007/BF00690438. [DOI] [PubMed] [Google Scholar]
- REMINGTON J. S., MELTON M. L., JACOBS L. Induced and spontaneous recurrent parasitemia in chronic infections with avirulent strains of Toxoplasma gondii. J Immunol. 1961 Nov;87:578–581. [PubMed] [Google Scholar]
- Remington J. S., Cavanaugh E. N. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N Engl J Med. 1965 Dec 9;273(24):1308–1310. doi: 10.1056/NEJM196512092732404. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Ruskin J., Rengton J. S. Role for the macrophage in acquired immunity to phylogenetically unrelated intracellular organisms. Antimicrob Agents Chemother (Bethesda) 1968;8:474–477. doi: 10.1128/AAC.8.4.474. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- WALLS K. W., TARASKA J. J., GOLDMAN M. ISOLATION OF TOXOPLASMA GONDII FROM CYSTS IN HUMAN BRAIN. J Parasitol. 1963 Dec;49:930–931. [PubMed] [Google Scholar]
- WANKO T., JACOBS L., GAVIN M. A. Electron microscope study of Toxoplasma cysts in mouse brain. J Protozool. 1962 May;9:235–242. doi: 10.1111/j.1550-7408.1962.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Witting P. A. Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z Parasitenkd. 1979;61(1):29–51. doi: 10.1007/BF00927085. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M. O. Experimental Toxoplasma retinitis: a light and electron microscopical study. Arch Pathol Lab Med. 1976 Sep;100(9):487–490. [PubMed] [Google Scholar]
- de ROEVER-BONNET Mice and golden hamsters infected with an avirulent and a virulent Toxoplasma strain. Trop Geogr Med. 1963 Mar;15:45–60. [PubMed] [Google Scholar]