Abstract
Internet-based educational and therapeutic programs (e-health applications) are becoming increasingly popular for a variety of psychological and physical disorders. We tested the efficacy of an online Chronic Pain Management Program, a comprehensive, fully self-directed and self-paced system that integrates social networking features and self-management tools into an interactive learning environment. Of 305 adult participants (196 women, 109 men), a total of 162 individuals with chronic pain were randomly assigned unsupervised access to the program for approximately 6 weeks; 143 were assigned to the wait-listed control group with treatment as usual. A comprehensive assessment was administered before the study and approximately 7 and 14 weeks thereafter. All recruitment, data collection, and participant involvement took place online. Participation was fully self-paced, permitting the evaluation of program effectiveness under real-world conditions. Intent-to-treat analysis that used linear growth models was used as the primary analytic tool. Results indicated that program utilization was associated with significant decreases in pain severity, pain-related interference and emotional burden, perceived disability, catastrophizing, and pain-induced fear. Further, program use led to significant declines in depression, anxiety, and stress. Finally, as compared to the wait-listed control group, the experimental group displayed a significant increase in knowledge about the principles of chronic pain and its management. Study limitations are considered, including the recognition that not all persons with chronic pain are necessarily good candidates for self-initiated, self-paced, interactive learning.
Keywords: Catastrophizing, Chronic pain management, Controlled trial, Coping with pain Treatment for chronic pain, Internet treatment, Pain adjustment, Self-management
1. Introduction
Many obstacles stand in the way of pain amelioration for millions of people, including financial barriers (both personal and insurance based), inadequacies in assessment (especially in primary care settings), patient reluctance to seek treatment, perceived communication barriers between patient and health care provider, and a paucity of comprehensive multidisciplinary treatment options [17,28,41,42,46]. Thus, there has been a movement to supplement and extend established therapeutic and educational interventions for chronic pain with innovative, cost-effective, and empirically sound alternatives such as automated telephone services, progress monitoring via regular mail and e-mail, electronic diaries, video-based psychoeducational programs, and online sites for the delivery of health information [18,19,21,22,26,30]. Across a range of health domains, including not only chronic pain but also depression [31], anxiety [5], eating disorders [45], and addictive disorders [14], Internet-mediated or computer-aided behavior change interventions based on well-tested cognitive behavior therapy principles are currently seeing wider application for children and adults, with the type of available online help ranging from text-based, information-only sites to custom-built software applications [9,20,27,32,39,43]. As noted in several systematic reviews of Web-based interventions for chronic pain [3,24], small but significant improvements in pain experience and reductions in functional disability have been reported, although the program effects on psychosocial outcomes (eg, depression, anxiety) have proved less consistent.
Notable also is the use of self-management methods in an on line format, with a literature that has progressed from case studies and uncontrolled feasibility testing [1,11] to randomized clinical trials [7,29,44]. Although early applications of interventions labeled ‘self-management’ tended to focus on biofeedback methods [40], contemporary approaches attempt to educate persons with pain in a variety of skills and motivational strategies presumed necessary for self-guided symptom management and everyday task persistence. Such skills include (but are not limited to): self-observation (aka self-monitoring), self-statement modification, goal setting, self-induced relaxation, physical exercise, attention control, emotion regulation, belief reappraisal, self-efficacy enhancement, planning, coping, and pacing [25,29,38].
The present research, building on previous efforts, evaluates a newly developed, multielement program for the self-management of chronic pain designed to be accessed via the Internet. The Chronic Pain Management Program (CPMP) leverages technical capabilities with program content and functionality derived from cognitive behavior therapy, interpersonal, and self-management approaches to address the adaptive burdens of chronic pain in adults. We provide the results of a preliminary test of the effectiveness of the program in the domains of pain-related outcomes, mental health, knowledge about the principles of pain management, and daily life functioning. By means of an intent-to-treat analysis and linear growth curve models, we sought to demonstrate, via a randomized controlled trial, that the CPMP would yield improvements in pain adaptation, in psychosocial adjustment, and in pain-related knowledge relative to wait-listed, treatment-as-usual control subjects.
2. Methods
2.1. Participants
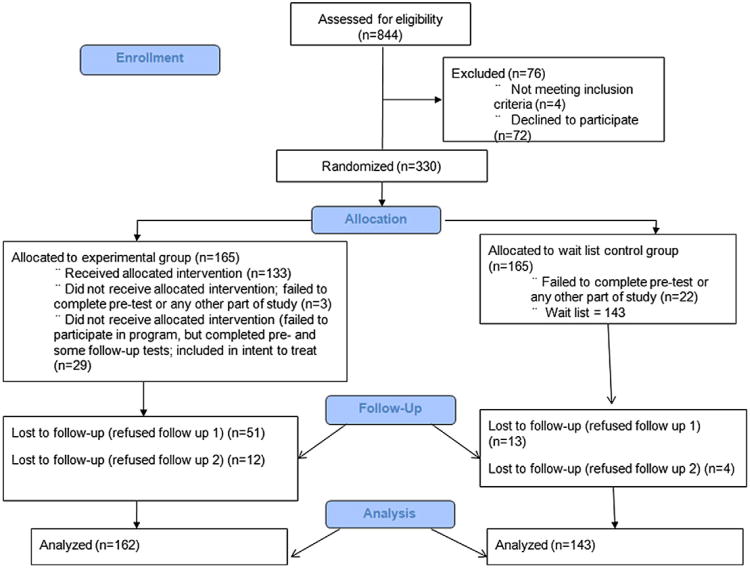
A participant flow diagram consistent with the CONSORT statement [2] is depicted in Fig. 1. As indicated, 330 participants were randomized. Twenty-five participants (3 experimental subjects and 22 control subjects) failed to take part in any aspect of the study upon randomization. The final sample therefore consisted of 305 individuals who completed the pretest and 1 or more follow-up tests, with 162 (53%) in the experimental group and 143 (47%) in the wait-listed/usual treatment control group. At the second (approximately 7 weeks) and third (approximately 14 weeks) data collection waves, 241 (79%; 111 in the experimental group and 130 in the control group) and 225 (73.8%; 99 in the experimental group and 126 in the control group) respondents provided data, respectively. Chi-square tests indicated that the probability of missing data differed across the 2 conditions, with the experimental group having the higher missing data rate. As described later, we used full information maximum likelihood estimation to deal with missing data. Maximum likelihood uses all of the available data to estimate the model parameters without the need for discarding cases or filling in the missing values [13]. Maximum likelihood yields unbiased parameter estimates under the so-called missing-at-random assumption, where the propensity for missing data at a particular wave is related to predictor variables (eg, treatment group membership, initial pain severity levels, age) or to the scores from previous assessments, but not to the scores that would have been obtained had the data been complete. Consequently, the unequal distribution of missing data is not necessarily problematic.
Fig. 1.
Participant flow diagram.
With regard to demographics, participants in this sample were predominantly white (82%), 6% were African American, 2% were American Indian, 2% were Asian, and 8% were more than one race or “other.” More than half of the sample was female (64%). Approximately 65% of the participants were married or living as married, 19% were single, and the remaining participants were either divorced (14.4%) or widowed (0.7%). Participant ages ranged between 19 and 78 years, with an average age of 44.93 years. With respect to education, 10.5% of the sample had a high school degree or lower, 70.8% reported attending at least some college, and 18.4% had advanced degrees. Employment status varied considerably; 39% were employed full time, 7.5% part time (due to pain 2.6%; not due to pain 4.9%), and the remaining participants were dispersed across a variety of categories, including unemployed (due to pain 14.4%; not due to pain 5.7%), retired (due to pain, none; not due to pain 4.6%), and disabled (due to pain 15.7%; not due to pain 3.9%). Most participants (91%) were under a doctor's for their pain problems. Most participants (89.5%) reported having pain for more than 2 years. A series of chi-square tests indicated that the experimental and control conditions did not differ significantly in the distribution of demographic or pain history characteristics.
At the beginning of the study, participants provided information about their medical diagnoses and pain problems. Table 1 presents the percentage of respondents who reported having 1 or more of 11 different pain-related diagnoses; the most common diagnoses were migraine headaches (65.5%) and back injury (60.5%). Tension headaches, fibromyalgia, osteoarthritis, face or jaw pain, and premenstrual pain were somewhat less common, with 20–40% of the participants reporting these. The average number of reported diagnoses was approximately 3 (range 0–8). An independent t test indicated that the experimental and control groups did not differ in their number of reported diagnoses (t(164) = 1.17, P = .25).
Table 1.
Diagnosis summary.
| Pain source | % |
|---|---|
| Migraine headaches | 65.5 |
| Back injury/disease | 60.5 |
| Tension headaches | 41.0 |
| Osteoarthritis | 31.1 |
| Facial/jaw pain | 29.1 |
| Premenstrual syndrome | 28.1 |
| Cluster headaches | 16.1 |
| Pelvic injury/disease | 12.2 |
| Rheumatoid arthritis | 7.1 |
| Cancer | 0.8 |
Participants were also asked to rate the amount of pain that they experienced from a variety of physical locations. Table 2 summarizes these ratings (scores ranged 0–5). The highest pain severity ratings were associated with head, shoulder/upper back, neck/ throat, lower back, and bones/joints; all produced ratings above 3.00 in magnitude (Table 2). The employment status question from the demographic survey also provided some indication of pain status, as 32.7% of the respondents reported being unemployed, disabled, or limited to working part time as a result of their pain.
Table 2.
Pain severity ratings by physical location.
| Pain location | M | SD |
|---|---|---|
| Head | 3.94 | 1.39 |
| Shoulder/upper back | 3.46 | 1.39 |
| Neck/throat | 3.37 | 1.49 |
| Lower back | 3.35 | 1.56 |
| Bones/joints | 3.34 | 1.42 |
| Muscles | 3.07 | 1.50 |
| Hip | 2.81 | 1.62 |
| Face | 2.65 | 1.60 |
| Stomach | 2.35 | 1.57 |
| Teeth/gums | 1.90 | 1.63 |
| Genital | 1.81 | 1.66 |
| Chest | 1.68 | 1.50 |
| Skin | 1.65 | 1.58 |
| Other | 2.75 | 1.92 |
2.2. Materials
2.2.1. Measures
Participants completed a battery of online assessments at each assessment interval. The battery consisted of the Center for Epidemiological Studies Depression Scale [33], the Depression, Anxiety, Stress Scales [23], a test of pain knowledge that assessed a wide range of topics addressed within the program (eg, the role of thought, emotion, social responses to pain, and behavior to the pain experience), and the Profile of Chronic Pain: Screen (PCP-S) and Profile of Chronic Pain Extended Assessment (PCP-EA) [36,37]. The 15-item PCP-S provides a multidimensional assessment of the individual's pain problem with scales that measure pain severity and interference, as well as the emotional burden of pain. The 95-item PCP-EA assesses pain location and prior diagnoses, pain characteristics (eg, worst daily pain), medication use, and health care status. In addition, the PCP-EA includes 13 multi-item subscales addressing aspects of coping (guarding, ignoring, task persistence, and positive self-talk), catastrophizing, pain attitudes and beliefs (including disability beliefs, belief in a medical cure for pain, belief in pain control, and pain-induced fear), and positive (tangible and emotional) and negative (insensitivity and impatience) social responses. Finally, functional limitations in 10 areas of daily living are evaluated. The PCP-S and PCP-EA have been found to have adequate reliability, validity, and low social desirability response bias; the factor structure of the various scales has been confirmed across several samples; age- and gender-based national norms are available [36,37].
2.2.2. Content development for the CPMP
Content development was an iterative process involving 3 teams: a team of 3 psychologists who created the initial content, a content review team of 3 professionals who treat chronic pain (2 psychologists and 1 employee assistance professional), and a team of 10 individuals with chronic pain to evaluate content. First, we examined the chronic pain literature to identify face-to-face forms of psychosocial interventions for chronic pain that have demonstrated efficacy. These fell easily into 4 basic categories: cognitive, behavioral, social, and emotional regulation. Four learning modules were created to reflect these content domains: “Thinking Better,” “Doing More,” “Relating Better,” and “Feeling Better.” Within each learning center, we created a multimedia presentation as an overview to the concepts within the center. Next, we created a series of interactive activities within the learning center to teach basic concepts and allow for both online and off-line practice. Naturally, depending on content domain, the nature of the learning activities varied widely and involved different media. For example, in “Thinking Better,” the program focuses on teaching basic concepts concerning the role of thought in pain, provides criteria for evaluating helpful vs self-defeating thinking, and engages the user in a series multimedia presentations and interactive online activities to practice evaluating thought content. A set of offline self-monitoring activities help the user to identify his or her own self-defeating thoughts. In contrast, a major goal of “Doing More” is assisting the user in developing an exercise program. This involved the creation of an interactive activity to help the user to learn about and create a realistic exercise plan. In addition, a physical medicine and rehabilitation physician and a physical therapist created 15 different strength and flexibility exercise programs. Models were photographed demonstrating each exercise within each of the 15 programs. As a third example of the diversity of content development tasks, we created a series of relaxation sessions in which it was necessary to develop the text for each session (eg, progressive muscle relaxation), record each session, and locate relaxing and appropriate photographs for each session. In some cases, additional functionality was created to augment content.
As content was created, formative evaluations were conducted by our teams of professionals and individuals with pain. All reviews were conducted online and included a large series of questions concerning content, user experience, and functionality. Most of the questions involved 0–5-point Likert-type rating scales; some open-ended questions were included as well. Feedback from the review teams was used to modify content; additional reviews were conducted until reviewers reached consensus that we had attained high quality (generally, average ratings of 4 or higher on 0–5-point scales).
2.2.3. Structure of the CPMP
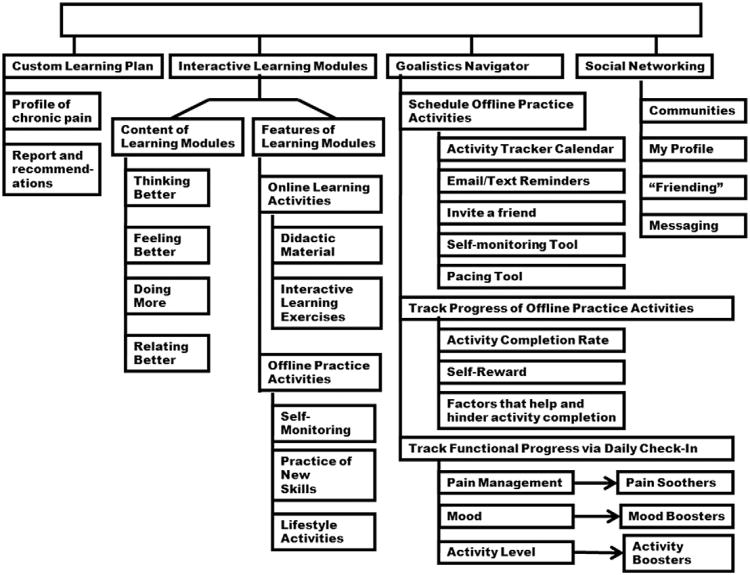
An overview of the structure of the program is presented in Fig. 2. A custom learning plan is created for each user after the online completion of the Profile of Chronic Pain (PCP). A user's responses on the PCP are automatically scored, compared to national age- and gender-based norms, and presented in an online report. Scores on the PCP map onto the 4 learning modules. Program recommendations are generated suggesting the order of completion of the 4 learning modules (based on normative analyses). Each of the 4 learning modules includes online and off-line activities. Online activities are a combination of didactic material and interactive exercises. For example, in Feeling Better, the user completes a number of interactive activities to learn about the role of emotion in pain management. Two types of off-line activities are akin to homework and include self-monitoring exercises and the practice of new skills. The third off-line activity type includes lifestyle activities such as exercise, relaxation, or implementation of goal-directed behavior.
Fig. 2.
Structure of the Chronic Pain Management Program.
The Navigator is a custom-built system that integrates self-management tools into the learning environment. The user may schedule off-line practice activities on the Calendar tool, send email/text reminders, or invite a friend to join in an activity. The Self-Monitoring Tool allows the user to create and track self-monitoring tasks. The Pacing Tool assists the user in the creation and tracking of a plan to gradually increase the duration of an activity that the user may find difficult to do. For example, if the user can only walk for 5 min but would like to walk for 30 min, the pacing tool will guide the user in the creation of a plan to increase walking by 10% each week. The user simply enters the baseline and target activity duration and the days and times to perform the activity. The Pacing Tool automatically creates a plan, schedules it on the calendar, and sends e-mail/text reminders.
The Navigator tool also allows for the tracking of progress of offline practice activities. At the end of each day, the user is encouraged to indicate whether each scheduled activity was completed, how helpful the activity was in pain management, and what factors helped or hindered activity completion. These data are stored and presented graphically in the My Progress section of the program. The user tracks functional progress via a second type of daily check-in. A graph displays the user's end of day ratings of pain management, mood, and activity level. Users who report low levels of activity, mood, or pain management are encouraged to use one of their custom activity boosters, mood boosters, or pain soothers. For example, a mood booster is defined as a brief, easy-to-complete activity that the user has found to provide a small boost to mood. The user can create an online list of such activities and schedule them on the Navigator Calendar along with a reminder (e-mail/ text) to complete them.
Finally, the social networking component of the CPMP allows the user to create a profile (with privacy options), join and participate in a variety of unmoderated community forums (eg, migraine, male and female forums, back pain, facial pain), invite friendship with other users, and send messages.
2.3. Procedure
The study was approved by the New England Institutional Review Board. Several online pain sites assisted in the recruitment of efficacy study participants. The sites were The National Headache Foundation, American Chronic Pain Association, National Pain Foundation, and the American Pain Foundation. The sites posted information about the study on their sites, sent e-mails to their members, and included information in their newsletter (if they distributed a newsletter). The recruitment request included information about the study, participation details, and the online address of the consent form. A consent database was created consisting of all of those who completed the online consent form and were eligible for participation (n = 532). Eligibility criteria included age 18 years or older, presence of a chronic pain problem for 6 or more months, access to a computer with high-speed Internet capabilities, and the ability to read and write English. We sought to include an equal number of men and women and a representative sample by ethnicity and race. Subgroups of potential participants were selected on the basis of gender, race, and ethnicity and assigned at random to either the control or experimental groups. First, to address difficulties in filling race and ethnicity enrollment quotas, all minorities were included and randomly assigned to either the experimental or control group. Having assigned all available minorities, we then sought to fill our gender quotas and randomly assigned nonminority male and female subjects to either the experimental or control group.
All 305 participants completed the pretest. Follow-up data were requested at approximately 7 and 14 weeks. Participants received $25 for completing each assessment across the 3 intervals (for a possible total of $75). Those who failed to complete one or more follow-up assessments were reminded on multiple occasions via e-mail and phone call until approximately 1 month after the target follow-up date. Upon completion of the study, the wait-listed control group was given full access to the program.
Interventions for chronic pain have primarily made use of the face-to-face channel of communication, with formally evaluated programs that typically are (a) manualized, (b) delivered and/or supervised by a team of trained health professionals, (c) fixed in terms of session duration and in total number of sessions, (d) externally monitored to ensure fidelity to protocol parameters, and (e) conducted in an academic, clinic, or hospital setting [15]. In contrast to such clinical outcome studies in which the subjects' participation is highly managed by an external team, we sought to test the effectiveness of the CPMP under real-world conditions. Participation by the experimental group was fully self-directed and self-paced. Participants were paid for completion of the assessments, but not for participation in the program. They were prompted to complete the assessments in a timely fashion, but were otherwise not contacted or managed by the research team.
3. Results
As noted previously, the final sample consisted of 305 individuals, with 162 (53%) in the experimental group and 143 (47%) in the wait-listed/usual treatment control group. In the experimental group, 29 individuals completed the initial assessment battery but failed to participate in the online pain program. Consistent with an intent-to-treat analysis, we included these individuals in the experimental condition for all statistical analyses. (Note that 25 participants, 3 experimental and 22 control, failed to take part in any aspect of the study upon randomization. Because no data were available for these individuals, they were not considered part of the study.) The means and standard deviations for the study variables are presented in Table 3.
Table 3.
Means and standard deviations for study variables.
| Variable | Experimental group, mean (SD) | Wait-listed control group, mean (SD) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pretest | 7-week follow-up | 14-week follow-up | Pretest | 7-week follow-up | 14-week follow-up | |
| Severity | 24.47 (3.11) | 22.75 (4.14) | 22.41 (4.31) | 23.93 (3.49) | 22.93 (4.25) | 22.34 (4.61) |
| Interference | 25.03 (6.98) | 22.31 (8.61) | 21.24 (8.96) | 23.19 (7.84 | 21.85 (8.47) | 21.72 (8.85) |
| Emotional burden | 18.03 (5.19) | 14.81 (6.09) | 14.10 (6.66) | 17.17 (5.49) | 15.25 (6.16) | 13.95 (7.11) |
| Perceived disability | 11.92 (5.92) | 10.31 (6.12) | 9.60 (6.28) | 10.64 (5.86) | 10.35 (5.80) | 9.73 (5.77) |
| Perceived control | 14.95 (5.40) | 16.14 (5.32) | 16.47 (6.02) | 14.78 (5.39) | 15.35 (5.25) | 15.19 (5.82) |
| Belief in a medical cure | 12.99 (5.88) | 12.97 (6.44) | 13.13 (6.82) | 12.07 (6.85) | 13.03 (6.20) | 12.48 (6.71) |
| Catastrophizing | 9.52 (5.48) | 8.18 (5.45) | 7.52 (5.50) | 8.73 (5.26) | 8.77 (5.19) | 8.18 (5.84) |
| CES-D | 25.58 (13.30) | 22.37 (12.51) | 21.98 (12.45) | 21.78 (13.13) | 21.49 (12.61) | 21.25 (14.36) |
| DASS–depression | 8.31 (6.32) | 6.38 (5.52) | 7.33 (5.53) | 7.27 (5.88) | 6.64 (5.61) | 6.97 (6.42) |
| DASS–stress | 8.84 (5.53) | 7.30 (5.01) | 7.36 (5.21) | 7.87 (5.44) | 7.67 (6.46) | 7.64 (5.63) |
| DASS–anxiety | 5.09 (4.79) | 4.50 (4.62) | 4.26 (4.08) | 4.73 (4.51) | 4.82 (4.74) | 4.93 (4.67) |
| Interference with social life | 3.66 (1.25) | 3.27 (1.39) | 3.12 (1.40) | 3.44 (1.22) | 3.33 (1.36) | 3.22 (1.38) |
| Interference with sex | 3.26 (1.73) | 3.18 (1.66) | 3.03 (1.67) | 3.22 (1.66) | 3.25 (1.72) | 3.07 (1.82) |
| Interference with sleep | 3.99 (1.23) | 3.70 (1.39) | 3.62 (1.47) | 3.85 (1.29) | 3.75 (1.26) | 3.75 (1.38) |
| Interference with recreation | 3.66 (1.32) | 3.33 (1.37) | 3.20 (1.54) | 3.49 (1.36) | 3.45 (1.36) | 3.34 (1.39) |
| Interference with chores | 3.80 (1.19) | 3.31 (1.37) | 3.19 (1.52) | 3.51 (1.38) | 3.38 (1.40) | 3.26 (1.47) |
| Interference with work | 3.55 (1.82) | 3.30 (1.89) | 3.01 (1.98) | 3.13 (1.73) | 3.16 (1.77) | 3.02 (1.80) |
| Interference with self-care | 1.55 (1.60) | 1.59 (1.58) | 1.40 (1.48) | 1.49 (1.59) | 1.38 (1.59) | 1.58 (1.62) |
| Interference with parenting | 1.40 (1.72) | 1.10 (1.61) | 1.07 (1.42) | 0.89 (1.45) | 0.88 (1.46) | 0.77 (1.33) |
| Interference with routine physical activities | 2.81 (1.74) | 2.40 (1.77) | 2.46 (1.72) | 2.60 (1.74) | 2.55 (1.66) | 2.38 (1.78) |
| Interference with exercise | 3.59 (1.38) | 3.30 (1.55) | 3.02 (1.56) | 3.52 (1.47) | 3.29 (1.54) | 3.23 (1.53) |
3.1. Analyses
A linear growth model was the primary analytic tool for the study. The linear growth model expresses the outcome variable as a function of a temporal predictor variable that captures time since the baseline assessment. Specifically, we used the following model:
| (1) |
where & β0 and β1 represent the baseline mean and the average growth rate for the control group, respectively, β2 is the baseline mean difference between the treatment and control groups, and β3 is the growth rate difference between groups (ie, the group-by-time interaction effect). Like a conventional linear regression model, the β terms are partial regression coefficients that control for the covariates. Finally, the model incorporates a pair of residuals that allow baseline scores and growth rates to vary across participants (ie, b0i and b1i respectively), and eti is a time-specific residual that captures the misfit between an individual's observed scores and idealized linear growth trajectory.
A series of initial analyses indicated that most of the outcomes exhibited nonlinear growth, such that the largest changes were observed between baseline and the first follow-up. Rather than move to a more complicated quadratic model, we chose to linearize the growth function by expressing change as a function of the square root of time since baseline. The timing of the assessments was such that this transformation effectively equated the change between the first and second assessment to the change between the second and final assessment (eg, a growth rate estimate of −5.00 implies that scores decreased by 5 points between the first and second assessment, on average, and scores decreased by another 5 points between the second and final assessment). To facilitate interpretation, we use the model-implied means from the final assessment to construct standardized mean difference effect size. Importantly, transforming the temporal predictor has a negligible impact on baseline and end-point mean estimates. Consequently, the effect size estimates from the transformed model were virtually identical to those from a more complicated quadratic growth curve model. The primary advantage of this transformation is that a single parameter quantifies the group-by-time interaction. Assessing this effect is more difficult with a quadratic model because differential growth requires 2 parameters (ie, group by linear and the group by quadratic coefficients).
We used Mplus 5.21 to estimate the growth models. Mplus accommodates missing data by using maximum likelihood estimation. This approach makes the so-called missing at random assumption where the probability of missing data at a particular assessment is related to scores at previous assessments or to scores on the covariates. Methodologists regard maximum likelihood as a state of the art missing data handling approach, and this method has a number of theoretical and empirical advantages (eg, less bias, greater power) over traditional approaches such as deletion of incomplete cases [13].
Several of the scales in this study were grouped together to form a smaller number of broader constructs, including the extent of the pain problem (pain severity, interference, and emotional burden), pain attitudes and beliefs (perceived disability, perceived control, belief in a medical cure, and pain-induced fear), psychological functioning (depression, anxiety, stress), and pain interference in 10 areas of daily functioning (social, sex, sleep, recreation, household chores, work, self-care, parenting, physical activities, and exercise). Two other constructs, catastrophizing and pain knowledge, were measured by single scales. Because it was of interest to obtain a fine-grained evaluation of the intervention, we analyzed each scale separately and made no attempt to model growth at the latent variable level (eg, by using scale scores as manifest indicators of a larger construct). To streamline the subsequent presentation, we present the results for groups of scales, highlighting the estimates from the analyses that produced a significant group-by-time interaction effect.
3.2. Changes in pain problem magnitude
Pain magnitude was characterized by the PCP-S scales that measured interference, severity, and emotional burden. Table 4 presents the regression coefficients and standard errors for selected parameters, omitting the coefficients for the covariates because these variables were not of substantive interest. Because the covariates were centered at their means, this omission has no bearing on the values or the interpretation of the growth model coefficients. To illustrate the interpretation of the growth model parameter estimates, we provide a detailed description of the interference scale results. Because many of the outcomes produced results that corresponded to those of the interference scale, we elected to condense the description of subsequent analyses.
Table 4.
Growth model parameter estimates for extent of pain outcomes.
| Parameter | Interference | Severity | Emotional burden | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Est. | SE | p | Est. | SE | p | Est. | SE | p | |
| Control baseline (β0) | 23.44 | 0.50 | .00 | 23.89 | 0.29 | .00 | 17.41 | 0.35 | .00 |
| Control growth (β1) | −0.65 | 0.25 | .01 | -0.75 | 0.16 | .00 | −1.68 | 0.21 | .00 |
| Baseline difference (β2) | 1.05 | 0.65 | .11 | 0.62 | 0.37 | .10 | 0.25 | 0.49 | .62 |
| Growth difference (β3) | −1.63 | 0.41 | .00 | -0.64 | 0.24 | .01 | −0.79 | 0.37 | .03 |
| Wave 3 effect size | 0.30 | 0.20 | 0.25 | ||||||
As seen in the table, the control group had an average interference score of 23.44 at the baseline assessment and decreased by approximately 0.65 points between each assessment, on average (ie, β0 and βa, respectively). After controlling for the covariates, the treatment group average was approximately 1.05 points higher at baseline (ie, β2) but this different was not statistically significant. Finally, the growth rate difference (ie, β3, the group-by-time interaction) was significant and indicated that the treatment group decreased by roughly 1.63 points more than the control group, on average (ie, the treatment group growth rate was −0.65 − 1.63 = −2.28).
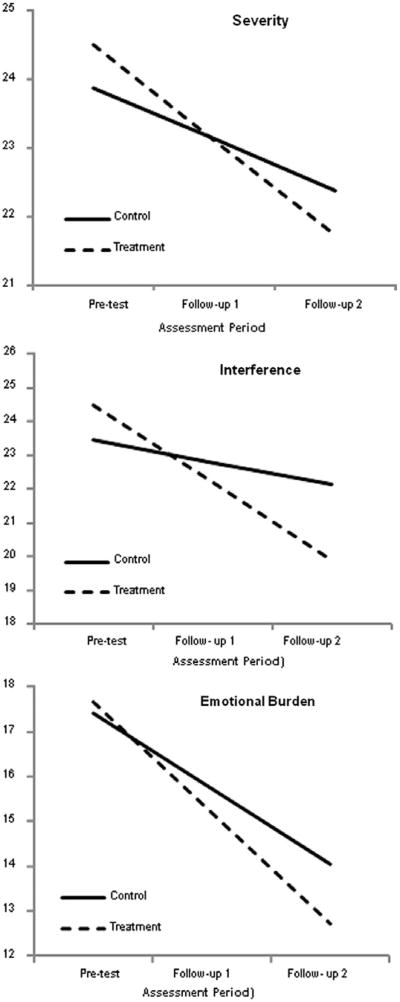
Consistent with a linear regression analysis, the parameter estimates in Table 4 combine to produce simple slopes that represent the linear trends for each group. Fig. 3 shows these growth curves, with the points on the lines representing the model-implied averages at each assessment. The simple slopes provide a graphic representation of the estimates in Table 4. For example, notice that for Interference the treatment group starts with a slightly higher average than the control group but decreases more rapidly over time. The vertical separation of the simple slopes at the final assessment is an estimate of the end-of-study mean difference. Dividing this difference by pooled within-group standard deviation from the baseline assessment yields a standardized mean difference effect size that is comparable to Cohen's d. As seen in the bottom row of Table 4 (wave 3 effect size), the standardized mean difference for the interference scale was 0.30, which indicates that the group means differed by nearly a third of a standard deviation at the final assessment. The magnitude of this effect size is consistent with Cohen's [8] benchmark for a small effect size (ie, d > 0.20). Again, it is important to reiterate that this effect size estimate is nearly identical to that from a more complex quadratic growth model.
Fig. 3.
Simple slopes for the extent of pain problems outcome measures. The graphs suggest that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graphs suggest that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
Table 4 also shows the parameter estimates for the severity and the emotional burden scales. The estimates are largely consistent with those from the interference scale. Specifically, the control group showed significant decreases over time, but the rates of change for the treatment group significantly exceeded the gains for the control group (ie, the group-by-time interactions were significant). Consistent with the interference scale, the mean differences at the final wave could be characterized as a small effect sizes. The top and bottom panels of Fig. 3 show the corresponding simple slopes for the severity and the emotional burden scales.
3.3. Changes in pain attitudes and beliefs
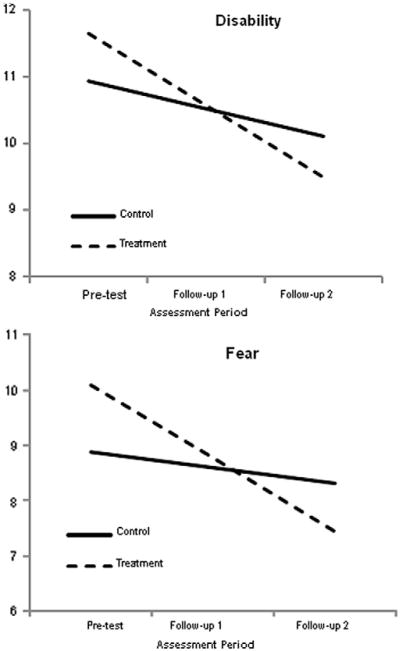
PCP-EA Battery scales measured perceived disability, perceived control, belief in a medical cure, and pain-induced fear reflect pain attitudes and beliefs. Two of these outcomes, perceived disability and pain-induced fear, produced significant group-by-time interaction effects, such that the treatment group decreased at a rate that was significantly different than the control. Table 5 gives the regression coefficients and standard errors for the parameters of substantive interest, and Fig. 4 displays the corresponding simple slopes for each intervention group.
Table 5.
Growth model parameter estimates for pain attitudes and beliefs outcomes.
| Parameter | Disability | Fear | Perceived control | Belief in medical cure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | |
| Control, baseline (β0) | 10.93 | 0.43 | .00 | 8.87 | 0.49 | .00 | 14.73 | 0.43 | 0.00 | 12.29 | 0.57 | .00 |
| Control, growth (β1 | −0.42 | 0.19 | .03 | −0.28 | 0.24 | .24 | 0.24 | 0.24 | 0.30 | 0.28 | 0.25 | .26 |
| Baseline, difference (β1) | 0.73 | 0.59 | .22 | 1.21 | 0.72 | .09 | 0.30 | 0.60 | 0.61 | 0.56 | 0.74 | .45 |
| Growth, difference (β3) | −0.66 | 0.29 | .02 | −0.03 | 0.37 | .00 | 0.63 | 0.38 | 0.10 | −0.22 | 0.36 | .55 |
| Wave 3 effect size | 0.10 | 0.12 | 0.29 | 0.02 | ||||||||
Fig. 4.
Simple slopes for the pain attitudes and beliefs outcomes. The graphs suggest that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graphs suggest that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
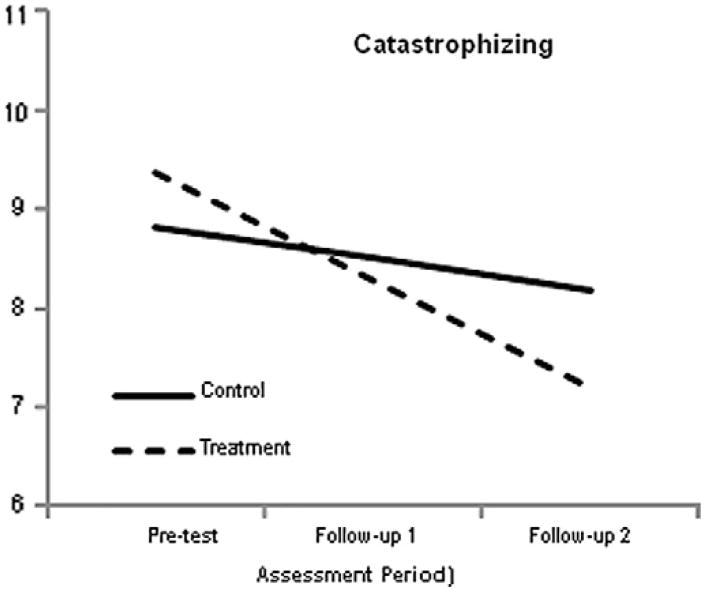
3.4. Changes in catastrophizing
Catastrophizing was assessed by the PCP-EA. The growth model parameter estimates are listed in Table 6. The results closely follow those from previous analyses. Specifically, the group-by-time interaction effect was significant, such that the treatment group decreased at a greater rate than the control group. To further illustrate these results, Fig. 5 shows the simple slopes for the 2 groups.
Table 6.
Growth model parameter estimates for catastrophizing.
| Parameter | Catastrophizing | ||
|---|---|---|---|
|
| |||
| Est. | SE | p | |
| Control baseline (β0) | 8.83 | 0.39 | .00 |
| Control growth (β1) | −0.32 | 0.19 | .09 |
| Baseline difference (β2) | 0.55 | 0.56 | .33 |
| Growth difference (β3) | −0.77 | 0.29 | .01 |
| Wave 3 effect size | 0.18 | ||
Fig. 5.
Simple slopes for the catastrophizing outcome. The graph suggests that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graph suggests that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
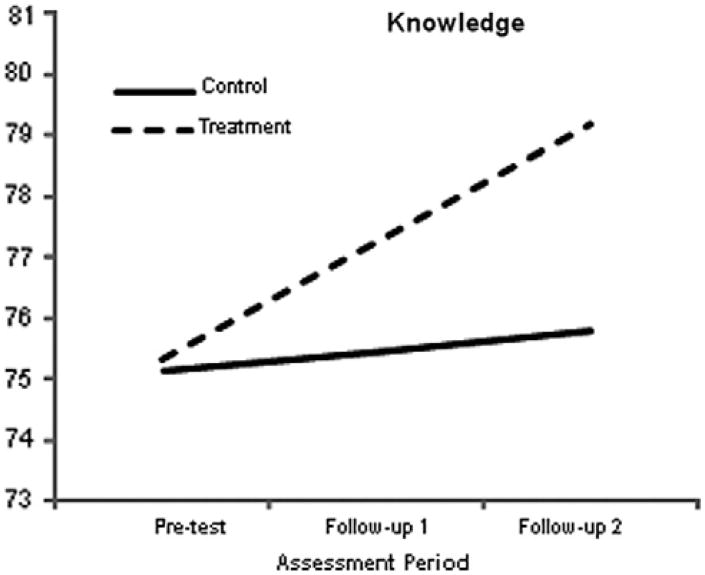
3.5. Changes in pain knowledge
Finally, pain knowledge was assessed by a single scale, the parameter estimates for which are provided in Table 7. Unlike most of the previous measures, a treatment effect for this outcome is evidenced by a score increase. Consistent with the previous outcomes, the growth model produced a significant group-by-time interaction, such that the treatment group knowledge scores improved at a higher rate than those of the control group. To further illustrate these results, Fig. 6 shows the simple slopes for the 2 groups.
Table 7.
Growth model parameter estimates for pain knowledge.
| Parameter | Pain knowledge | ||
|---|---|---|---|
|
| |||
| Est. | SE | p | |
| Control baseline (β0) | 75.14 | 1.06 | .00 |
| Control growth (β1) | 0.31 | 0.51 | .54 |
| Baseline difference (β2) | 0.20 | 1.46 | .89 |
| Growth difference (β3) | 1.60 | 0.78 | .04 |
| Wave 3 effect size | 0.27 | ||
Fig. 6.
Simple slopes for the pain knowledge outcome. The graph suggests that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graph suggests that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
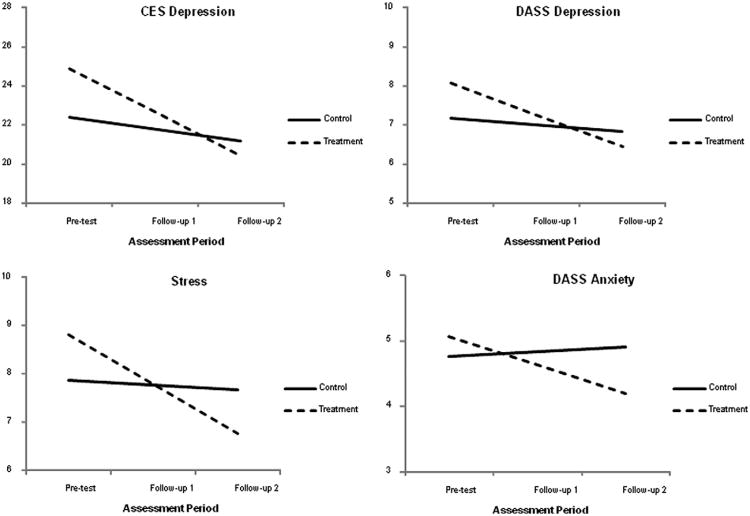
3.6. Changes in psychological functioning
Depression, stress, and anxiety were measured via the Center for Epidemiological Studies Depression scale (CES-D; [33]) and the Depression, Anxiety, Stress Scales (DASS; [23]). Statistically significant interaction effects were observed for all of the 4 scales: depression (both the CES-D and the DASS subscales), stress, and anxiety. Table 8 lists the regression coefficients and standard errors from the analyses. Because the pattern of estimates was largely similar across the 4 significant outcomes, we use the CES-D to illustrate the results. As seen in Table 8, the control group had an average baseline depression score of β0 = 22.40. This group's average change rate was β1 = −0.61, which was nonsignificant (the control group did not show significant change on any of the psychological outcomes). The treatment group had a higher baseline average, although the difference was not statistically significant. Finally, the negative group-by-time interaction coefficient (β3 = −1.64) indicated that the treatment group experienced a greater decrease in psychological problems relative to the control group. To illustrate the results graphically, Fig. 7 shows the average growth trajectories for each of the 4 significant outcomes. The modest end-of-study effect sizes estimates (the values in Table 8 fall below Cohen's small effect benchmark) may be because the treatment group had higher baseline means than the control group.
Table 8.
Growth model parameter estimates for psychological outcomes.
| Parameter | CES depression | DASS stress | DASS anxiety | DASS depression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | |
| Control baseline (β0) | 22.40 | 1.02 | .00 | 7.86 | 0.44 | .00 | 4.76 | 0.35 | .00 | 7.15 | 0.47 | .00 |
| Control growth (β1) | −0.61 | 0.44 | .16 | -0.09 | 0.19 | .63 | 0.07 | 0.17 | .66 | −0.16 | 0.19 | .41 |
| Baseline difference (β2) | 2.50 | 1.45 | .08 | 0.95 | 0.59 | .11 | 0.31 | 0.51 | .54 | 0.92 | 0.66 | .16 |
| Growth difference (β3) | −1.64 | 0.74 | .03 | −0.93 | 0.30 | .00 | −0.51 | 0.26 | .05 | −0.65 | 0.32 | .04 |
| Wave 3 effect size | 0.06 | 0.17 | 0.15 | 0.06 | ||||||||
Fig. 7.
Simple slopes for the CES-D and DASS scales. The graphs suggest that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graphs suggest that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
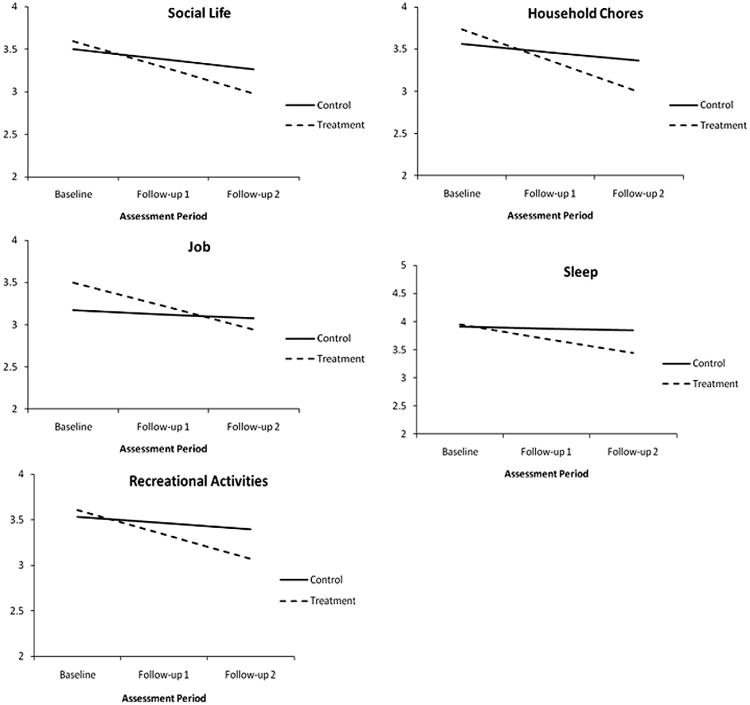
3.7. Changes in pain interference in 10 areas of functioning
The degree to which pain interferes with daily functioning was assessed via interference ratings over 10 different areas of daily functioning (sleep, sex, recreation, social life, work, household chores, self-care, parenting, routine physical activity, and exercise). As seen in Table 9, there was a significant group-by-time interaction effect for the following items: interference with social life, sleep, recreational activities, household chores, and work. With the exception of the social life variable, the control group exhibited nonsignificant change during the course of the study. Furthermore, the baseline mean difference between the intervention and the control group was nonsignificant for all items that produced a significant interaction effect. Fig. 8 depicts the simple slopes for interference with social life, sleep, recreational activities, chores, and work.
Table 9.
Growth model parameter estimates for interference items.
| Parameter | Social life | Sex life | Sleep | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Est. | SE | p | Est. | SE | p | Est. | SE | p | |
| Control baseline (β0) | 3.50 | 0.09 | .00 | 3.28 | 0.13 | 0.00 | 3.91 | 0.09 | .00 |
| Control growth (β1) | −0.12 | 0.05 | .01 | −0.04 | 0.06 | 0.50 | −0.03 | 0.05 | .47 |
| Baseline difference (β2) | 0.09 | 0.13 | .46 | −0.05 | 0.18 | 0.76 | 0.04 | 0.13 | .78 |
| Growth difference (β3) | −0.19 | 0.08 | .01 | −0.14 | 0.10 | 0.18 | −0.22 | 0.08 | .01 |
| Wave 3 effect size | 0.24 | 0.19 | 0.32 | ||||||
| Recreational activities | Household chores | Job | |||||||
|
|
|
|
|||||||
| Est. | SE | p | Est. | SE | p | Est. | SE | p | |
|
|
|||||||||
| Control baseline (β0) | 3.53 | 0.10 | .00 | 3.56 | 0.10 | .00 | 3.17 | 0.14 | .00 |
| Control growth (β1) | −0.07 | 0.06 | .27 | −0.10 | 0.06 | .10 | −0.05 | 0.09 | .59 |
| Baseline difference (β2) | 0.07 | 0.14 | .59 | 0.17 | 0.14 | .22 | 0.33 | 0.19 | .09 |
| Growth difference (β3) | −0.20 | 0.10 | .04 | −0.27 | 0.10 | .01 | −0.23 | 0.12 | .05 |
| Wave 3 effect size | 0.24 | 0.29 | 0.07 | ||||||
| Self-care | Parenting | Physical activities | |||||||
|
|
|
|
|||||||
| Est | SE | p | Est. | SE | p | Est. | SE | p | |
|
|
|||||||||
| Control baseline (β0) | 1.54 | 0.12 | .00 | 0.91 | 0.12 | .00 | 2.71 | 0.13 | .00 |
| Control growth (β1) | 0.04 | 0.06 | .55 | −0.05 | 0.05 | .38 | −0.08 | 0.06 | .22 |
| Baseline difference (β2) | −0.04 | 0.17 | .81 | 0.47 | 0.18 | .01 | −0.01 | 0.18 | .96 |
| Growth difference (β3) | -0.12 | 0.10 | .20 | −0.10 | 0.08 | .23 | −0.12 | 0.10 | .23 |
| Wave 3 effect size | 0.18 | 0.17 | 0.15 | ||||||
| Exercise | |||||||||
|
|
|||||||||
| Est. | SE | p | |||||||
|
|
|||||||||
| Control baseline (β0) | 3.56 | 0.11 | .00 | ||||||
| Control growth (β1) | −0.12 | 0.06 | .03 | ||||||
| Baseline difference (β2) | 0.01 | 0.15 | .96 | ||||||
| Growth difference (β3) | −0.15 | 0.09 | .12 | ||||||
| Wave 3 effect size | 0.20 | ||||||||
Fig. 8.
Simple slopes for areas of interference. The graphs suggest that the amount of time between pretest and follow-up 1 was identical to the time between follow-up 1 and follow-up 2, which it was not. The growth curve analyses modeled change as a function of the square root of time since pretest. This transformation effectively equated the amount of elapsed time between the 2 pairs of assessments. Although the graphs suggest that change was constant, the greatest changes occurred between pretest and follow-up 1. However, this transformation does not affect the end-point differences (ie, the vertical separation of the simple slopes at follow-up 2).
4. Discussion
The present findings suggest that a self-paced, interactive pain management training program undertaken by persons with a variety of pain problems and recruited not from clinics or hospitals but from pain Web sites can achieve measurable, albeit modest, effects on varied indicators of pain adjustment, mental health, and learning. The conditions under which the CPMP was carried out were designed to mirror, as much as possible, those that would obtain in the daily lives of persons with pain. That is, except for the fact that our participants received a small financial incentive for providing us with preprogram and postprogram data, they were free to determine if and when they would avail themselves of all program elements. It is therefore noteworthy that even when including individuals assigned to the experimental group who failed to participate in the program (n = 29), our growth model analyses revealed significant decreases in perceived pain magnitude (severity, interference, and emotional burden), disability, catastrophizing, pain-induced fear, depression, anxiety, stress, interference with areas of living, and increases in pain knowledge.
Generally speaking, our findings relevant to program effects mirror what has been reported in recent reviews/meta-analyses of Web-based interventions [3,24]. That is, the CPMP revealed significant albeit modest reductions in pain-related variables relative to wait-listed control subjects. Notably, however, our attrition rate (25 of 330, 7.6%) was smaller than the average of 26% (yet ranging as high as 99%) reported in several reviews [3,16,35], and the effects for psychosocial outcomes (depression, anxiety, and stress) were more apparent than previously noted. That our participants showed an increase in pain knowledge after program exposure seems to be novel.
Because the growth curve model used as our data analytic strategy has not been widely used in the pain literature, its value may not seem immediately obvious. However, it should be noted that its ability to handle missing data and its capacity to obtain more precise estimates of individual change parameters relative to typical analysis of variance procedures enhances our confidence that the CPMP altered pain adjustment and pain learning. That our effect sizes tended to be modest is not surprising in light of the unsupervised nature of the program.
Despite the comparatively low attrition rate in the present study, it would be premature to conclude that professional support for program participation would not have added significantly to the efficacy of the CPMP. Whether self-paced training such as that provided by the CPMP is employed as an adjunct to face-to-face treatment, or whether clinician participation is used to support or bolster the effects of a fully self-contained e-health program, the combination of online and off-line learning and motivation may well maximize the program's ultimate adaptive yield. Further research is clearly needed that focuses on the pragmatics of merging Internet-based and traditional methods of treatment delivery for persons with chronic pain.
Several interpretive limitations of our findings must also be noted. First, whereas our recruitment from Internet sites is fully consistent with the expected mode of access to and delivery of the CPMP, we are nonetheless restricted in our ability to address the generalizability of the present set of outcomes. As there is no logical or clinical warrant for precluding patient recruitment from hospital and clinic sites, future investigations are needed to address program utility for individuals so recruited. Of course, because most (91%) of our participants reported being under a doctor's care, we anticipate that our results are not seriously population specific. Second, as we sought to be minimally intrusive, we were unable to obtain the sorts of data that would allow us to address fidelity of program use, such as actual time spent on individual program components, or to address possible moderators of program success. But, once again, apparent program success tends to offset (but not fully justify) these omissions. In addition, as we had no control over the treatment-as-usual regimens available to our control participants, we cannot offer strong comparative evaluations of the potency of the CPMP. Interestingly, a recent study comparing an interactive Web site for chronic back pain management [6] against a structured text-based control condition, likewise found support for the relative superiority of the e-program on varied measures of pain adjustment. Nonetheless, although we have some reason to be optimistic that the CPMP provides more than mere expectancy enhancement, we must acknowledge the finding [20] that studies of computer-based intervention with lower levels of methodological quality tend to have a higher likelihood of reporting significant treatment effects relative to control conditions. Thus, we are cognizant of the need to enhance the assessment of treatment fidelity in a fluid medium such as the Internet.
Additionally, despite the differences in slopes between our experimental and control conditions (all in the hypothesized directions) as depicted in Figs. 3–8, future research would need to extend the postprogram follow-up interval to permit us to make justified comparisons between the CPMP and traditional therapist-led pain treatment modalities. Nonetheless, it is important to bear in mind that the CPMP, unlike interventions of fixed session length and fixed program duration, is designed to be used for as long as needed and at the convenience of the individual patient. Indeed, the concept of “follow-up” per se derives from the convention that treatment duration is necessarily restricted by third party payment options, patient finances, and patient-clinician scheduling constraints. By contrast, Internet-based training, retraining, and/or progress monitoring is meant to be a mouse-click away, deliverable on an as needed basis. Thus, computer-aided psychoeducational assessment and training might best be evaluated on a regular and continuous schedule, one defined by patient need rather than logistics or pragmatics.
We also acknowledge that the use of portable interactive interventions depends on patient readiness to assume a level of initiative and responsibility that may not characterize a large segment of children and adults with chronic pain. In fact, even the most conscientious users of the CPMP (ie, the most dedicated and compliant) may not have accrued maximal benefit from its many interactive features (Fig. 2). Future research will need to carefully track program adherence among participants who differ in (among other things) types of pain, levels of pain-related disability, interest in self-managed change, and computer comfort in order to clarify which training elements work best for which types of patients.
Despite the acknowledged limitations of the present study, the present findings offer a sense of cautious optimism in response to Eccleston's [12] query as to whether e-health technology can truly deliver on its promise for persons with chronic pain. Unlike several previous investigations, ours revealed that several psychosocial outcomes (indices of anxiety, depression, stress, and pain-related knowledge) could be improved along with the expected program-induced decreases in pain severity, fear, disability, functional interference, perceived emotional burden, and catastrophizing. It is also worth noting that, unlike several other computer-assisted pain intervention programs that have been formally described and evaluated, the CPMP remains available on the Internet.
Finally, it must be acknowledged that the Internet as a means of clinical services delivery is still in its infancy. The lure of technical advancements in patient monitoring and online program delivery should not overshadow the continuing need to ensure program quality and minimize risk [4,10,34]. Advocates of electronic programs for chronic pain will, in the future, need to (among other things): provide evidence of their effectiveness relative to credible alternatives (not just wait-listed control subjects), ensure that social networking options do not lead to violations of ethics and privacy, address differences in user sophistication and attrition potential, maximize the safety of data storage, and better educate practitioners and the public about the benefits and challenges of computer technology relative to its costs and practical limitations.
Acknowledgments
Supported in part by award R44NS048743 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or those of the National Institutes of Health. The support for this research was administered via the Small Business Innovation Research (SBIR) mechanism of NIH, which expressly encourages the development of a commercial product. The pain program herein described is that product, fully owned by the first 2 authors. The third author declares neither ownership nor conflict of interest
References
- 1.Appel PR, Bleiberg J, Noiseux J. Self-regulation training for chronic pain: can it be done effectively by telemedicine? Telemed J E Health. 2002;8:361–8. doi: 10.1089/15305620260507495. [DOI] [PubMed] [Google Scholar]
- 2.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized control trials. The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 3.Bender JL, Radhakrishnan A, Diorio C, Englesakis M, Jadad AR. Can pain be managed through the Internet? A systematic review of randomized controlled trials. Pain. 2011;152:1740–50. doi: 10.1016/j.pain.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Black AD, Car J, Pagliari C, Anandan C, Cresswell K, Bokun T, McKinstry B, Procter R, Majeed A, Sheikh A. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med. 2011;8:e1000387. doi: 10.1371/journal.pmed.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlbring P, Westling BE, Ljungstrand P, Ekselius L, Andersson G. Treatment of panic disorder via the Internet: a randomized trial of a self-help program. Behav Ther. 2001;32:751–64. [Google Scholar]
- 6.Chiauzzi E, Pujol LA, Wood M, Bond K, Black R, Yiu E, Zacharoff K. painACTION-back pain: a self-management website for people with chronic back pain. Pain Med. 2010;11:1044–58. doi: 10.1111/j.1526-4637.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen S, Oettingen G, Dahme B, Klinger R. A short goal-pursuit intervention to improve physical capacity: a randomized clinical trial in chronic back pain patients. Pain. 2010;149:444–52. doi: 10.1016/j.pain.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. Statistical power for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 9.Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med. 2008;31:169–77. doi: 10.1007/s10865-007-9144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMonaco HJ, von Hippel E. Reducing medical costs and improving quality via self-management tools. PLoS Med. 2007;4:e104. doi: 10.1371/journal.pmed.0040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiIorio C, Escoffery C, McCarty F, Yeager KA, Henry TR, Koganti A, Reisinger EL, Wexler B. Evaluation of WebEase: an epilepsy self-management website. Health Ed Res. 2009;24:185–97. doi: 10.1093/her/cyn012. [DOI] [PubMed] [Google Scholar]
- 12.Eccleston C. Can ‘ehealth’ technology deliver on its promise of pain management for all? Pain. 2011;152:1701–2. doi: 10.1016/j.pain.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Enders CK. Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- 14.Gainsbury S, Bkaszczynski A. A systematic review of Internet-based therapy for the treatment of addictions. Clin Psychol Rev. 2011;31:490–8. doi: 10.1016/j.cpr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Gatchel RJ. Clinical essentials of pain management. Washington, DC: American Psychological Association; 2005. [Google Scholar]
- 16.Geraghty AWA, Wood AM, Hyland ME. Attrition from self-directed interventions: investigating the relationship between psychological predictors, intervention content, and dropout from a body dissatisfaction intervention. Soc Sci Med. 2010;71:30–7. doi: 10.1016/j.socscimed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Jamison RN, Gintner L, Rogers JF, Fairchild DG. Disease management for chronic pain: barriers of program implementation with primary care physicians. Pain Med. 2002;3:92–101. doi: 10.1046/j.1526-4637.2002.02022.x. [DOI] [PubMed] [Google Scholar]
- 18.Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain. 2001;91:277–85. doi: 10.1016/S0304-3959(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 19.Keogh E, Rosser BA, Eccleston C. e-Health and chronic pain management: current status and developments. Pain. 2010;151:18–21. doi: 10.1016/j.pain.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kulik BD, Sugarman DE, Nich C, Gibbons CJ, Martino S, Rounsaville BJ, Carroll KM. A methodological analysis of randomized clinical trials of computer assisted therapies for psychiatric disorders: toward improved standards for an emerging field. Am J Psychiat. 2011;168:790–9. doi: 10.1176/appi.ajp.2011.10101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorig KR, Laurent DD, Deyo RA, Marnell ME, Minor MA, Ritter PL. Can a back pain e-mail discussion group improve health status and lower health care costs? Arch Intern Med. 2002;162:792–6. doi: 10.1001/archinte.162.7.792. [DOI] [PubMed] [Google Scholar]
- 22.Lorig KR, Laurent DD, Ritter PL. Can the Internet affect outcomes? A randomized one year back pain trial. Paper presented at the 109th annual meeting of the American psychological association; San Francisco, CA. 2001. [Google Scholar]
- 23.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2nd. Sydney: Psychology Foundation; 1995. [Google Scholar]
- 24.Macea DD, Gajos K, Calil YAD, Fregni F. The efficacy of Web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J Pain. 2010;11:917–29. doi: 10.1016/j.jpain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Maes S, Karoly P. Self-regulation assessment and intervention in physical health and illness: a review. Appl Psych. 2005;54:267–99. [Google Scholar]
- 26.Marceau LD, Link C, Jamison RN, Carolan S. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Med. 2007;8:S101–9. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 27.Marks I, Cavanagh K. Computer-aided psychological treatments: evolving issues. Ann Rev Clin Psychol. 2009;5:121–41. doi: 10.1146/annurev.clinpsy.032408.153538. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. The tragedy of needless pain. Sci Am. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 29.Moss-Morris R, McAlpine L, Didbury LP, Spence MJ. A randomized controlled trial of a cognitive behavioural therapy based self-management intervention for irritable bowel syndrome in primary care. Psych Med. 2010;40:85–94. doi: 10.1017/S0033291709990195. [DOI] [PubMed] [Google Scholar]
- 30.Naylor MR, Helzer JE, Naud S, Keefe FJ. Automated telephone as an adjunct for the treatment of chronic pain: a pilot study. J Pain. 2002;3:429–38. doi: 10.1054/jpai.2002.129563. [DOI] [PubMed] [Google Scholar]
- 31.Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for anxiety and depression: is human contact necessary for therapeutic efficacy? Clin Psychol Rev. 2010;31:89–103. doi: 10.1016/j.cpr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146:205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;3:385–401. [Google Scholar]
- 34.Rosser BA, McCullagh P, Davies R, Mountain GA, McCracken L, Eccleston C. Technology-mediated therapy for chronic pain management: the challenge of adapting behavior change interventions for delivery with pervasive communication technology. Telemed J E Health. 2011;17:211–6. doi: 10.1089/tmj.2010.0136. [DOI] [PubMed] [Google Scholar]
- 35.Rosser BA, Vowles KE, Keogh E, Eccleston C, Mountain GA. Technologically assisted behavior change: a systematic review of studies of novel technologies for the management of chronic pain. J Telemed Telecare. 2009;15:327–38. doi: 10.1258/jtt.2009.090116. [DOI] [PubMed] [Google Scholar]
- 36.Ruehlman LS, Karoly P, Newton C, Aiken L. The development and preliminary validation of a brief measure of chronic pain impact for use in the general population. Pain. 2005;113:1–10. doi: 10.1016/j.pain.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Ruehlman LS, Karoly P, Newton C, Aiken LS. The development and preliminary validation of the profile of chronic pain: extended assessment battery. Pain. 2005;118:380–9. doi: 10.1016/j.pain.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Sauer SE, Burris JL, Carlson CR. New directions in the management of chronic pain: self-regulation theory as a model for integrative clinical psychology practice. Clin Psych Rev. 2010;30:805–14. doi: 10.1016/j.cpr.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Strom L, Pettersson R, Andersson G. A controlled trial of self-help treatment of recurrent headache conducted via the Internet. J Consult Clin Psych. 2000;68:722–7. [PubMed] [Google Scholar]
- 40.Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: a cognitive-behavioral perspective. New York: Guilford Press; 1983. [Google Scholar]
- 41.Turk D, Rudy T. Neglected topics in the treatment of chronic pain patients–relapse, noncompliance, adherence enhancement. Pain. 1991;44:5–28. doi: 10.1016/0304-3959(91)90142-K. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M. Perspectives on management of back pain in primary care. In: Gebhardt GF, Hammond DL, editors. Proceedings of the seventh world congress on pain, vol. 2. Progress in pain research and management; Seattle, WA. IASP Press; 1994. pp. 97–112. [Google Scholar]
- 43.Webb TL, Joseph J, Yardley L, Michie S. Using the Internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical bases, use of behavior change techniques, and mode of delivery on efficacy. J Med Internat Res. 2010;12:e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ. Internet-enhanced management of fibromyalgia: a randomized controlled trial. Pain. 2010;151:694–702. doi: 10.1016/j.pain.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winzelberg AJ, Eppstein D, Eldredge KL, Wilfley D, Dasmahapatra R, Dev P, Taylor CB. Reducing risk factors for eating disorders: comparison of an Internet- and a classroom-delivered psychoeducational program. J Consult Clin Psych. 2000;68:346–50. [PubMed] [Google Scholar]
- 46.Yates PM, Edwards HE, Nash RE, Walsh AM, Fentiman BJ, Skerman HM, McDowell JK, Najman JM. Barriers to effective casncer management: a survey of hospitalized cancer patients. J Pain Symptom Manag. 2001;23:393–405. doi: 10.1016/s0885-3924(02)00387-1. [DOI] [PubMed] [Google Scholar]