Abstract
Estrogen-negative (ER−) breast cancers have limited treatment options and are associated with earlier relapses. Because glucocorticoid receptor (GR) signaling initiates anti-apoptotic pathways in ER− breast cancer cells, we hypothesized that activation of these pathways might be associated with poor prognosis in ER− disease. Here we report findings from a genome-wide study of GR transcriptional targets in a pre-malignant ER− cell line model of early breast cancer (MCF10A-Myc) and in primary early-stage ER− human tumors. Chromatin immunoprecipitation with massively parallel sequencing (ChIP-seq) coupled to time course expression profiling led us to identify epithelial-to-mesenchymal transition (EMT) pathways as an important aspect associated with GR activation. We validated these findings by performing a meta-analysis of primary breast tumor gene expression from 1378 early stage breast cancer patients with long-term clinical follow-up, confirming that high levels of GR expression significantly correlated with shorter relapse-free survival in ER− patients who were treated or untreated with adjuvant chemotherapy. Notably, in ER+ breast cancer patients, high levels of GR expression in tumors were significantly associated with better outcome relative to low levels of GR expression. Gene expression analysis revealed that ER− tumors expressing high GR levels exhibited differential activation of EMT, cell adhesion and inflammation pathways. Our findings suggest a direct transcriptional role for GR in determining the outcome of poor-prognosis ER− breast cancers.
Keywords: Breast cancer, glucocorticoid receptor, estrogen receptor, ChIP-sequencing, prognosis, meta-analysis
Introduction
Breast cancer, the most frequent cancer in women, is generally classified into estrogen receptor (ER)-positive (ER+) and ER-negative (ER−) subtypes because of the strong prognostic and predictive importance of ER-alpha (1, 2). ER-alpha expression (henceforth, ER+) is associated with a significantly lower five year recurrence rate in part because of the effectiveness of ER-targeted treatments (3) and in part because of a generally more indolent and well-differentiated phenotype when compared to ER− tumors (2, 4). As a consequence, identifying ER positivity (usually through immunohistochemistry or IHC) has become routine clinical practice. Unfortunately, the progress made in treating ER− breast cancer has been less successful. Firstly, ER− tumors are often intrinsically more aggressive and of higher grade than ER+ tumors; secondly, underlying signaling pathways driving ER− breast cancer have been difficult to identify in part because of ER− tumor heterogeneity. Compared to ER+ tumors, relatively few prevention and treatment strategies are available for ER− breast cancer patients (5, 6). Until recently, the only clinically effective treatment for ER− breast cancer was cytotoxic chemotherapy. More recently, anti-angiogenic and PARP-inhibitors have been explored as treatments for ER− breast cancer (7), although their ultimate effectiveness in the ER− breast cancer subgroup is not yet clear. Despite the fact that many “triple-negative” [i.e. ER−, progesterone receptor-negative (PR−) and Human Epidermal Growth Factor Receptor 2-negative (HER2−)] breast cancers are highly chemotherapy-sensitive, a significant proportion demonstrate resistance to chemotherapy and progress rapidly (8).
Recently, the stress hormone (glucocorticoid) receptor (GR) was shown to play an important role in breast epithelial and breast cancer cell biology, particularly in ER− premalignant and breast cancer cell lines (9–11). Using ER−/GR+ breast epithelial and cancer cell lines (e.g. MCF10A-Myc and MDA-MB-231) and global gene expression studies, our laboratory identified several GR-regulated target genes whose protein products mediate cell survival, including serine/threonine-protein kinase1 (SGK1) (12) and the dual specificity protein phosphatase 1 (DUSP1/MKP1) (13). In addition, using a mouse xenograft model of MDA-MB-231 breast cancer cell tumor growth, we found that tumor cell apoptosis induced by paclitaxel is inhibited by pre-treatment with systemic dexamethasone (dex, a synthetic glucocorticoid) (14). We also discovered that increased endogenous glucocorticoid exposure is associated with greater ER− mammary tumor growth in the C3(1) SV40 Tag transgenic mouse model of ER−/PR−/GR+ invasive breast cancer (15). These observations suggest that GR signaling may be an important pathway influencing ER− breast cancer biology.
To further understand the role of GR signaling in ER− breast cancer, we performed genome-wide GR chromatin immunoprecipitation-sequencing (ChIP-seq) and time course gene expression analysis using the ER−/GR+ pre-malignant breast epithelial cell line MCF10A-Myc. We found that GR target genes were enriched in many cancer-related pathways, including epithelial to mesenchymal transition (EMT), cell adhesion, and cell survival. In addition, to examine whether GR-mediated gene expression associates with worse outcome in ER− breast cancer patients, we compiled and analyzed a meta-dataset of early-stage breast cancer patients with associated tumor gene expression and long-term clinical outcome data. In this retrospective analysis of primary tumor gene expression data in n=1378 patients, we found that high GR gene (NR3C1) expression was associated with a significantly shorter relapse-free survival (RFS) in both adjuvant chemotherapy-treated and untreated early stage ER− patients. Unexpectedly, we also found that in ER+ breast cancer, higher GR expression was associated with longer RFS. Thus, both the cell line and primary human tumor data analyses suggest that the GR directly mediates gene expression pathways associated with aggressive behavior and early relapse in the ER− breast cancer subtype.
Materials and Methods
Cell culture
Early passage MCF10A-Myc cells were derived from MCF10A cells (ATCC) transduced with the viral oncogene c-Myc as previously described (9), and checked for Myc expression. Cells were cultured in a 1:1 mixture of DMEM and Ham’s F12 (BioWhittaker) supplemented with hydrocortisone (0.5 µg /ml), human recombinant epidermal growth factor (10 ng/ml), insulin (5 ng/ml), 5% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin. Identical conditions were used for gene expression profiling after dex treatment (16).
GR ChIP assay
MCF10A-Myc cells (1×107) were serum-starved for 3 days and then treated with either dex or ethanol for 1 hour as previously described (9). Cellular DNA and protein were then cross-linked for 20 minutes with formaldehyde (1% final concentration) followed by addition of glycine to a final concentration of 125 mM for 5 minutes. We also took 1% of each lysate to evaluate as input protein and DNA. ChIP experiments were performed according to a standard protocol (Upstate Biotechnology, Milipore). ChIP-grade rabbit polyclonal anti-GR (sc-1003x, Santa Cruz) antibodies were used for immunoprecipitation. We also used normal rabbit IgG (sc-2027, Santa Cruz) as a negative control.
An anti-GR Western blot analysis was performed on a small percentage of the input lysate and the anti-GR immunoprecipitate to assess enrichment of GR by the immunoprecipitation process (see Supplemental Fig.1). Cell lysates or immunoprecipitated complexes were resuspended in 2× Laemmli lysis buffer. The proteins were resolved by SDS-PAGE, transferred to nitrocellulose membrane, blocked with 5% skim milk in TBS-T (0.1% Tween-20 in TBS), and probed with a 1:1000 dilution anti-GR antibody (sc-1003, Santa Cruz). Goat anti-rabbit-horseradish peroxidase (HRP) (Santa Cruz, 1:5000) was used as the secondary antibody. Proteins were visualized using enhanced chemiluminescence (Amersham Pharmacia Biotech, Arlington Heights, IL). After confirming efficient GR immunoprecipitation (Supplemental Figures 1 A and B), we reversed the crosslinks of the immunoprecipitated protein-chromatin complexes according to the manufacturer’s instructions. ChIP DNA was then purified using Qiagen’s PCR Purification KIT.
Quantitative Real-Time PCR
To ensure the quality of the anti-GR antibody ChIP’d DNA, we performed quantitative real time PCR (Q-RT-PCR) as previously described (13) on the promoter regions of SGK1 and TSC22D3/GILZ genes containing established GR binding regions (17, 18). The primers for SGK1’s promoter regions were: 5’-CCCCTCCCTTCGCTTGTT-3’ (sense) and 5’-GGAAGAAGTACAATCTGCATTTCACT-3’ (antisense) (19) ; the primers for TSC22D3/GILZ’s promoter regions were: 5’-GATACCAGTTAAGCTCCTGA-3’ (sense) and 5’-AGGTGGGAGACAATAATGAT-3’ (antisense) (20). Dex- and ethanol-treated ChIP DNA and input samples were analyzed in triplicate.
Deep sequencing and data analysis for ChIP-sequencing
After the quality control experiments described above proved to be successful, the GR ChIP DNA was deep-sequenced using Illumina’s Solexa sequencer [National Center for Genome Resources (NCGR)]. We used the Maq program (Version 0.7.1) (21) to align the resulting sequenced 36-bp tags to the Human Genome (NCBI/b36). Tags that were mapped to more than one location in the human genome were removed. The GR-binding regions (GBRs) were identified using the Model-based analysis of ChIP-Seq (MACs) program (version 1.3.7.1) (22). Input DNA tags were used to call GBRs.
A p-value cutoff of p ≤10−3 was initially used to call the GBRs in both dex and ethanol-treated samples. We then developed more stringent criteria for accurately detecting significant peak signals based on known GR promoter occupancy regions for the two GR target genes, SGK1 (19) and TSC22D3/GILZ (20). In the initial p≤10−3 GBR dataset, we found the number of tags per binding-region (Ntag) in all the SGK1 and TSC22D3/GILZ–associated GBRs in ethanol-treated samples was always ≤ 6, while Ntag in dex-treated samples was always ≥5 (see Supplemental Table 1 for the summary of SGK1 and TSC22D3/GILZ GBR data). Since ethanol-treated samples should not demonstrate significant GR occupancy for these two previously-described dex-dependent GBRs, an Ntag >6 was determined as the cut-off for removing non-dex-dependent GBRs associated with ethanol treatment. Similarly, we identified −log10(p-value) >5.8 (the maximum value of −log10(p) in ethanol-treated samples) as the minimum cut-off for removing likely non-specific GBRs associated with ethanol treatment. Therefore, Ntag>6 and −log10(p)>5.8 became our lower-bound cutoffs for removing likely false-positive (i.e. ethanol-associated) GBRs in the MCF10A-Myc cell ChIP-seq experiments.
We also validated the dex-dependent GR occupancy of the same established promoter region GBRs in SGK1 and GILZ using a directed ChIP-PCR assay (Supplemental Figure 1B). In the same sample subjected to dex-treated ChIP-seq, we found that the Ntag for these two GBRs was Ntag=22 (SGK1) and Ntag=25 (GILZ), respectively. For the promoter GBR for SGK1 and GILZ, the −log10(p-value) was 13.9 and 15.5, respectively. Both values were much higher than the lower-bound cutoffs used to filter out ethanol-associated GBRs (i.e. Ntag> 6 and −log10(p) >5.8). Since SGK1 and GILZ GBRs are known to have very high GR occupancy, Ntag > 22 and −log10(p) >13.9 were likely overly stringent cut-offs for genome-wide identification of GBRs. We therefore took the average of the Ntag for the lower-bound cut-off and for the more stringent cut-off as the final cut-off [(6+22)/2 =14], i.e. Ntag≥15]. Likewise we averaged the −log10(p-value) cut-offs to determine the final cut-off value [(5.8+13.9)/2=9.85 and rounded to 10], i.e. −log10(p-value) ≥10 or p ≤10−10. The final set of GBRs was then identified using both Ntag≥15 and p ≤10−10.
All other statistical analyses and data plotting were done using the R language. Gene pathways were identified using MetaCore database and software suite version 6.6 build 28323 (GeneGo Inc.) Enrichment analysis of transcription factor binding motifs located in GBRs was performed using TRANSFAC version 2009.3 (23, 24).
Data analysis for time course gene expression profiling
Microarray CEL files of the MCF10A-Myc cell time course (2 hrs, 4 hrs and 24 hrs) gene expression profiling following dex or ethanol treatment (16) were downloaded from the Gene Expression Omnibus (GEO) database (GEO ID is GSE4917). We then re-normalized and reanalyzed the original data using the latest version of Robust Multiarray Average (RMA) method (25) in the Bioconductor’s Affy package (26). Up- and down- regulated genes were identified as those with greater than a ±1.5 fold change in the average of dex- or ethanol-treated values from three independent experiments at any one time point.
Compiling and normalizing tumor expression microarray meta-dataset
Breast cancer gene expression microarray CEL files derived from early stage primary tumors with clinical follow-up from eight well-annotated studies (Table 1) were downloaded from GEO. To minimize potential cross-platform bias, we only used data obtained from Affymetrix’s U133A and/or U133+2 platforms (Affymetrix’s U133A and U133+2 are essentially the same chip except that U133+2 contains more probesets than U133A). Duplicate patient samples were identified by both GEO ID and gene expression and removed from the dataset. Assuming that tumor microarray analyses within the same “batch” or hospital/lab source were performed under similar conditions, we first grouped samples by institution. We then used the unscaled standard error (NUSE) analysis in Bioconductor’s AffyPLM package (27, 28) to remove poor quality arrays within each institutional batch. Next, we performed an RMA normalization (25) within each study group using Bioconductor’s Affy package (26) to adjust the background noise. To remove batch effects between study groups, we performed a within-array normalization by subtracting the array average expression of five well-established housekeeping genes (ACTB, GAPDH, GUSB, RPLP0 and TFRC, altogether 19 probesets) from the expression of each probeset. We confirmed that the variation of the mean expression of these five housekeeping genes was very small in individual datasets (all coefficients of variation were <0.03), ensuring that the normalized values were comparable across the groups. A similar normalization strategy for Q-RT-PCR assessment of mRNA has been used on the commercially available Oncotype DX assay (29).
Table 1.
Datasets of early stage invasive breast cancer used for the meta-analysis
| Study | GEO ID | Platform | ESR1+/ESR1− | Adjuvant Chemo |
Tamoxifen | Reference |
|---|---|---|---|---|---|---|
| 1 | GSE2034 | U133A | 200/80 | 0 | 0 | Wang et al. 2005 (42) |
| 2 | GSE2603 | U133A | 35/38 | “Vast Majority” | Unknown | Minn et al. 2005 (43) |
| 3 | GSE2990 | U133A | 148/33 | 0 | 59 | Sotiriou et al. 2006 (44) |
| 4 | GSE6532 | U133A | 105/9 | 0 | 114 | Loi et al. 2007 (45) |
| U133+2 | 82/1 | 0 | 83 | |||
| 5 | GSE7390 | U133A | 115/74 | 0 | 0 | Desmedt et al. 2007 (46) |
| 6 | GSE9195 | U133+2 | 74/3 | 0 | 77 | Loi et al. 2008 (47) |
| 7 | GSE11121 | U133A | 162/29 | 0 | 0 | Schmidt et al. 2008 (48) |
| 8* | GSE12276 | U133+2 | 103/87 | 14 | 17 | Bos et al. 2009 (49) |
| Total | 1024/354 | |||||
Treatment information was only available for a subset of these patients.
Determination of ER (ESR1) and GR (NR3C1) expression in the meta-dataset
We performed a receiver operating characteristic (ROC) analysis using the Bioconductor’s genefilter package (30) on the known ER (IHC) status of tumors in the meta-dataset (799 ER+ and 208 ER−) and confirmed that the ESR1 205225_at probeset was most accurate for predicting ER IHC status (31) (Supplemental Figure 2A). We then calculated the Youden Index [J = MAX(specificity+sensitivity−1)] of the 205225_at probeset ROC curve (32, 33), and took the corresponding normalized expression of the probe as the cut-off point to determine ER status. This cutoff value was −3.416, meaning that if ESR1 expression was greater than −3.416, the tumor was identified as ESR1+; otherwise it was considered to be ESR1−.
Because there are no available GR IHC datasets coupled to gene expression data, we were unable to identify a GR gene (NR3C1) expression cut-off using ROC analysis. There are four NR3C1 probesets: 201865_x_at, 201866_s_at, 211671_s_at and 216321_s_at on the Affymetrix’s U133A and U133+ arrays. We used 216321_s_at to represent the expression of NR3C1 because: 1) only 216321_s_at hybridizes with all NR3C1 mRNA isoforms (Supplemental Figure 2B); 2) only the probe sequences (11 for each probeset) of 216321_s_at and 211671_s_at can be all aligned perfectly (100% score) with NR3C1 (by Blat (34), human genome NCBI36/hg18); 3) based on BioGPS (35), 216321_s_at shows the most diverse tissue expression of the four probesets. We then used the highest and lowest quartiles (25%) of NR3C1 probeset expression as a cutoff to identify “high” and “low” GR (NR3C1) tumor expression, respectively.
Relapse-free survival and gene pathway analysis for the meta-dataset
Relapse-free survival (RFS) was estimated using the Kaplan-Meier method. RFS between groups was compared using the log-rank test. Hazard ratios were calculated using the Cox proportional hazards regression model. All survival analyses were performed using the survival package in R (36).
Genes that were differentially expressed between NR3C1 (GR)-high versus -low tumors were identified using SAM analysis (37) in R’s siggene package (38). We also used the MetaCore software suite (GeneGo Inc.) to identify significant gene pathways from differentially expressed genes between GR-high and -low tumors. All other statistical analyses were done using the R language.
Results
GR ChIP-sequencing reveals evidence of EMT and immune gene regulation
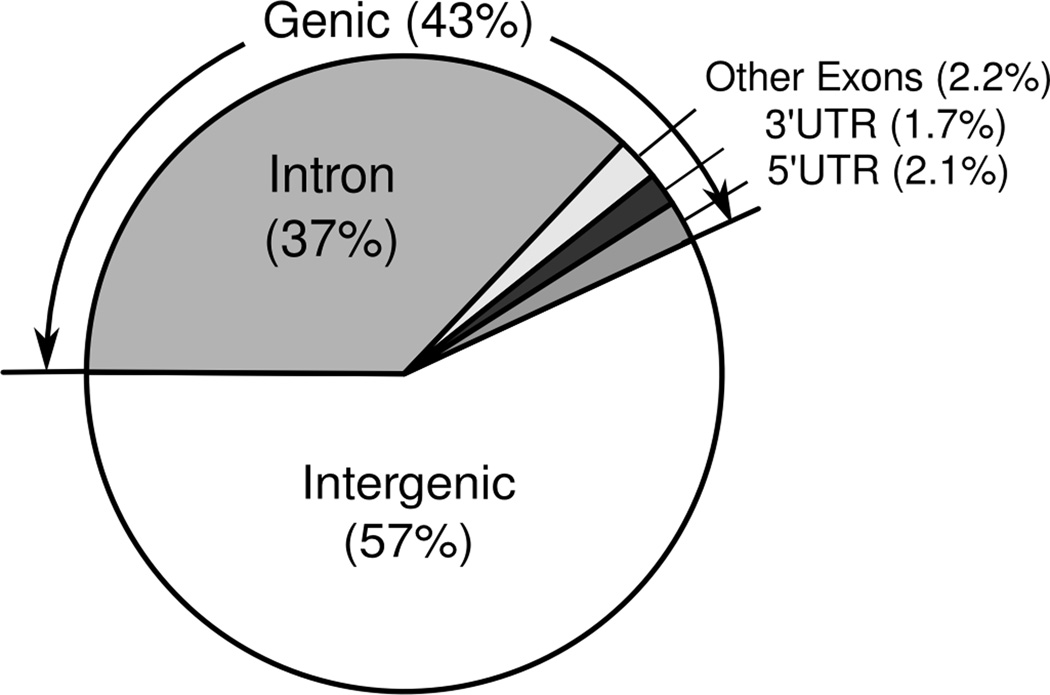
To examine the genome-wide GR binding regions in a model of ER− breast cancer, MCF10A-Myc cells were subjected to GR ChIP-sequencing (39). The genomic locations of the sequence tags obtained from deep-sequencing were mapped using the UCSC’s human genome version NCBI36/hg18. The number of uniquely mapped 36 bp tags was 1.34 × 107 in the dex-treated sample and 0.83 × 107 in the ethanol-treated sample, suggesting significantly more GR binding events following dex treatment. Using our customized threshold values developed from well-validated GBRs (SGK1 and GILZ), we identified 1533 GBRs following dex treatment and 126 GBRs following ethanol treatment. Only 18 dex-associated GBRs were found within 100kb of any ethanol-associated GBRs. The remaining 1515 GBRs appeared to be highly specific for ligand-bound GR (a list of dex-specific GBRs is provided in Supplemental Table 2). Figure 1 shows that more than half of these dex-specific 1515 GBRs were located in intergenic regions. Even within genes, most of the GBRs were located in introns, showing the typical distribution of genome-wide binding sites reported previously for ligand-bound nuclear receptors such as ER-alpha (40). GR response elements (GREs) were found in 66% (1000/1515) of the dex-dependent GBRs identified, making the canonical GR-binding motifs the 3rd most commonly identified transcription factor. The ten most common transcription factor motifs identified using TRANSFAC analysis are shown in Supplemental Table 3.
Figure 1. Relative genomic locations of GR binding regions (GBRs) identified by ChIP-seq.
MCF10A-Myc cells were treated with dex (10−6M) or vehicle for one hour followed by GR ChIP-sequencing. Dex-specific GBRs (n=1515) were identified and then mapped to the human genome (NCBI36). The relative percentage of GR binding regions (GBRs) in genic vs. intergenic locations is indicated.
GR-target gene identification was based on the presence of at least one GBR within 100kb of a gene’s transcript starting site (TSS). GR target genes were further classified into two categories based on the physical distance of the GBR from the TSS: 1) 0 to 10 kb (a proximal and traditional “promoter” location); and 2) 10 to 100 kb (a distal and traditional “enhancer” location). Using these criteria, 286 target genes had proximal GBRs and 2044 genes had distal GBRs; 133 genes demonstrated GR occupancy in both regions. Therefore, GR-binding occurred more frequently in the distal/enhancer regions of target genes, although the density of GR occupancy was higher in the more proximal regions [28.6 (proximal) vs. 22.7 (distal) hits/kb].
Because GR-chromatin occupancy does not guarantee a transcriptional event, we refined our list of likely direct GR target genes using time-course gene expression data from MCF10A-Myc cells (GEO accession ID is GSE4917) (16). This represents the steady-state mRNA expression following treatment with dex (10−6 M) versus ethanol for 2, 4 and 24 hrs. In total, 680 genes were significantly regulated (an average of ≥ 1.5 or ≤ −1.5 fold change compared to the ethanol vehicle at any one or more time points). Among them, 517 genes were upregulated and 163 genes were downregulated. There was no overlap between the gene sets of upregulated and downregulated genes.
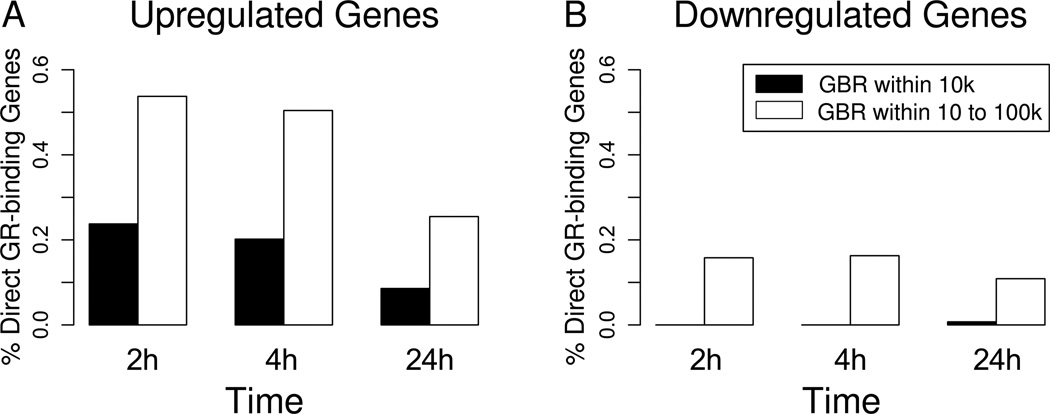
By examining the MCF10A-Myc cell line ChIP-seq target genes and the gene profiling list, we identified n=187 overlapping genes, among which 51 had proximal GR binding regions (GBRs), 160 had distal GBRs, and 24 had both. Putative direct target genes identified by overlapping the ChIP-seq-identified genes with the gene profiling lists (either up or downregulated) were compared to the total number of differentially expressed genes at each time point (Figure 2). This revealed a much larger proportion of directly upregulated genes composing the more immediate 2 and 4 hour time points compared to the later 24 hour time point (Figure 2A). This finding is consistent with the direct GR-mediated transcriptional activation of these earlier genes and was true regardless of the proximal versus distal location of the GBR. Interestingly, a much lower proportion of repressed genes (Figure 2B) were identified as direct GR target genes. This suggests that the GR more commonly mediates transcriptional repression through indirect mechanisms (e.g. due to sequestration of activating transcription factors such as NF-Kappa B), rather than through direct GR occupancy of “repressive” DNA elements. In those downregulated genes that were identified by gene expression studies and also demonstrated GR occupancy by ChIP-seq, the GR bound almost exclusively to regions >10kb distal to the TSS, consistent with previous observations in lung cancer cells (41). Finally, unlike upregulated genes which mainly showed significantly increased expression 2 and 4 hours following Dex treatment, the proportion of genes with downregulated mRNA steady-state levels following GR activation was approximately equal at all time points, suggesting that the kinetics of GR-mediated gene repression may be significantly influenced by variable mRNA degradation rates following repression of gene transcription.
Figure 2. Relative percentage of glucocorticoid-regulated genes with proximal (0–10kb) versus distal (10–100kb) GBR locations.
GR direct target genes were identified as having at least one GBR within 100kb of the transcription start site (TSS) and as showing at least 1.5-fold gene expression change at the designated time point following treatment with dex (10−6M) relative to vehicle. The percentage represents the proportion of upregulated (or downregulated) genes with associated GBRs mapping within 10kb (black bars) versus 10 to 100kb (white bars) of the TSS.
We next performed a gene enrichment pathway analysis to identify the functional pathways associated with the 187 GR “direct” target genes that were identified in the MCF10-A Myc pre-malignant breast cancer cell line following GR activation (Table 2). The top 10 most significant pathways included: GnRH activation, epithelial mesenchymal transition (EMT), immune regulation and proliferation, all of which are critical pathways associated with clinical outcome in breast cancer. Taken together, the MCF10A-Myc cell line data suggested that GR activation directly regulates genes associated with aggressive breast cancer biology.
Table 2.
Top 10 gene enrichment pathways identified from GR-target genes in MCF10A-Myc cells*
| NO. | GeneGo Pathway | pValue | Differentially expressed genes in the pathway |
|---|---|---|---|
| 1 | GnRH signaling | 1.54e-5 | JunB, MEF2D, DUSP-1, DUSP-2, PER1, PLC-beta, p90Rsk |
| 2 | Serpin F1 signaling | 1.69e-5 | JunB, NFKBIA, c-FLIP (L), c-FLIP (S), c-IAP1, c-IAP2 |
| 3 | ECM remodeling | 2.40e-5 | Fibronectin, IGF-2, Matrilysin, PAI1, TIMP3, VIL2 |
| 4 | NOTCH1-mediated pathway for NF-KB activity modulation | 3.59e-5 | I-kB, IL-1RI, NFKBIA, SAP30, SMRT |
| 5 | IL-17 signaling pathways | 5.49e-5 | C/EBPbeta, C/EBPdelta, ENA-78, GCP2, GRO-1, I-kB |
| 6 | Regulation of epithelial-to-mesenchymal transition (EMT) | 7.92e-5 | Caldesmon, Fibronectin, IL-1RI, PAI1, SLUG, TGIF |
| 7 | Thrombopoetin signaling via JAK-STAT pathway | 9.64e-5 | PIAS1, PIAS3, SERPINA3, SOCS1 |
| 8 | Signaling pathway mediated by IL-6 and IL-1 | 2.22e-4 | C/EBPbeta, I-kB, IL-1RI, SOCS1 |
| 9 | ATM/ATR regulation of G1/S checkpoint | 4.35e-4 | GADD45alpha, GADD45beta, I-kB, Nibrin |
| 10 | TNFR1 signaling pathway | 1.36e-3 | I-kB, c-FLIP (S), c-IAP1, c-IAP2 |
Genes (n=187) that were 1.5-fold differentially expressed [upregulated (n=168) or downregulated (n=19)] following glucocorticoid (dexamethasone 10−6 M) treatment and demonstrated GR occupancy within 100kb of their TSS by GR ChIP-seq analysis were analyzed for pathway enrichment using GeneGO’s Metacore Version 6.6 (see Materials and Methods for details). Bolded genes appear in more than one pathway.
Meta-analysis of primary patient tumor gene expression
Given the pathways revealed by GR transcriptome analysis above, and because GR activation in ER− breast cancer cell lines has been shown to promote cell survival, chemotherapy resistance, and increased tumor growth in a pre-clinical xenograft model of human breast cancer (14), we next asked whether high versus low GR expression in primary breast cancers is associated with worse outcome in early stage ER− breast cancer patients. To test this hypothesis, we compiled a meta-dataset of the eight publically available Affymetrix gene expression studies of early-stage primary breast tumors in patients with long-term clinical follow-up (42–49) (n = 1378, summarized in Table 1).
Because the longer time to relapse in ER+ versus ER− breast cancers suggests clinically different disease entities, we wished to analyze these tumor subsets independently. However, ER IHC information was not available for all tumors; therefore we used ER-alpha gene (ESR1) expression to determine ER status. Using the expression of ESR1 probeset 205225_at as determined by ROC analysis (AUC=0.939), we identified 1024 ESR1+ and 354 ESR1− tumors. For GR expression, we were not able to identify a GR gene (NR3C1) expression cut-off using ROC analysis because there are no publicly available GR IHC datasets coupled with NR3C1 gene expression data. Published GR IHC studies, however, have shown a range of 15% – 40% GR positivity in invasive breast cancers; this variation is likely dependent on tumor subtype, patient age, ancestry, sample conditions and antibody (10, 50, 51). As described in the Materials and Methods, because most studies show that at least 25% of breast cancers express significant GR by IHC, we used the highest and lowest quartiles (top and bottom 25%) of NR3C1 probeset 216321_s_at expression to identify high vs. low tumor GR expression.
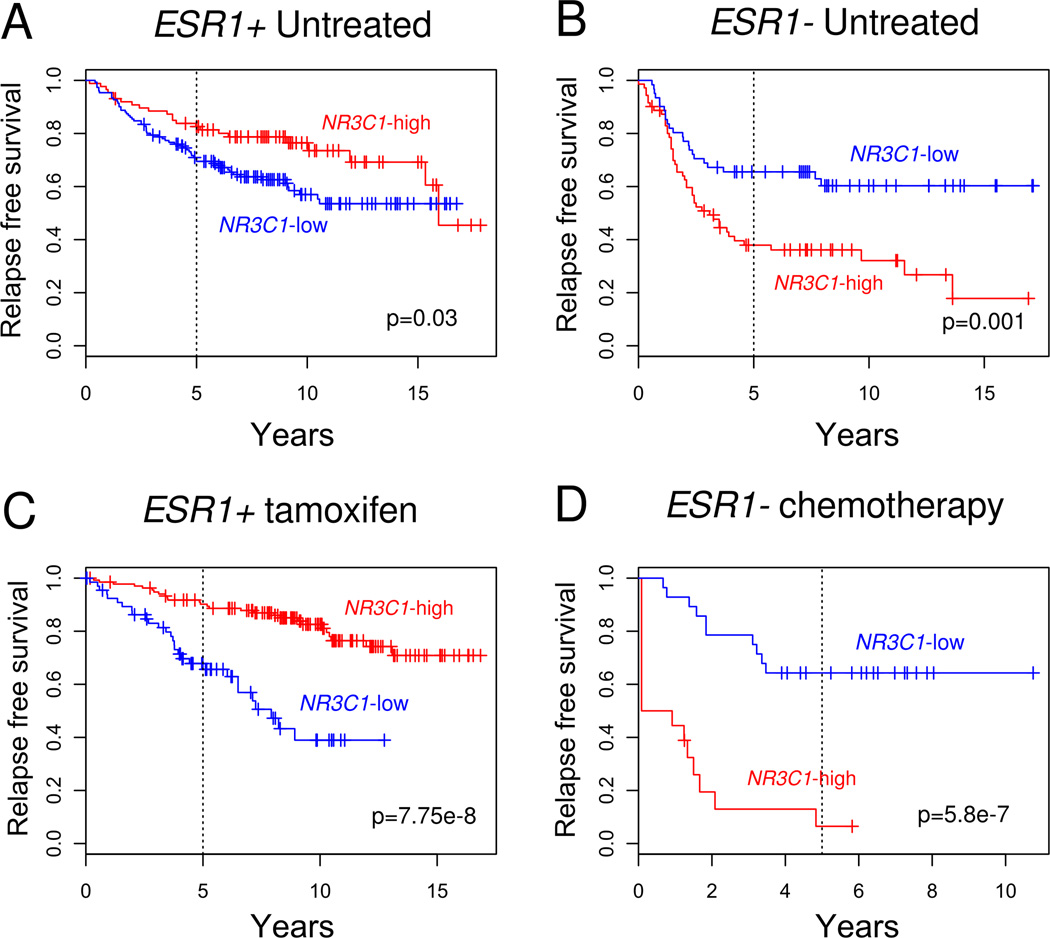
Next we used the Kaplan-Meier (KM) method to estimate relapse-free survival (RFS) for patients with NR3C1-high versus -low expressing tumors (Figure 3). We stratified patients into untreated and adjuvant-treated (tamoxifen treatment for ESR1+ patients and adjuvant chemotherapy for ESR1− patients) groups. As hypothesized, we found that in ESR1− tumors, high NR3C1 expression was indeed associated with significantly worse outcome in both the untreated (p = 0.001, log-rank test; HR=2.23) and adjuvant chemotherapy-treated (p= 5.8e-7, log-rank test; HR= 6.83) groups. Because the analysis was not performed prospectively, the predictive ability of GR expression (with respect to chemotherapy) in ER− breast cancer cannot be assessed, but the data suggest that high GR expression is associated with a relatively poor prognosis regardless of adjuvant chemotherapy treatment (Figure 3B and 3D). Unexpectedly, we observed the reverse association between high NR3C1 gene expression and clinical outcome in ESR1+ patients (Figures 3A and 3C). In ESR1+ cancer, both untreated (p=0.03, log-rank test; HR=0.60) and tamoxifen-treated (p=7.75e-8, log-rank test; HR=0.25) patients with high NR3C1 tumors had better outcome compared to patients with low NR3C1-expressing tumors. Therefore, the phenotypic context of ER expression appears to reverse the association of high GR expression with a poor outcome in breast cancer.
Figure 3. Kaplan-Meier estimates of relapse-free survival (RFS) for early stage breast cancer patients with NR3C1-high versus -low tumors, by ESR1 status.
Tumors in the top quartile of NR3C1 expression were identified as “NR3C1-high,” while tumors in the bottom quartile of NR3C1 expression were identified as “NR3C1-low.” A, ESR1+ untreated NR3C1-high patients (n=87) had a better outcome (p=0.03, log-rank test; HR=0.60) than NR3C1-low untreated patients (n=151). B, ESR1−/ NR3C1-high patients who did not receive adjuvant chemotherapy (n=71) had a worse outcome (p=0.001, log-rank test; HR=2.23) than NR3C1-low untreated patients (n=61). C, ESR1+/NR3C1-high patients (n=136) who received adjuvant tamoxifen treatment had a significantly better outcome (p=7.75e-8, log-rank test; HR=0.25) than ESR1+ / NR3C1-low adjuvant tamoxifen treated patients (n=68). D, ESR1− / NR3C1-high patients who received adjuvant chemotherapy treatment (n=18) had a significantly worse RFS (p=5.8e-7, log-rank test; HR=6.83) than ESR1− / NR3C1-low adjuvant chemotherapy-treated patients (n=28).
To be certain that these results were not confounded by an association of NR3C1 expression with the expression of other receptors used in breast cancer prognosis, we also calculated the individual correlation coefficients between GR gene expression (216321_s_at) and genes encoding the progesterone receptor (PR), androgen receptor (AR) and the Human Epidermal Growth Factor Receptor 2 (HER2). We found the correlation between GR expression and other receptors to be weak (Table 3), making it unlikely that expression of these receptors influenced our results. One of the HER2 probesets (210930_s_at) showed about a 30% negative correlation with NR3C1 expression. The influence of this group of HER2+ tumors on patient clinical outcome was therefore examined by excluding those patients in the top HER2 expression quartile from the ESR1− dataset and then reanalyzing the remaining patients. Similar results, i.e. a significantly worse prognosis in NR3C1-high tumors, were observed when we excluded the HER2-high patients (see Supplemental Figure 3), suggesting that HER2-positivity does not confound the association of high NR3C1 expression with a poor prognosis among ESR1− patients.
Table 3.
Low correlation between NR3C1 (216321_s_at) expression and genes encoding clinically relevant breast cancer receptors
| NR3C1(216321_s_at) vs. | Correlation coefficient | ||
|---|---|---|---|
| Protein/Gene | Probeset ID | ESR1+ | ESR1− |
| PR/PGR | 208305_at | 0.0088 | −0.2026 |
| AR/AR | 211110_s_at | −0.1387 | 0.0338 |
| HER2/ERBB2 | 210930_s_at | −0.2929 | −0.1444 |
| HER2/ERBB2 | 216836_s_at | −0.0242 | −0.0146 |
Gene pathways enriched in NR3C1-high versus -low tumors
We next examined the differences in global gene expression between high GR− versus low GR-expressing tumors among ER+ and ER− breast cancers as determined by tumor ESR1 expression. We performed a Significance Analysis of Microarray (SAM) (37) and identified genes demonstrating a significant difference in expression (≥ 1.5 or ≤−1.5 fold change, FDR ≤ 1%) in NR3C1-high vs. NR3C1-low tumors (Fig. 4).
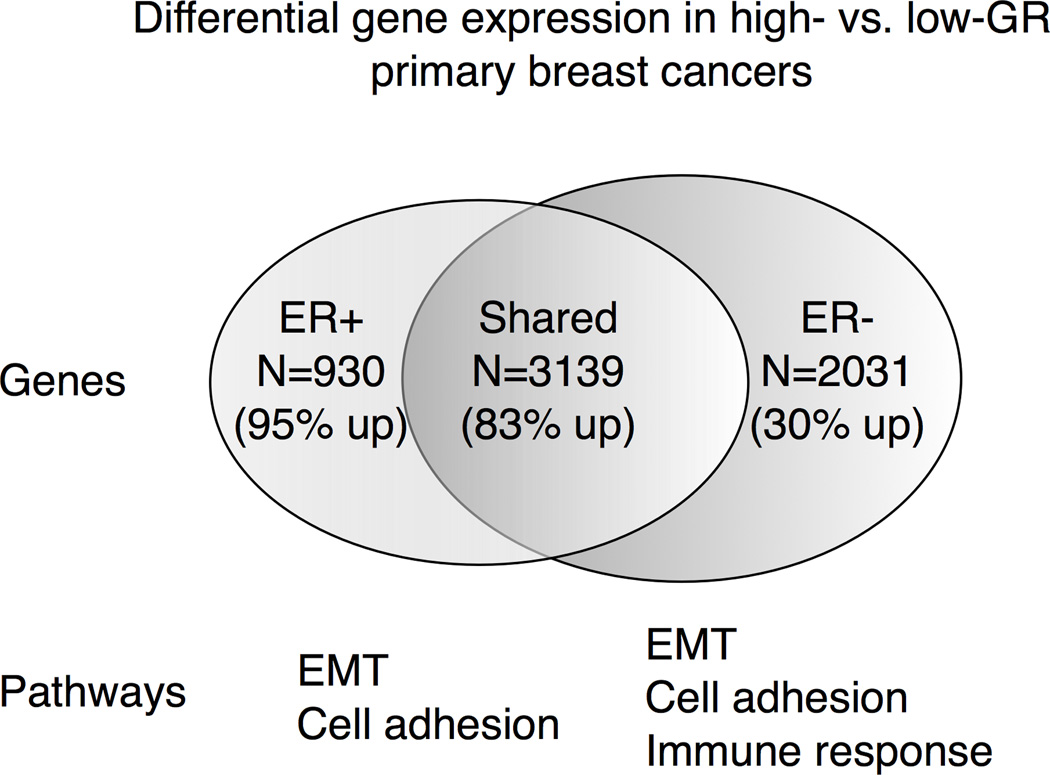
Figure 4. Genes and gene expression pathways associated with NR3C1-high (versus NR3C1-low) tumors divided by ESR1 (ER) status.
The number of differentially expressed genes (either 1.5-fold induced or repressed) between the highest versus lowest NR3C1 expression quartiles in ESR1+ and ESR1− breast cancers is shown. GeneGo’s MetaCore gene enrichment pathway analysis was then used to identify highly significant biological pathways from these differentially expressed genes in (ESR1) ER+ and (ESR1) ER− tumors.
We found that in ESR1+ tumors, n=4069 genes were differentially expressed in NR3C1-high versus NR3C1-low tumors. Among these genes, n=3488 were significantly upregulated and n=581 were downregulated. In ESR1− tumors, n=5170 genes were significantly differentially expressed, among which n=3209 were upregulated and n=1961 genes were downregulated. We then classified these differentially regulated genes into three groups: (1) Genes regulated exclusively in ESR1+ tumors (n=930, 882 upregulated and 48 downregulated); (2) genes regulated exclusively in ESR1− tumors (n=2031, 603 upregulated and 1428 downregulated) and (3) shared genes commonly regulated in both ESR1 subtypes (n=3139, 2606 upregulated and 533 downregulated) (Fig. 4). Thus, the majority of genes associated with high NR3C1 expression were shared between ESR1− and ESR1+ tumors, although there were important differences among the uniquely expressed genes. For example, among the genes uniquely expressed in ESR1+ breast cancers, the majority of genes were upregulated, while in ESR1− tumors, the majority were downregulated, suggesting an influence of ER context on modulating the direction of GR-associated gene expression.
We then performed a gene enrichment pathway analysis using GeneGo’s MetaCore software suite to identify the most significant GR-associated pathways found in the ESR1+ and ESR1− breast cancers (Fig. 4). Top pathways that were identified involved EMT, cell adhesion and the immune response. Somewhat surprisingly, we found that EMT-associated genes were differentially expressed in both the ESR1+ and ESR1− breast cancer subtypes, suggesting that high GR expression influences EMT–associated gene expression in an ER-independent fashion. Many cell adhesion pathway genes were also identified in both ESR1+ and ESR1− breast cancers, but the majority of genes in these pathways were upregulated in ESR1+ breast cancer and downregulated in ESR1− tumors. Interestingly, a significant number of immune response pathway genes were only identified in ESR1− breast cancers, suggesting that GR-associated modulation of the immune response may play a specific role in ER− breast cancer outcome.
Overlap of ER− cell line and ESR1− primary human tumor data reveals GR-associated biology
We next asked how the ER− cell line data and ESR1− human primary tumor data compared with respect to overlapping GR-associated gene expression. We examined genes that were common to both the n=2031 differentially regulated genes from ESR1−/GR-high tumors (Figure 4) and the gene set (n=187) derived from our cell line ChIP-sequencing and gene expression data. We found that n=90 genes were identified in both analyses and likely represented direct GR target genes (Table 4). As hypothesized, these genes encoded anti-apoptotic proteins previously identified as critical to GR-mediated tumor cell survival and chemotherapy resistance such as SGK1 and DUSP1/MKP1. Unexpectedly, genes encoding proteins central to EMT (e.g. SNAI2/SLUG), chromatin remodeling (e.g. SMARCA2), and epithelial cell-inflammatory cell interactions (e.g. IL1R1 and PTGDS) were also identified. Thus, the combined cell line and primary human tumor data analysis suggest that the GR directly mediates pathways associated with aggressive breast cancer biology. Taken together with the high recurrence rate found in ER− patients with high GR-expressing early breast cancers, these finding suggest that GR expression may be a useful marker of high-risk early stage ER− breast cancer as well as a potential therapeutic target in these cancers.
Table 4.
GR target genes (n=90) common to 1) MCF10A-Myc ChIP-seq, 2) MCF10A-Myc gene expression profiling and 3) ER−/GR-high (versus GR-low) primary human tumor gene expression
| GBR location relative to TSS |
Target genes found among all three datasets | ||
|---|---|---|---|
| Related Functions | Genes | ||
| From 0 to 10kb | Upregulated | Cell Survival/ Proliferation/Apoptosis | C5AR1, DUSP1, RPS6KA2, SGK1 |
| Transcription/Chromatin Remodeling | PDE4DIP, SFRS13A, SMARCA2, ZFAND5, SAP30 | ||
| EMT/Cell Shape | PTGDS, SNAI2, EZR | ||
| Inflammation | F3, NFKB1A, SAA1 | ||
| Lipid/fatty acid metabolism | PLCB4 | ||
| Miscellaneous | GRAMD3, SLC46A3 | ||
| Down-regulated | None | None | |
| From 10 to 100kb | Upregulated | Cell Survival/Proliferation | BIRC3, CYR61, DUSP1, GADD45A, GADD45B, IGF2, IRS2, LEPR, LYRM1, MAP4K3, MCL1, NBN, NEDD9, PDE4DIP, PIAS1, PLCB4, PTGDS, RGS2, RHOBTB3, RPS6KA2, RTN1, SESN1 |
| Transcription/Chromatin Remodeling | CEP350, GTF2H1, NBN, NNMT, SMARCA2, TSC22D3, ZFAND5, ZFP36L2, ZNF395 | ||
| EMT/Cell Shape | ACTR2, ADI1, CALD1, CDC42EP3, CYR61, DPT, GPM6B, HPS5, IQGAP1, NEBL, NEDD9, PALLD | ||
| Inflammation, Macrophage Function | CPM, CXCR4, CPM, IL1R1, SAA2 | ||
| Ion Transport, Energy | ATP2B4, SLC26A2, STOM | ||
| Lipid/Fatty Acid Metabolism | ACSL1, STXBP3, TFPI | ||
| Miscellaneous | COPS8, GRAMD3, LASS6, NACC2, PDLIM5, PICALM, RTN1, TTC37 | ||
| Down-regulated | Transcription/Chromatin Remodeling | ARID1A | |
| Apoptosis | LIF | ||
Bolded genes demonstrate GR occupancy in both proximal and distal genomic regions.
Discussion
In the clinical treatment of breast cancer, tumors are initially classified by ER, PR and HER2 expression. This classification guides both therapy and prognosis. Compared to early stage ER+ breast cancer, similar stage ER− breast cancer is more aggressive and relapses earlier. In addition, ER− patients have relatively few options for adjuvant treatment because only ER+ patients benefit significantly from anti-estrogen agents (5, 6). As a follow-up to our previous studies examining the molecular targets of GR activation that promote cell survival in ER− breast epithelial and cancer cells (12–15), this study identified both genome-wide GR target genes in an ER− breast epithelial cell line and GR-associated genes in early ER− breast cancers. In our meta-analysis of 1378 early stage breast cancer patients, we found that patients with ER−/GR-high tumors have significantly increased risk of early relapse compared to patients with ER−/GR-low tumors. Unexpectedly, we also found that high GR expression was associated with better outcome in ER+ breast patients, suggesting potential cross-talk between the ER and GR (52, 53). Recently, loss of the E2F1-associated gene signature was also found to associate with a reversed prognosis in ER+ versus ER− breast cancers, similarly suggesting the importance of ER expression in the activity and function of transcriptional networks in breast cancer (54). The unexpected finding of an improved RFS time for breast cancer patients with GR-high/ER+ tumors may in part be due to antagonism between ER and GR signaling (55). For example, in ER+ MCF-7 cells, glucocorticoid treatment inhibits the growth-stimulatory effect of estrogen (56, 57). Conversely, ER activation can also inhibit glucocorticoid action. Specifically, activated ER can induce the expression of the protein phosphatase 5 (PP5) gene, which in turn mediates the dephosphorylation and inactivation of the GR at Ser211 (52). Therefore, unlike ER− tumors, where GR regulates genes independently of estrogen and ER-alpha action, in ER+ tumors, ER may alter GR action and the GR may in turn also alter a subset of important ER-regulated genes involved in tumor growth. This hypothesis will be explored in further studies examining the role of ligand-activated ER-alpha expression in GR chromatin occupancy and transcriptional activity.
Analysis of gene expression pathways based on our meta-dataset of human tumors revealed that the EMT pathway is associated with high GR expression in both ER− and ER+ tumors, suggesting that GR expression may mediate transcriptional activation of fundamental cytoskeletal genes. Interestingly, however, cell adhesion pathway gene expression was differently regulated between ER− and ER+ tumor subtypes; in ER+ tumors, genes encoding cell adhesion proteins were generally upregulated while in ER− tumors they were downregulated. In contrast, inflammatory and macrophage-associated genes (i.e. immune response) were uniquely identified as differentially expressed only in the ER− tumors. Furthermore, the upregulation of several genes encoding proteins involved in epithelial cell - macrophage attraction such as DAP12 and Syk was observed in the gene set common to both the ER− cell line data and ER− primary tumor gene expression. This interesting finding suggests that GR activity plays an important role in tumor epithelial cell communication with the microenvironment in the ER− breast cancer subtype (58).
Based on these findings, the role of GR overexpression in ER− breast cancer should be evaluated prospectively in trials of early stage patients undergoing adjuvant therapy. Eventually, the presence of high GR expression might be a useful tool to determine a recurrence threshold for employing systemic treatment in ER− patients with <1.0 cm node-negative breast cancer who currently do not routinely receive adjuvant systemic therapy but appear likely to relapse if they have the ER−/GR+ subtype. Furthermore, GR expression may represent a novel therapeutic target for chemotherapy-resistant ER− breast cancer that should be explored with the new second generation specific GR antagonists currently under development.
Supplementary Material
Acknowledgments
We thank Drs. Gini Fleming and Geoffrey Greene for valuable discussions and Dr. John Foekens for sharing unpublished data.
Grant funding: The AVON Foundation for Women, NIH R01CA089208 (SDC), pilot project funding from P50CA125183 (SPORE), and the University of Chicago Comprehensive Cancer Center Core Facility Support P30CA014599-35.
References
- 1.Samaan NA, Buzdar AU, Aldinger KA, Schultz PN, Yang KP, Romsdahl MM, et al. Estrogen receptor: a prognostic factor in breast cancer. Cancer. 1981;47:554–560. doi: 10.1002/1097-0142(19810201)47:3<554::aid-cncr2820470322>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 3.Anan K, Mitsuyama S, Koga K, Tanabe R, Saimura M, Tanabe Y, et al. Disparities in the survival improvement of recurrent breast cancer. Breast Cancer. 2010;17:48–55. doi: 10.1007/s12282-009-0103-2. [DOI] [PubMed] [Google Scholar]
- 4.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:181–202. [PubMed] [Google Scholar]
- 5.Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005;18:26–35. doi: 10.1038/modpathol.3800255. [DOI] [PubMed] [Google Scholar]
- 6.Correa Geyer F, Reis-Filho JS. Microarray-based gene expression profiling as a clinical tool for breast cancer management: are we there yet? Int J Surg Pathol. 2009;17:285–302. doi: 10.1177/1066896908328577. [DOI] [PubMed] [Google Scholar]
- 7.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 8.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 9.Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60:867–872. [PubMed] [Google Scholar]
- 10.Belova L, Delgado B, Kocherginsky M, Melhem A, Olopade OI, Conzen SD. Glucocorticoid receptor expression in breast cancer associates with older patient age. Breast Cancer Res Treat. 2009;116:441–447. doi: 10.1007/s10549-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conzen SD. Minireview: nuclear receptors and breast cancer. Mol Endocrinol. 2008;22:2215–2228. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276:16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]
- 14.Pang D, Kocherginsky M, Krausz T, Kim SY, Conzen SD. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–940. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 15.Williams JB, Pang D, Delgado B, Kocherginsky M, Tretiakova M, Krausz T, et al. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res (Phila Pa) 2009;2:850–861. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu W, Zou M, Brickley DR, Pew T, Conzen SD. Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol. 2006;20:2304–2314. doi: 10.1210/me.2006-0131. [DOI] [PubMed] [Google Scholar]
- 17.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5'-flanking region. American journal of physiology. 2002;283:E971–E979. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- 18.Burkhart BA, Kennett SB, Archer TK. Osmotic stress-dependent repression is mediated by histone H3 phosphorylation and chromatin structure. J Biol Chem. 2007;282:4400–4407. doi: 10.1074/jbc.M609041200. [DOI] [PubMed] [Google Scholar]
- 19.Hebbar PB, Archer TK. Chromatin-dependent cooperativity between site-specific transcription factors in vivo. J Biol Chem. 2007;282:8284–8291. doi: 10.1074/jbc.M610554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Rogatsky I, Garabedian MJ. MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor. Mol Endocrinol. 2006;20:560–572. doi: 10.1210/me.2005-0318. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Ruan J, Durbin R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 2008;18:1851–1858. doi: 10.1101/gr.078212.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, et al. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wingender E, Dietze P, Karas H, Knuppel R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996;24:238–241. doi: 10.1093/nar/24.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics (Oxford, England) 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics (Oxford, England) 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 27.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brettschneider J, Collin F, Bolstad BM, Speed TP. Quality assessment for short oligonucleotide arrays. Technometrics. 2007;50:241–264. [Google Scholar]
- 29.Cronin M, Sangli C, Liu ML, Pho M, Dutta D, Nguyen A, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 30.Gentleman R, Carey V, Huber W, Hahne F. genefilter: methods for filtering genes from microarray experiments. R package [Google Scholar]
- 31.Gong Y, Yan K, Lin F, Anderson K, Sotiriou C, Andre F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 2007;8:203–211. doi: 10.1016/S1470-2045(07)70042-6. [DOI] [PubMed] [Google Scholar]
- 32.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kent WJ. BLAT--the BLAST-like alignment tool. Genome research. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome biology. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Therneau T, Lumley T. survival: Survival analysis, including penalised likelihood. R package [Google Scholar]
- 37.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwender H. siggenes: Multiple testing using SAM and Efron's empirical Bayes approaches. R package [Google Scholar]
- 39.Luca F, Kashyap S, Southard C, Zou M, Witonsky D, Di Rienzo A, et al. Adaptive variation regulates the expression of the human SGK1 gene in response to stress. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000489. e1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 41.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 43.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 45.Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 46.Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007;13:3207–3214. doi: 10.1158/1078-0432.CCR-06-2765. [DOI] [PubMed] [Google Scholar]
- 47.Loi S, Haibe-Kains B, Desmedt C, Wirapati P, Lallemand F, Tutt AM, et al. Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics. 2008;9:239. doi: 10.1186/1471-2164-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 49.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conde I, Paniagua R, Fraile B, Lucio J, Arenas MI. Glucocorticoid receptor changes its cellular location with breast cancer development. Histol Histopathol. 2008;23:77–85. doi: 10.14670/HH-23.77. [DOI] [PubMed] [Google Scholar]
- 51.Buxant F, Engohan-Aloghe C, Noel JC. Estrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancer. Appl Immunohistochem Mol Morphol. 2010;18:254–257. doi: 10.1097/PAI.0b013e3181c10180. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009;284:24542–24552. doi: 10.1074/jbc.M109.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2011;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He J, Cheng Q, Xie W. Minireview: Nuclear receptor-controlled steroid hormone synthesis and metabolism. Mol Endocrinol. 2010;24:11–21. doi: 10.1210/me.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou F, Bouillard B, Pharaboz-Joly MO, Andre J. Non-classical antiestrogenic actions of dexamethasone in variant MCF-7 human breast cancer cells in culture. Mol Cell Endocrinol. 1989;66:189–197. doi: 10.1016/0303-7207(89)90031-2. [DOI] [PubMed] [Google Scholar]
- 57.Gong H, Jarzynka MJ, Cole TJ, Lee JH, Wada T, Zhang B, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68:7386–7393. doi: 10.1158/0008-5472.CAN-08-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends in cell biology. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.