Abstract
Objective
Given that the repetitive loss and regain of body weight, termed weight cycling, is a prevalent phenomenon that has been associated with negative physiological and psychological outcomes, the purpose of this study was to investigate weight change and physiological outcomes in women with a lifetime history of weight cycling enrolled in a 12-month diet and/or exercise intervention.
Methods
439 overweight, inactive, postmenopausal women were randomized to: i) dietary weight loss with a 10% weight loss goal (N=118); ii) moderate-to-vigorous intensity aerobic exercise for 45 min/day, 5 days/week (n=117); ii) both dietary weight loss and exercise (n=117); or iv) control (n=87). Women were categorized as non-, moderate-(≥3 losses of ≥4.5 kg), or severe-cyclers (≥3 losses of ≥9.1 kg). Trend tests and linear regression were used to compare adherence and changes in weight, body composition, blood pressure, insulin, C-peptide, glucose, insulin resistance (HOMA-IR), C-reactive protein, leptin, adiponectin, and interleukin-6 between cyclers and non-cyclers.
Results
Moderate (n=103) and severe (n=77) cyclers were heavier and had less favorable metabolic profiles than non-cyclers at baseline. There were, however, no significant differences in adherence to the lifestyle interventions. Weight-cyclers (combined) had a greater improvement in HOMA-IR compared to non-cyclers participating in the exercise only intervention (p=0.03), but no differences were apparent in the other groups.
Conclusion
A history of weight cycling does not impede successful participation in lifestyle interventions or alter the benefits of diet and/or exercise on body composition and metabolic outcomes.
Keywords: lifestyle intervention, insulin resistance, inflammation, adipokines
INTRODUCTION
The repetitive loss and regain of body weight, referred to as weight cycling, appears to be a prevalent phenomenon. No uniform definition exists; however, estimates commonly range from 10–40% of the population in Westernized countries (1–5).
Repetitive weight loss followed by regain has been associated with unfavorable physiological and psychological outcomes including effects on body composition, metabolic rate, immune function, and lower body esteem (4, 6–11). However, the degree to which repetitive fluctuations in body weight represent an independent risk factor for adverse health outcomes, including successful future weight loss, is not clear. Studies have reported mixed findings on behavioral and physiological changes over successive weight loss attempts, including worse compliance (12) and effects on body fat distribution, energy expenditure, and specific comorbidities (13, 14). It has been suggested that weight cycling may increase preference for dietary fat (15) and the likelihood of weight gain over time (2, 16–18). Repeated unsuccessful attempts at weight loss maintenance may therefore affect future weight loss through metabolic adaptation or factors related to program adherence (14). However, few studies have been able to examine this, and it remains unclear whether repetitive cycles of weight loss and regain have any significant effect on subsequent success in achieving weight loss, or on the metabolic changes that typically accompany it.
Given the well-documented difficulty of maintaining weight loss (19, 20), the potentially deleterious effects of weight cycling would be an important consideration in formulating treatment recommendations and population-based approaches to address obesity. The purpose of this study was to explore whether postmenopausal women with a lifetime history of repetitive weight cycling exhibit different body composition or physiologic responses to 12 months of diet and/or exercise intervention compared to non-cyclers.
METHODS
The Nutrition and Exercise in Women (NEW) study, conducted from 2005 to 2009, was a 12-month randomized controlled trial to test the effects of dietary weight loss and/or exercise on circulating hormones and other variables (21). Study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board in Seattle, WA, and all participants provided informed consent.
Participants
Participants were overweight or obese (BMI ≥25.0 kg/m2 or ≥23.0 kg/m2 if Asian-American), postmenopausal women (50–75 y). Specific exclusion criteria included: >100 min/week of moderate activity, diagnosed diabetes, fasting blood glucose ≥7mmol/L or use of diabetes medications; use of postmenopausal hormone therapy; history of breast cancer or other serious medical condition(s); alcohol intake ≥2 drinks/day or current smoking; contraindication to participating in the study interventions for any reason (e.g. an abnormal exercise tolerance test), participation in another structured weight loss program, or use of weight loss medications.
Study Design
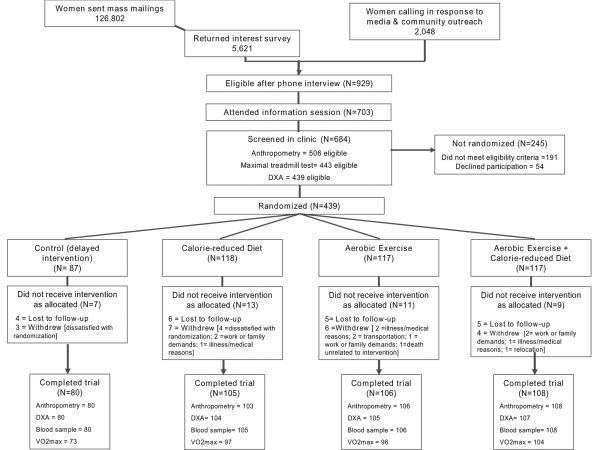
Women were recruited through targeted mass mailings and media, and underwent several screening activities (Figure 1). Eligible women were randomized into one of four study arms: 1) dietary weight loss (N=118); 2) moderate-to-vigorous intensity aerobic exercise (N=117); 3) combined aerobic exercise and dietary weight loss (N=117); or 4) control (no intervention) (N=87). The random assignment was computer-generated stratified according to BMI (<30 or ≥30 kg/m2) and participants' self-reported race/ethnicity (Black, non-Hispanic White, or other). To achieve a proportionally smaller control group, a permuted blocks randomization with blocks of 4 was used, wherein the control assignment was randomly eliminated from each block with a probability of approximately 1 in 4. The NEW trial was designed to have at least 80% power to detect a difference of 10% in serum estrone (trial's primary endpoint) over 12 months, making three primary pairwise comparisons: diet + exercise vs. exercise; diet + exercise vs. diet; and diet vs. exercise intervention groups. One participant randomized to diet + exercise intervention was excluded from this analysis due to missing baseline blood measures.
Figure 1.
Flow of participants through recruitment and participation in the Nutrition & Exercise in Women (NEW) Trial.
footnote: One participant randomized to diet + exercise intervention was excluded from present study due to missing baseline blood measure
The dietary weight-loss intervention comprised our modification of the Diabetes Prevention Program (22) and Look AHEAD (23) lifestyle behavior change programs with the following goals: total daily energy intake of 1200–2000 kcal/day based on baseline body weight, <30% daily energy intake from fat, and a 10% reduction in body weight by 6 months with maintenance thereafter to 12 months. Participants met individually with a study dietitian at least twice, followed by weekly group meetings (5–10 women), for 6 months. Thereafter (months 7–12), participants attended monthly groups meetings, in addition to phone and email contact with study dietitians. Women were asked to record all food eaten daily for at least 6 months, or until they reached their individual weight loss goal (10%). During the first 6 months, food journals were collected weekly and returned with feedback. Food journals, weekly weigh-ins and session attendance were used to promote and track adherence to the diet intervention.
The exercise intervention progressed to 45 minutes of moderate-to-vigorous intensity exercise at a target heart rate of 70–85% observed maximum, 5 days per week (225 min/week) by the 7th week. Throughout the intervention, participants attended 3 supervised sessions/week at the study facility and exercised 2 days/week at home. Participants recorded the mode and duration of exercise, peak heart rate (Polar Electro, Lake Success, NY) and relative perceived exertion at each session. Activities of at least 4 metabolic equivalents (METs) according to the Compendium of Physical Activities (24) were counted towards the prescribed exercise target of 225 mins/week. Weight loss was not a specific goal of the exercise intervention.
Participants randomized to the diet + exercise group received separate nutrition sessions and were instructed not to discuss the diet intervention during supervised exercise sessions. The control group was asked not to change their diet or exercise habits for 12 months. At study completion, they were offered 4 group nutrition classes and 8 weeks of individualized exercise training.
Weight cycling
Study participants were asked to answer the following question in a baseline questionnaire: “Since you were 18 years old, how many different times did you lose each of the following amounts of weight on purpose (excluding pregnancy or illness): 5–9 lbs, 10–19 lbs, 20–49 lbs, 50–79 lbs, 80–99 lbs, ≥100 lbs?” Possible responses were 0, 1–2, 3–4, 5–6, or 7+ times for each magnitude of weight loss. No specific information was available to quantify the magnitude of weight regain following each weight loss; however, given that all participants were overweight/obese at baseline, the assumption of at least partial weight regain, particularly among women who reported ≥3 episodes was felt to be acceptable, as has been done in other studies (9, 16, 25, 26). To be consistent with previous studies (4, 9, 16, 27), women who reported losing ≥20 lbs (≥9.9 kg) on three or more occasions were classified as severe weight cyclers; women who reported losing ≥10 lbs (≥4.5 kg) on three or more occasions, but did not meet the criteria for severe cycling, were classified as moderate weight cyclers. All other women were considered non-cyclers.
Study measures
All study measures were obtained and analyzed by trained personnel who were blinded to the participants' randomization status.
Demographic information, medical history, health habits, reproductive and body weight history, psychosocial attributes, dietary intake (via a validated 120-item self-administered food frequency questionnaire (28)), and physical activity patterns (via a modified, interview-administered Minnesota Physical Activity Questionnaire (29)) were collected at baseline (prior to randomization) and 12 months. At both timepoints, participants wore pedometers (Accusplit, Silicon Valley, CA) while awake for 7 consecutive days to determine an average daily step count. Cardiorespiratory fitness (VO2max) was assessed using a maximal graded treadmill test with a modified branching protocol (30). Heart rate and oxygen uptake were continuously monitored with an automated metabolic cart (MedGraphics, St. Paul, MN).
Anthropometric measures were obtained from participants in hospital gowns without shoes. BMI (kg/m2) was calculated from weight and height, measured to the nearest 0.1 kg and 0.1 cm, respectively, with a balance beam scale and stadiometer. Waist circumference was measured to the nearest 0.5 cm at the minimal waist. Body composition was measured using a DXA whole-body scanner (GE Lunar, Madison, WI). Resting blood pressure was measured from the brachial artery while participants were seated comfortably. To facilitate analysis, mean arterial pressure (MAP = 1/3[systolic pressure-diastolic pressure] + diastolic pressure) was calculated and used as a single, continuous variable.
Fasting venous blood samples (50 mL) were collected during clinic visits prior to randomization and at 12 months. Participants consumed no food or drink other than water for 12 hours prior, and were requested not to exercise for 24 hours preceding the blood draw. Blood was processed within 1 hour and samples stored at −70°C.
Serological assays
Blood samples were analyzed in batches such that each participant's samples were: assayed simultaneously, the number of samples from each intervention group was approximately equal, participant randomization dates were similar, and the sample order was random. All but four samples were analyzed for insulin and C-peptide in a single batch. Exclusion of these samples did not affect the results.
C-reactive protein (CRP) was measured at the University of Washington Clinical Nutrition Research Unit Laboratory. All other assays were performed at the University of Washington Northwest Lipid Research Laboratories. CRP was assayed on a Roche Mira Plus Chemistry Analyzer using Genzyme CRP Ultra Wide Range Reagent. Intra- and inter-assay coefficients of variation (CVs) were 4.1% and 4.7%, respectively. Interleukin-6 (IL-6) was quantified with an ultra-sensitive ELISA assay (Quantikine HS ELISA, Minneapolis, MN) using a quantitative sandwich enzyme immunoassay technique. Intra- and inter-assay CVs were 9.7% and 12.4%, respectively. Adiponectin was measured using a radioimmunoassay (Linco Research) with 125I-labeled murine adiponectin and anti-adiponectin antibody; leptin was quantified using a Linco Research Human Leptin radioimmunoassay that utilizes 125I-labeled Human Leptin, and the double antibody/PEG technique (Millipore, Billerica, MA). Intra- and inter-assay CVs were 8.4% and 9.8%, respectively, for adiponectin; they were 9.1% and 14.4%, respectively, for leptin.
Insulin was quantified by a 48-hour, polyethylene glycol-accelerated, double antibody radioimmunoassay. C-peptide was analyzed using a two-site immunoenzymometric assay performed in a Tosoh AIA 1800 auto analyzer (Tosoh Bioscience, Inc., San Francisco). Glucose was quantified using a ClinicalChemistry Autoanalyzer with the hexokinase method. The intra-assay CVs were 4.5% for insulin and 4.3% for C-peptide. The intra- and inter-assay CVs for glucose measurement were 1.1% and 3.5%, respectively.
The homeostasis assessment model (HOMA-IR= fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5)(31) was calculated as a surrogate measure of whole-body insulin resistance (32). In cases where the difference between baseline and 12-month CRP values exceeded approximately 10× their other value, data were excluded (n=4).
Statistics
In cases of missing values, no change from baseline was assumed. Repeating the main analyses using only available data did not meaningfully affect any of the results. On account of skewed distributions, MAP and serum measures were log transformed prior to analysis. General linear models were used to test for trends in baseline body composition, blood pressure (MAP), serum blood measures, and intervention adherence across non-, moderate-, and severe weight cyclers. A Chi-square test was used to examine between-group differences in study retention.
Mean 12-month changes in body composition and physiological variables among weight-cyclers and non-cyclers were compared separately within the exercise, diet, and exercise + diet groups. The generalized estimating equations (GEE) modification of linear regression was used to account for the correlation within individuals over time (33). Differences between moderate and severe cyclers and non-cyclers were examined in unadjusted models and in models that included baseline BMI as a covariate. To increased statistical power, these analyses were repeated after combining moderate and severe weight cyclers.
All statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
At 12 months, 398 of 438 participants completed physical exams and provided blood samples, 397 underwent a DXA scan, and 371 completed a maximal treadmill test; 39 women did not complete the study (diet=13, exercise=12, diet + exercise=7, controls=7) (Figure 1).
Weight Cycling
Overall, 103 (24%) women met the criteria for moderate weight cycling and an additional 77 (18%) met the criteria for severe weight cycling. There were no significant differences in demographic characteristics, dietary intake, physical activity, or fitness levels between weight cycling groups at baseline (Table 1). On average, moderate and severe weight cyclers were heavier, had larger waist circumferences, and a greater percentage of body weight as fat (% body fat) (p< 0.001). Weight cyclers also had less favorable metabolic and hormonal profiles than non-cyclers; however, these differences disappeared after adjusting for BMI (Table 1).
Adherence to interventions
Overall adherence to the dietary weight loss and aerobic exercise interventions has been previously described in detail (21). Journaling, session attendance, weekly weight loss, and changes in % calories from fat and fiber intake (g) were used as measures of adherence to the diet intervention. Total minutes of facility and home-based exercise, average pedometer steps/day and the change in VO2max were used as indicators of adherence to the exercise intervention.
A history of weight cycling was not significantly associated with any measure of dietary or exercise adherence (Table 2). Similar results were observed when the association between a history of weight cycling and adherence was examined in all dieters (n=234) and all exercisers (n=233). History of weight cycling was not associated with study retention in any of the intervention arms or in the study as a whole.
Changes in response to diet and/or exercise intervention
Table 3 summarizes the anthropometric and body composition changes in response to 12 months of diet and/or exercise intervention in weight cyclers compared to non-cyclers. Table 4 provides the absolute and % change in physiologic variables in weight cyclers compared to non-cyclers within each of the 4 study arms.
When all weight cyclers (moderate and severe) were combined, weight cyclers in the exercise alone group had a smaller reduction in % body fat (p= <0.01) and smaller corresponding increase in % lean mass (p=<0.01) compared to their non-cycling counterparts, despite a similar reduction in overall body weight (−2.4%). These women also had a significantly greater improvement in HOMA-IR, even after adjusting for differences in body weight (p = 0.04). No significant differences in outcomes were detected between weight cyclers (combined) and non-cyclers in the other intervention arms.
DISCUSSION
In this study of overweight postmenopausal women, a history of weight cycling did not appear to impede successful participation in diet and/or exercise interventions or alter their benefits on body composition and physiological outcomes. Although weight cyclers had less favorable metabolic profiles at baseline compared with non-cyclers, these differences could be accounted for by differences in body composition (higher BMI, larger waist circumference, and greater % body fat) rather than any independent effect of weight cycling per se.
While a few significant differences were detected between moderate and severe weight cyclers compared to non-cyclers (Tables 3 and 4), these differences were not consistent across groups and could be expected by chance alone. When all women with a history of weight cycling were combined, no differences were observed between cyclers and non-cyclers assigned to diet alone or diet + exercise. In women randomized to exercise alone, weight cyclers and non-cyclers had a similar mean reduction in body weight (−2.4%), yet cyclers had a smaller reduction in % body fat and a greater improvement in insulin sensitivity, even after adjusting BMI. Given that exercise may affect insulin resistance even in the absence of significant weight loss (34, 35), the greater magnitude of change observed may be owed to higher initial levels of metabolic dysregulation among the weight cyclers. Unfortunately, we did not have a sufficient sample size to perform a stratified analysis to test this hypothesis. Given that the NEW trial was not specifically designed to examine differences in response to lifestyle interventions among women with and without a lifetime history of weight cycling, this analysis should be considered exploratory. It is likely that the current study was underpowered to adequately test the large number of comparisons performed within each intervention arm. Nevertheless, given the relatively small differences in the absolute magnitude of the observed changes between weight cyclers and non-cyclers within each intervention group, the overall results of this study do not suggest that weight cycling has deleterious effects on the physiological changes induced by 12 months of dietary weight loss and/or aerobic exercise.
To our knowledge, no previous studies have examined the effect of prior weight cycling on the body composition, metabolic, and hormonal changes induced by a comprehensive lifestyle intervention in free-living women. The higher baseline BMI, larger waist circumference, and greater body fat among moderate and severe cyclers are consistent with previously reported cross-sectional associations (4, 11, 16) and the observation that fat mass is regained to a greater degree than lean mass after weight loss (36). We did not observe lower adiponectin in weight cyclers compared to non-cyclers as reported by Strychar et al. (11) in a sample of 121 overweight/obese postmenopausal women; however, our other findings are consistent with the aforementioned study in that no differences in blood pressure, glucose, insulin sensitivity, or leptin concentrations were observed across weight cycling groups once BMI was accounted for. A history of weight cycling has been identified as an important predictor of future weight gain (27, 37). We did not observe a significant difference in weight change between cyclers and non-cyclers in the control group. However, the small sample size and 12 month follow-up period may have been insufficient to detect differences in this regard. In the intervention arms, good adherence was achieved in all groups and was similar between non-, moderate-, and severe cyclers when caloric restriction and exercise were targeted separately or in tandem.
Provencher et al. (38) previously reported that women with a history of dieting were more likely to have a higher fat and lower carbohydrate dietary pattern than non-dieters. We found no significant difference in dietary pattern according to history of weight cycling in the present study, nor in dietary changes throughout the intervention. Moderate weight cyclers appeared to reduce their carbohydrate intake less than non-cyclers and severe cyclers; however, this difference was not statistically different from the other groups in a pooled analysis of all participants who received the dietary intervention (p = 0.34, results not shown).
Weight loss stimulates adjustments in energy homeostatic hormones, including lower levels of insulin and leptin (39, 40), yet the long-term effect of repetitive weight cycling on these parameters has not been widely examined. We did not detect an independent effect of weight cycling on baseline insulin, leptin, or adiponectin levels, nor did we detect differences in the hormonal response to 12 months of diet and/or exercise induced weight loss in weight cyclers compared to non-cyclers. Changes in these parameters beyond the 12-month intervention remain an important area for future study, with the potential to improve our understanding of how these hormones influence long-term weight loss maintenance. Longer-term studies will also help evaluate the relative benefit of caloric restriction and aerobic exercise on weight loss maintenance, and assess the degree to which women with a history of weight cycling continue to maintain healthy behavior change after participation in a structured program.
Additional research is also required to better characterize the degree to which the magnitude and frequency of weight fluctuations influence the metabolic and hormonal response to weight loss and may contribute to weight gain over time. The absence of a standard definition for weight cycling makes comparisons between studies difficult. Moreover, many studies have been unable to distinguish intentional from unintentional weight loss, which is important given that these are known to be differentially associated with health outcomes (3, 41). For the purpose of this study, we opted to identify women with a history of weight cycling based on both the magnitude and number of intentional weight losses throughout adulthood. However, we could not characterize the degree or time-course of weight regain, nor assess potential effects of variability in this regard on the outcomes of interest. This represents a limitation of the current analysis and will only be able to be examined in longitudinal studies with serial measurements and/or detailed self-reporting.
In a recent cross-sectional analysis of 159 overweight postmenopausal women (25), ghrelin levels were 11%, 22%, and 44% higher in women who reported ever losing ≥10 lbs (≥4.5 kg), ≥20 lbs (≥9.9 kg), and ≥50 lbs (≥23 kg), respectively compared to women who reported never intentionally losing ≥10 lbs. More frequent weight loss was also associated with higher ghrelin and modest trends towards lower insulin and glucose. Thus, it is possible that magnitude and frequency of weight cycling episodes have differential effects on metabolic and hormonal responses to weight loss that are not maximally captured with a combined measure.
The strengths of the current study include its large sample size, comprehensive collection of physiological measures, and high retention and adherence rates, although the potential for differential over-reporting of non-supervised exercise between weight cyclers and non-cyclers cannot be ruled out. The classification of weight cycling was based on self-reported episodes that were collected retrospectively; thus, some degree of misclassification due to recall bias is possible. Given the current sample included women who volunteered to participate in a trial targeting weight loss, a higher prevalence of weight cycling compared to population-based samples is not surprising (1–4), yet is still slightly lower than reported in another sample of post-menopausal women enrolled in a 6-month weight loss intervention (11). The high prevalence of weight cycling among overweight post-menopausal women underscores the importance of understanding its impact on weight loss and metabolic improvements in this population. Two-thirds of the U.S. population is currently overweight or obese (42), and available estimates indicate that nearly half of American women are currently dieting to lose weight (43). Repeated unsuccessful attempts at maintaining weight loss are frustrating for both individuals as well as health care practitioners. Reports that weight cycling may be associated with increased risk of particular health outcomes have called into question the prudence of recommending weight loss in otherwise healthy men and women, particularly in light of the difficulty of weight loss maintenance (19, 20). Yet, several analyses where unintentional and intentional weight loss have been clearly distinguished have failed to show an independent association between weight cycling and risk of morbidity or mortality (1, 5, 16, 44). Moreover, the risk of future weight gain previously associated with weight cycling was recently shown to be greater among women who employ unhealthy weight control strategies rather than ones generally encouraged in most structured behavior change programs (37).
Our results suggest that a history of weight cycling does not impede successful participation in lifestyle interventions or alter the benefits of diet and/or exercise on anthropometric and metabolic outcomes in women. Thus, a history of unsuccessful weight loss should not dissuade an individual from future attempts at weight loss nor diminish the role of a healthy diet and regular physical activity in successful weight management. However, healthy and sustainable approaches to weight loss should be promoted. Given that lost lean mass is not recovered during weight regain (36), the benefit of exercise for preventing lean mass loss during caloric restriction is an especially important message for patients and clinicians in order to minimize the detrimental effects of weight cycling on future weight gain.
Acknowledgments
FUNDING This work was supported by the National Cancer Institute at the National Institutes of Health (grant number: R01 CA102504, U54-CA116847, 5KL2RR025015-03 to K.F.S, R25 CA94880 and 2R25CA057699-16 to A.K.); and the Canadian Institutes of Health Research (Fellowship to CM & K.L.C). None of the funding agencies were involved in the trial design or conduct. While working on the trial, Dr. Alfano was employed at The Ohio State University, and located to NCI following completion of her effort on the NEW trial.
LIST OF ABBREVIATIONS
- NEW
Nutrition and Exercise in Women
- BMI
Body Mass Index
- METs
Metabolic Equivalents
- VO2max
Maximal Oxygen Uptake
- DXA
Dual-energy X-ray Absorptiometry
- MAP
Mean Arterial Pressure
- CRP
C-Reactive Protein
- CV
Coefficient of Variation
- ELISA
Enzyme-linked immunosorbent assay
- IL-6
Interleukin-6
- HOMA-IR
Homeostasis Model Assessment—Insulin Resistance
- GEE
General Estimating Equation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
DISCLOSURES: The authors have no disclosures.
AUTHOR CONTRIBUTIONS: Drs Mason and McTiernan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Mason, Foster-Schubert, Wang, Alfano, Ulrich, Blackburn, McTiernan; Acquisition of data: Kong, Bain, Campbell, Blackburn, McTiernan; Analysis and interpretation of data: Mason, Xiao, Alfano, McTiernan; Drafting of the manuscript: Mason; Critical revision of the manuscript for important intellectual content: Imayama, Kong, Campbell, Wang, Alfano, Ulrich, Blackburn, McTiernan; Statistical analysis: Xiao, Mason; Obtained funding: McTiernan; Administrative, technical, or material support: Bain, Xiao; Study supervision: McTiernan
REFERENCES
- 1.Field AE, Byers T, Hunter DJ, Laird NM, Manson JE, Williamson DF, et al. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol. 1999;150(6):573–9. doi: 10.1093/oxfordjournals.aje.a010055. [DOI] [PubMed] [Google Scholar]
- 2.Kroke A, Liese AD, Schulz M, Bergmann MM, Klipstein-Grobusch K, Hoffmann K, et al. Recent weight changes and weight cycling as predictors of subsequent two year weight change in a middle-aged cohort. Int J Obes Relat Metab Disord. 2002;26(3):403–9. doi: 10.1038/sj.ijo.0801920. [DOI] [PubMed] [Google Scholar]
- 3.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27(12):1447–52. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 4.Lahti-Koski M, Mannisto S, Pietinen P, Vartiainen E. Prevalence of weight cycling and its relation to health indicators in Finland. Obes Res. 2005;13(2):333–41. doi: 10.1038/oby.2005.45. [DOI] [PubMed] [Google Scholar]
- 5.Stevens VL, Jacobs EJ, Sun J, Patel AV, McCullough ML, Teras LR, et al. Weight Cycling and Mortality in a Large Prospective US Study. Am J Epidemiol. 2012;175(8):785–92. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 6.Weight cycling National Task Force on the Prevention and Treatment of Obesity. Jama. 1994;272(15):1196–202. [PubMed] [Google Scholar]
- 7.French SA, Jeffery RW, Folsom AR, Williamson DF, Byers T. Relation of weight variability and intentionality of weight loss to disease history and health-related variables in a population-based sample of women aged 55–69 years. Am J Epidemiol. 1995;142(12):1306–14. doi: 10.1093/oxfordjournals.aje.a117598. [DOI] [PubMed] [Google Scholar]
- 8.French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 1997;21(3):217–23. doi: 10.1038/sj.ijo.0800390. [DOI] [PubMed] [Google Scholar]
- 9.Shade ED, Ulrich CM, Wener MH, Wood B, Yasui Y, Lacroix K, et al. Frequent intentional weight loss is associated with lower natural killer cell cytotoxicity in postmenopausal women: possible long-term immune effects. J Am Diet Assoc. 2004;104(6):903–12. doi: 10.1016/j.jada.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Foreyt JP, Brunner RL, Goodrick GK, Cutter G, Brownell KD, St Jeor ST. Psychological correlates of weight fluctuation. Int J Eat Disord. 1995;17(3):263–75. doi: 10.1002/1098-108x(199504)17:3<263::aid-eat2260170307>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Strychar I, Lavoie ME, Messier L, Karelis AD, Doucet E, Prud'homme D, et al. Anthropometric, metabolic, psychosocial, and dietary characteristics of overweight/obese postmenopausal women with a history of weight cycling: a MONET (Montreal Ottawa New Emerging Team) study. J Am Diet Assoc. 2009;109(4):718–24. doi: 10.1016/j.jada.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Smith DE, Wing RR. Diminished weight loss and behavioral compliance during repeated diets in obese patients with type II diabetes. Health Psychol. 1991;10(6):378–83. doi: 10.1037//0278-6133.10.6.378. [DOI] [PubMed] [Google Scholar]
- 13.Rodin J, Radke-Sharpe N, Rebuffe-Scrive M, Greenwood MR. Weight cycling and fat distribution. Int J Obes. 1990;14(4):303–10. [PubMed] [Google Scholar]
- 14.Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Arch Intern Med. 1994;154(12):1325–30. [PubMed] [Google Scholar]
- 15.Drewnowski A, Holden-Wiltse J. Taste responses and food preferences in obese women: effects of weight cycling. Int J Obes Relat Metab Disord. 1992;16(9):639–48. [PubMed] [Google Scholar]
- 16.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169(9):881–6. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field AE, Wing RR, Manson JE, Spiegelman DL, Willett WC. Relationship of a large weight loss to long-term weight change among young and middle-aged US women. Int J Obes Relat Metab Disord. 2001;25(8):1113–21. doi: 10.1038/sj.ijo.0801643. [DOI] [PubMed] [Google Scholar]
- 18.Korkeila M, Rissanen A, Kaprio J, Sorensen TI, Koskenvuo M. Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. Am J Clin Nutr. 1999;70(6):965–75. doi: 10.1093/ajcn/70.6.965. [DOI] [PubMed] [Google Scholar]
- 19.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 20.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24(1):58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster-Schubert KE, Alfano CM, Duggan C, Xiao L, Campbell KL, Kong A, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity. 2011 Apr 14; doi: 10.1038/oby.2011.76. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–68. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Hooper LE, Foster-Schubert KE, Weigle DS, Sorensen B, McTiernan A, Ulrich CM. Frequent intentional weight loss is associated with higher ghrelin and lower glucose and androgen levels in p[ostmenopusal women. Nutr Res. 2010;30(3):163–70. doi: 10.1016/j.nutres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Wye G, Dubin JA, Blair SN, Di Pietro L. Weight cycling and 6-year weight change in healthy adults: The Aerobics Center Longitudinal Study. Obesity. 2007;15(3):731–9. doi: 10.1038/oby.2007.598. [DOI] [PubMed] [Google Scholar]
- 27.Field AE, Manson JE, Taylor CB, Willett WC, Colditz GA. Association of weight change, weight control practices, and weight cycling among women in the Nurses' Health Study II. Int J Obes Relat Metab Disord. 2004;28(9):1134–42. doi: 10.1038/sj.ijo.0802728. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HL, Jacobs DR, Jr., Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 30.Pate R, Blair S, Durstine J. Guidelines for Exercise Testing and Prescription. Lea & Febinger; Philadelphia, Pa: 1991. pp. 70–72. [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 34.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 35.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122–31. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 36.Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr. 2011;94(3):767–74. doi: 10.3945/ajcn.110.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage JS, Birch LL. Patterns of weight control strategies predict differences in women's 4-year weight gain. Obesity (Silver Spring) 2010;18(3):513–20. doi: 10.1038/oby.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provencher V, Drapeau V, Tremblay A, Despres JP, Bouchard C, Lemieux S. Eating behaviours, dietary profile and body composition according to dieting history in men and women of the Quebec Family Study. Br J Nutr. 2004;91(6):997–1004. doi: 10.1079/BJN20041115. [DOI] [PubMed] [Google Scholar]
- 39.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 40.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 41.French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women's Health Study. Am J Epidemiol. 1999;149(6):504–14. doi: 10.1093/oxfordjournals.aje.a009844. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data. 2004;(347):1–17. [PubMed] [Google Scholar]
- 43.Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, 3rd, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res. 2005;13(3):596–607. doi: 10.1038/oby.2005.64. [DOI] [PubMed] [Google Scholar]
- 44.Field AE, Manson JE, Laird N, Williamson DF, Willett WC, Colditz GA. Weight cycling and the risk of developing type 2 diabetes among adult women in the United States. Obes Res. 2004;12(2):267–74. doi: 10.1038/oby.2004.34. [DOI] [PubMed] [Google Scholar]