Abstract
Background
Disulfiram has been an effective cocaine addiction pharmacotherapy, and one of its possible mechanisms of efficacy is through copper chelation and inhibition of an enzyme involved in catecholamine metabolism, dopamine β-hydroxylase (DβH), which converts dopamine to norepinephrine. A variant in the gene encoding DβH leads to reduced DβH activity and as such, disulfiram may not be an effective treatment of cocaine dependence for individuals with this variant. This study explored that potential matching.
Methods
Seventy-four cocaine and opioid co-dependent (DSM-V) subjects were stabilized on methadone for two weeks and subsequently randomized into disulfiram (250 mg/day, N =34) and placebo groups (N =40) for 10 weeks. We genotyped the DBH gene polymorphism, −1021C/T (rs1611115), that reduces DβH enzyme levels and evaluated its role for increasing cocaine free urines with disulfiram.
Results
Using repeated measures analysis of variance, corrected for population structure, disulfiram pharmacotherapy reduced cocaine positive urines from 80% to 62% (p = .0001), and this disulfiram efficacy differed by DBH genotype group. Patients with the normal DβH level genotype dropped from 84% to 56% on disulfiram (p = .0001), while those with the low DBH level genotype showed no disulfiram effect.
Conclusions
This study indicates that a patient’s DBH genotype could be used to identify a subset of individuals for which disulfiram treatment may be an effective pharmacotherapy for cocaine dependence.
Keywords: Genes, disulfiram, polymorphism, cocaine, treatment, dependence
Introduction
Cocaine dependence is common with over 1.5 million actively cocaine dependent people in 2011 who have substantial social and economic morbidity from it, but it has no FDA approved pharmacotherapy (1, 2)., In methadone maintenance programs rates of cocaine use range from 30% to 50% and lead to poorer outcomes and higher incidence of HIV risk behaviors (3–13). Although a number of innovative pharmacological approaches have had limited success in reducing cocaine use, (e.g.,(14–16), disulfiram has shown some initial promise in treating cocaine dependence in both non opioid-dependent (17–19) and opioid-dependent cocaine abusers (20, 21).
Tailoring pharmacological treatment to a person’s genetic background can enhance therapeutic response (22), increase compliance (23) and decrease drug toxicity (24–26). Since cocaine addiction has a strong genetic basis, with the vulnerability to develop an addiction estimated to be as high as 72% (27), pharmacotherapy of this relapsing brain disease may be better treated using a molecular genetics approach (28–31). Applying a molecular genetics approach to disulfiram may involve its inhibitory action on the copper-containing glycoprotein enzyme dopamine β-hydroxylase (DβH), which transforms dopamine to norepinephrine (32). Inhibiting DβH decreases peripheral and central norepinephrine levels, and increases dopamine levels (33, 34).
Based on twin and family studies, plasma levels of DβH vary between unrelated individuals (35–37). Some of these differences are due to polymorphisms in close proximity to the DBH gene. Indeed, studies link the C-1021T (−1021C>T) variant to differences in circulating DβH levels (38–41). The variant C-1021T is positioned ~1000 nucleotides upstream from the initiation codon of the DBH gene (41). Several studies indicate that the C-1021T variant is a functional polymorphism, which alters transcription and decreases plasma levels of DβH (41–44). This variant accounts for up to 52% of overall variation in the enzyme levels (41, 43–45). Individuals that are homozygous for the T allele have the lowest levels of plasma DBH activity. Variable DβH enzyme levels or activity is linked with a number of psychiatric disorders ranging from psychotic (46) to conduct disorders (47–51). The cerebrospinal fluid level of the dopamine metabolite homovanillic acid (HVA) is an indirect measure of monoamine concentration in the brain and is correlated with the DBH C-1021T genotype (52). Several, complementary mechanisms probably contribute to disulfiram's efficacy and interact with this polymorphism: increased cocaine aversion by causing dopamine receptor hypersensitivity, reversal of a dopaminergic deficiency and dysphoria by increasing dopamine production in noradrenergic neurons during withdrawal, and preventing relapse by lowering norepinephrine levels and attenuating signaling via adrenergic receptors. All of these mechanisms might be enhanced by genetically determined baseline levels of the enzyme DβH and will be returned to in the Discussion. However, no simple genetic association can be ascertained a priori for enhancing disulfiram’s efficacy, and it requires direct testing in a clinical trial, as we have done.
Arguments can be made for disulfiram’s efficacy in cocaine dependence being enhanced in individuals who have the C-1021T allele that is associated with normal DβH or low levels. However, the potential importance of this functional variant in treatment outcome merits testing. Thus, we tested this hypothesis of its importance in a placebo controlled randomized clinical trial of disulfiram at 250 mg daily by comparing disulfiram’s efficacy at reducing cocaine abuse in methadone maintained patients with the CC genotype and normal DBH levels to those carrying the T allele and lower DβH levels.
Methods and Materials
Subjects
Our sample of 74 opioid and cocaine dependent subjects (10 African American, 8 Hispanic, 56 Caucasian) were drawn from a sample of 93 candidates who entered into a two week screening period for stabilization on methadone maintenance between 2005 to 2006 at Yale University (n =40) and then from 2006 to 2008 at the Baylor College of Medicine (n = 53). During these two weeks, we obtained thrice-weekly urine toxicologies for opiates and cocaine metabolites, and subjects needed to have at least one urine sample showing cocaine use for entry into the randomized clinical trial. Eleven subjects had all six urines free of cocaine and were excluded. Another eight subjects dropped out during this screening. All subjects met DSM-IV criteria for opioid and cocaine dependence after interview by a psychiatrist or clinical psychologist. Exclusions included a current diagnosis of other drug or alcohol physical dependence (other than tobacco), current major medical illness unstablized on medications, a history of major psychiatric disorder (psychosis, schizophrenia, bipolar), current suicidality, and an inability to read and understand the consent form. Women of childbearing age were included provided they had a negative urine pregnancy test, agreed to use adequate contraception to prevent pregnancy during the study, and agreed to monthly pregnancy tests. All participants signed an informed consent approved by Yale University and the Baylor College of Medicine Institutional Review Boards that gave specific consent for genetic studies. Ethnicity was based self-report of ethnic/cultural background of the subjects.
Study Design and Medications
The 74 subjects were randomly assigned one to one by computer to disulfiram 250 mg daily or placebo while stabilized on methadone maintenance at 60 mg daily. We enrolled methadone maintained subjects in order to maximize our treatment retention during this study, since primary cocaine abusers not in methadone treatment have had poor treatment retention with over half of the subjects leaving treatment within 3 months (17–19). Subjects ingested methadone daily, 7 days per week. Methadone was administered orally in a colored liquid and ingested at the dispensing window under observation of the dispensing nurse (except on Sundays, for which take home doses were provided). During induction onto methadone, participants initially received 25 mg of methadone, which was increased by 5 mg at each subsequent daily dosing until participants received a 60 mg maintenance dose. During the 10 weeks after stabilization, subjects attended the clinic daily for oral methadone administration with either disulfiram or placebo (lactose) dissolved in their liquid methadone. Double blinding of patients, providers and clinical staff and treatment assignment were maintained through the research pharmacy, and the individual patient’s bottles liquid methadone looked and tasted identical with lactose added to both active and placebo. Clinical staff enrolled participants and advised them not to drink alcohol or use alcohol-containing products during the study. Supervised urine samples were obtained thrice weekly and tested for the presence of opiates and cocaine metabolite (benzoylecgonine) using an Olympus AU 640 Emit system (Olympus America Inc., Melville, NY) with a cut-off concentration of 300 ng/ml. We obtained saliva samples for genotyping. At study entry, we completed the Addiction Severity Index (ASI) on all subjects to assess baseline characteristics and to compare them across treatment and genotype groups (53). The ASI includes seven interviewer ratings of problem severity in medical, employment, legal, drug, alcohol, family and psychological problems. All participants received weekly manual-driven individual cognitive behavioral therapy and had excellent participation with less than 10% missed sessions across all groups (54). At the end of the study, participants either transferred to a local opioid maintenance program or underwent detoxification from methadone over a 4–6 week period.
Genotyping
DNA was purified from buccal cells. Briefly, 10 ml Scope mouthwash was swished in the subject’s mouth for 60 seconds and recovered. Cells were isolated by centrifugation at 2,000×g for 5 min. DNA was isolated from the pellet using the Gentra Puregene Buccal Cell Kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. DNA was rehydrated in 300 µl DNA hydration solution.
Genotypes were determined using 5’-fluorogenic exonuclease assays (TaqMan®, Applied Biosystems, Foster City, CA). The DBH −1021C/T genetic variant was genotyped using the TaqMan® primer-probe sets (Applied Biosystems) DBH rs1611115, Assay ID C_2535786_10. PCR amplifications were performed using Platinum® quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA) on a GeneAmp® PCR system 9700. Samples were amplified at 50°C for 2 min, 95° C for 10 min, and then 50 cycles of 95°C for 15s and 60°C for 1 min. The amplification products were analyzed using an Applied Biosystems Prism® 7900 sequence detection system and SDS 2.2 software (Applied Biosystems). All genotype analyses were performed by an individual unaware of the clinical status of the subjects. The DBH genotypes did not show significant evidence for deviation from Hardy-Weinberg Equilibrium (χ2 = 0.686, p = 0.4074). An SRY PCR assay that identifies the presence of the Y chromosome-specific SRY gene was used to confirm the subject’s sex (55). Ten ancestry informative markers were evaluated using the TaqMan® primer-probe sets (rs722869, C_7566096_20; rs1858465, C_11417706_10; rs1876482, C_11640969_10; rs1344870, C_8767848_10; rs1363448, C_3169933_1_; rs952718, C_8844929_10; rs2352476, C__26357333_20; rs714857, custom order; rs1823718, C__12080106_10; rs735612, C___2043758_10, Applied Biosystems). The TaqMan® assays were performed in duplicate and had a concordance of 100%.
Statistical Analysis
Sample size of 35 per group, which was met, used a power of 0.8 with alpha 0.05 based on effect sizes from three previous Yale studies of disulfiram for cocaine. We compared baseline differences in demographics and drug use history using chi squared or t-test. A repeated measures analysis of variance (ANOVA) used the number of cocaine positive urines over the total number of samples (six) for each two week period to compare disulfiram to placebo over time and to determine if the effect of disulfiram is modulated by the DBH locus using R version 2.9.1 (56). We compared condition (disulfiram or placebo), DBH genotype (0 = CT/TT genotype, 1 = CC genotype), time (each two week period), and interactions between condition and time, and between condition and DBH. We analyzed all individuals who had complete data (n = 61) and unbalanced repeated measures ANOVA for all individuals (n = 74). The two analyses yielded almost identical results.
To determine population structure, our cohort was compared against CEPH-HGDP samples (1,035 subjects of 51 populations). The CEPH-HGDP cohort is a collection of 1,035 subjects derived from 51 populations from America, Europe, the Middle East, Central and East Asia, and Oceania, and sub-Saharan Africa. Genotypic data for the ancestral informative markers and population codes for this cohort were kindly provided by Oscar Lao (57). The STRUCTURE 2.3.3 software (58, 59) was run using four ancestral populations (K = 4), a burnin period of 100,000 iterations, and 1 million MCMC replications after burnin to determine population substructure. For all analyses, we corrected for any possible confounding effects by including the proportion of each subject from the founder populations as well as gender and site effects as covariates in the model. The obtained p-values were very similar to those obtained when we did not correct for these covariates. Furthermore, analyses were performed with the total group then within the two DBH subgroups.
Results
Baseline characteristics by treatment and DBH genetics
We enrolled 74 patients from the 93 screened for this study and randomized 34 to disulfiram and 40 to placebo. The patients included 38 with the CC, 32 with the CT, and 4 with the TT genotype. The patients were mostly Caucasian males with a mean age of 39 years and 13 years of opiate abuse. Forty (54%) patients had been previously treated with methadone maintenance. They used cocaine for a mean of 12 years and for 19 days in the month before entering the study. Only 29 patients (39%) reported any alcohol abuse history reflecting our exclusion criteria, and 39 patients (53%) reported marijuana use. As shown in Table 1, we found no significant baseline differences among the four treatment by genotype groups in any clinical characteristics including the ASI interviewer problem severity ratings (p >.05).
Table 1.
Demographic and clinical characteristics by treatment and DBH genotype
| Characteristic | Placebo | Disulfiram | ||
|---|---|---|---|---|
| CC | CT/TT | CC | CT/TT | |
| N | 21 | 19 | 17 | 17 |
| % Male | 71 | 63 | 70 | 53 |
| % Caucasian | 67 | 79 | 82 | 77 |
| % Employed | 67 | 53 | 47 | 71 |
| Age years (s.d.) | 43 (10) | 37 (10) | 38 (11) | 37 (10) |
| Cocaine last 30 days | 14 (7) | 18 (8) | 17 (9) | 18 (9) |
| Cocaine years | 14 (10) | 11 (7) | 9 (7) | 9 (8) |
| Heroin years | 11 (11) | 7 (6) | 9 (7) | 9 (8) |
| % Alcohol abuse | 33 | 42 | 40 | 47 |
| % Marijuana abuse | 33 | 37 | 70 | 35% past |
| Methadone | 71 | 48 | 47 | 47 |
| ASI medical | 2.4 (1.6) | 2.8 (1.9) | 4.2 (2.1) | 2.3 (1.3) |
| ASI employment | 2.4 (4.2) | 0.8 (2.6) | 0.9 (2.0) | 1.4 (3.1) |
| ASI alcohol | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ASI drug | 8.8 (0.6) | 8.8 (0.7) | 8.8 (0.4) | 8.8 (0.4) |
| ASI legal | 0 (0) | 0 (0) | 0 (0) | 0.3 (1.0) |
| ASI family | 0.4 (1.0) | 1.7 (3.2) | 0 (0) | 1.4 (3.1) |
| ASI psychological | 0 (0) | 0.2 (0.6) | 1.1 (2.3) | 0.1 (0.3) |
Retention by Treatment Condition
Treatment retention for the full 12 weeks was 82% (61/74) with no significant difference between disulfiram (77% = 26/34) and placebo (87% = 35/40) (p >.05). The mean numbers of weeks completed was 11.2 ± 3.6. The reasons for dropout were incarceration in two patients (both disulfiram) and nine others left for community treatment programs mostly near the end of the study (four disulfiram). Only two disulfiram patients left the study for adverse effects. Subjects who completed the full 12 week trial did not differ demographically from the 13 who did not complete (p >.05).
Adverse Events
We only had four significant adverse events, and no patient reported an adverse interaction with alcohol, although some patients did report drinking alcohol. One disulfiram patient left the study for reduced sexual functioning, but this was considered as related to the methadone. Other adverse events were two disulfiram cases of arm numbness that resolved spontaneously during the trial without any changes in medication. One of them completed 12 weeks, but the other patient also had a back rash and left at week 6. One placebo patient also made a suicide gesture of superficially cutting his wrist, but was not hospitalized and completed the 12 weeks.
Cocaine Treatment Outcomes by Genotype
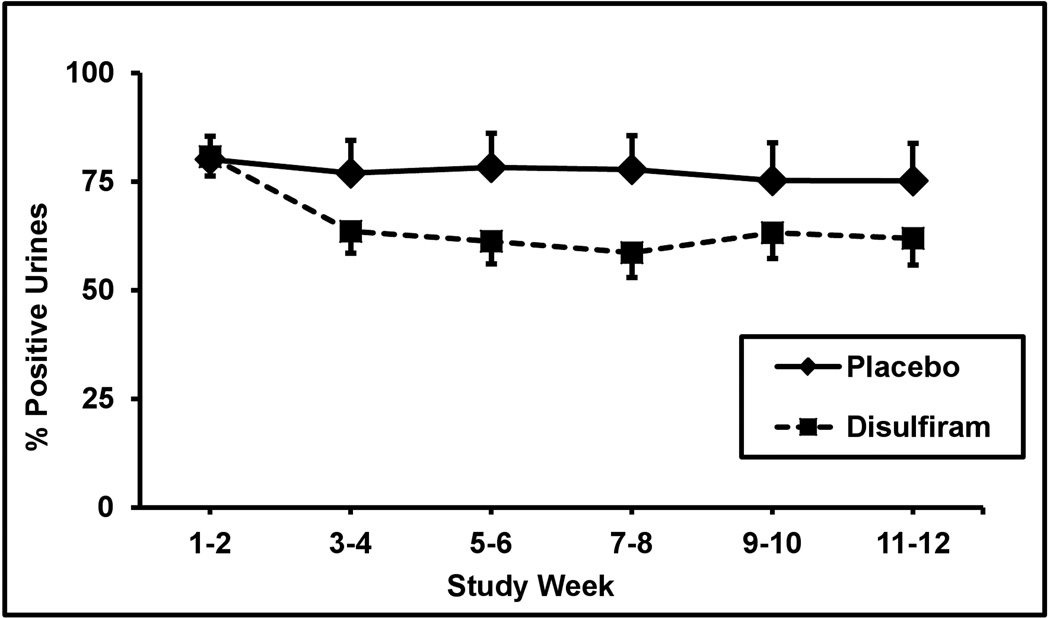
Cocaine positive urine screens showed a significant difference between treatment groups as the overall cocaine urine rates decreased from 80% during the baseline two weeks to 69% during the last two weeks of treatment (F = 12.4; df = 1,440; p <.0005). As shown in Figure 1 with SEM of 3.4% to 5%, the mean cocaine rates during the two baseline weeks were 80% for disulfiram and 80% for placebo. These rates dropped during the last two weeks of treatment to 62% for disulfiram and 75% for placebo. When we only included the 61 subjects who completed the study, the disulfiram treatment effect remained highly significant (F = 15.2; df = 1,364; p <.0002). The interaction between treatment and the SNP was also significant (F=2.6; df=2, 440; P<0.05), although the SNP × weeks and treatment × weeks were not significant.
Figure 1.
Percentage of cocaine positive urine toxicology screens for two-week time blocks across the 12-week trial for the placebo (solid line, n = 40) versus disulfiram (250 mg/day) (dashed line, n = 34) treatment groups. Standard error bars are shown at each time point.
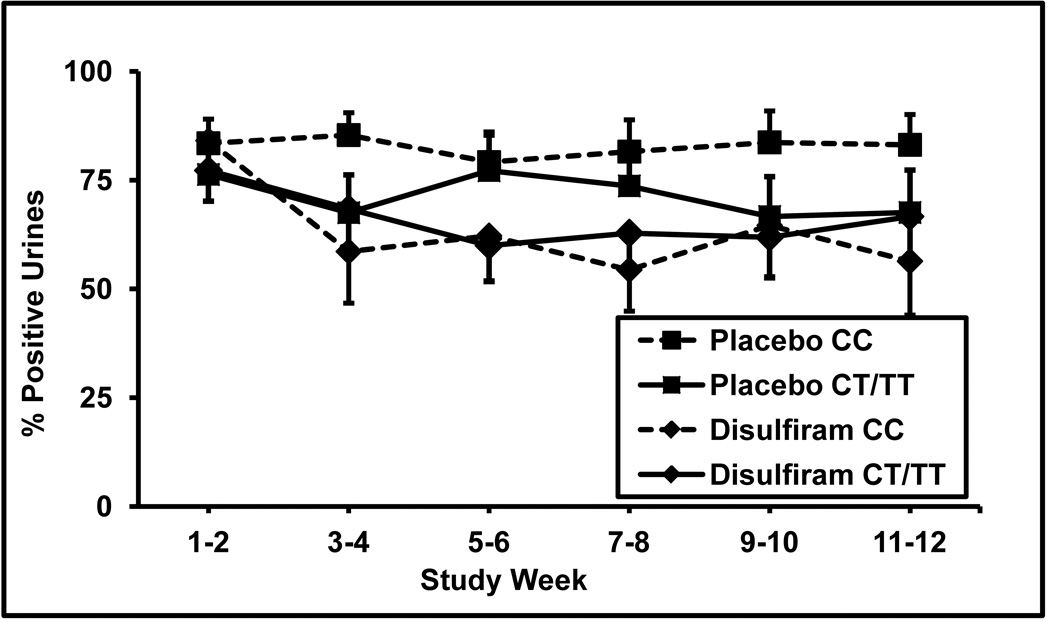
Subjects were divided into two DBH genotype groups: those subjects without a T allele (CC genotype group) and those with a T allele (CT/TT genotype group). When separated into these two genotype groups, cocaine positive urine rates differed between the treatment groups for patients having the CC genotype (F = 17.2; df = 1,236; p <.00005), but did not differ for those having the CT or TT genotypes (F = 1.12; df = 1,234; p >.05). As shown in Figure 2, cocaine positive urines for the CC patients during the two baseline weeks were 84% for disulfiram and 84% for placebo. These rates dropped during the last two weeks of treatment to 56% for disulfiram and were unchanged at 84% for placebo. In Figure 2 for comparison, cocaine urines for the CT/TT patients during the two baseline weeks were 77% for disulfiram and 76% for placebo. These rates dropped during the last two weeks of treatment to 67% for disulfiram and to 68% for placebo. When we only included the 61 subjects who completed the study, the disulfiram treatment effect remained highly significant only among the CC patients (F = 24.2; df = 1,176; p <.0002).
Figure 2.
Percentage of cocaine positive urine toxicology screens for two-week time blocks across the 12-week trial for the placebo versus disulfiram (250 mg/day) treatment groups. Subjects with the CC genotype (square symbols, dashed line, n = 21) and those with CT/TT genotypes (square symbols, solid lines, n = 19) in the placebo group, and subjects the CC genotype (diamond symbols, dashed line, n = 17) and the CT/TT genotypes (diamond symbols, dashed line, n = 17) in the disulfiram group are shown. Standard error bars are shown at each time point.
Opioid Treatment Outcomes by Genotype
Opioid positive urine screens decreased over time, but did not significantly differ between treatment groups. The mean opioid positive rates during the two baseline weeks were 50% for disulfiram and 49% for placebo. These rates dropped during the last two weeks of treatment to 35% for disulfiram and 25% for placebo. When separated into the two genotype groups, patients in neither group showed a difference between the treatment regimens. We also found no significant correlation between the rates of opiate and cocaine positive urines (r=0.08).
Discussion
We found a significant reduction in cocaine positive urines with 250 mg of disulfiram compared to placebo, which is consistent with several other previous studies in cocaine abusers (17–21). This reduction in cocaine use was associated with a specific functional genetic polymorphism in the gene that codes for the enzyme dopamine β-hydroxylase (DβH) (rs1611115). We found that patients having two of the alleles associated with normal levels of DβH (CC) responded to disulfiram, while those with the genotypes encoding lower levels (CT and TT) showed no difference from placebo. Genotype made no difference in the reduction in opioid use.
The different treatment response to disulfiram between those patients with low and high DβH activity may reflect differences in brain dopamine receptors. Minimal DβH activity reduces norepinephrine, but also reduces basal extracellular dopamine in the nucleus accumbens and caudate-putamen (34, 60). This reduction upregulates high-affinity postsynaptic dopamine receptors as much as six-fold and produces behavioral hypersensitivity to psychostimulants (61). Psychostimulant induced locomotor, reinforcing, and aversive effects are enhanced in DBH knockout mice (34, 62). These findings suggest that modest reductions of norepinephrine and dopamine transmission from disulfiram may not attenuate the behavioral responses to psychostimulants in those individuals who have upregulated dopamine receptors because of their genetically low DβH levels.
The number of DBH alleles affects dopamine and norepinephrine levels in the prefrontal cortex of mice when they are treated with disulfiram (Bourdelat-Parks et al., 2005). Disulfiram increased dopamine and decreased norepinephrine levels in their prefrontal cortex of mice with two normal alleles, while disulfiram showed relatively little effect on these levels in mice with null alleles (33). Like these mice, our human study participants with low DβH appeared less affected by disulfiram-induced inhibition of DβH than those with high DβH activity.
Lowering DβH activity through disulfiram may increase aversive symptoms from acute cocaine use, as one mechanism for its efficacy. Although none of these outpatients reported aversive symptoms from cocaine as an adverse event, disulfiram has increased cocaine-associated negative effects including anxiety and paranoia and reduced positive subjective effects during acute laboratory cocaine administration in humans (63–66). Low DBH levels have been associated with psychotic symptoms in psychiatric disorders (see review (67)). For instance, schizophrenic or depressed patients who have low plasma or cerebrospinal fluid levels of DβH exhibit more positive psychotic symptoms compared to those with higher levels of DβH (68–72). Moreover, patients diagnosed with unipolar depression plus psychotic features have lower DβH levels than those without psychotic features (73). In addition, the genetic predisposition for lower levels of DβH protein is associated with cocaine-induced paranoia (39).
This trial has several limitations. First, the sample size is small for the genetic association studies and larger replications are needed of this preliminary study. Second, the genetic associations reflect a modest reduction in cocaine use to a mean proportion of 0.56 cocaine positive urines. However, this reduction for the normal DβH (CC genotype) patients treated with disulfiram was a 33% reduction compared to no change with placebo. The low DβH patients showed only a 13% reduction, which was the same as the 13% reduction with placebo. Thus, we had at least a doubling in efficacy with this genetic selection. Third, most cocaine abusers are not also opioid dependent, which limits the generalization of our findings. Fourth, alcohol abuse can be common among cocaine abusers and our rates of alcohol abuse were low reflecting our exclusion criteria. Fifth, an alternative rationale that may also explain the effectiveness of disulfiram involves ALDH-2 inhibition leading to generation of tetrahydropapaveroline (THP). This chemical inhibits activated tyrosine hydroxylase and suppresses cocaine induced dopamine production and release (74). Future studies might examine the polymorphisms in the gene coding for ALDH-2 as well as the gene for tyrosine hydroxylase and the role of these as potential pharmacogenetic targets. Finally, disulfiram may not be the optimal medication for attaining DβH inhibition, but another DβH inhibitor, nepicastat is being developed that does not inhibit aldehyde dehydrogenase or produce aversive interactions with alcohol (75). Future studies should investigate this more selective DβH inhibitor’s efficacy, since this compound attenuates cocaine-seeking during relapse-like behavior in rats (76) and reduces some positive subjective effects of cocaine in humans (77).
Supplementary Material
Acknowledgements
Supported by: NIH/NIDA 5 P50 DA018197-05 (TK), the Veterans Health Administration, and the David Toomim Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 2.SAMSHA. Results from the 2010 National Survey on Drug Use and Health (NSDUH) In: Rockville MD, editor. H-41 NS. 2011. [Google Scholar]
- 3.Wolf BC, Lavezzi WA, Sullivan LM, Flannagan LM. Methadone-related deaths in Palm Beach County. J Forensic Sci. 2004;49:375–378. [PubMed] [Google Scholar]
- 4.Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Depend. 1995;37:29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- 5.MacGowan RJ, Fichtner RR, Swanson N, Collier C, Kroliczak A, Cole G. Factors associated with client-reported HIV infection among clients entering methadone treatment. AIDS Educ Prev. 1997;9:205–217. [PubMed] [Google Scholar]
- 6.Meandzija B, O'Connor PG, Fitzgerald B, Rounsaville BJ, Kosten TR. HIV infection and cocaine use in methadone maintained and untreated intravenous drug users. Drug Alcohol Depend. 1994;36:109–113. doi: 10.1016/0376-8716(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 7.Hartel DM, Schoenbaum EE, Selwyn PA, Kline J, Davenny K, Klein RS, et al. Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am J Public Health. 1995;85:83–88. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J Behav Health Serv Res. 2003;30:290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- 9.Kosten TR, Rounsaville BJ, Kleber HD. Antecedents and consequences of cocaine abuse among opioid addicts. A 2.5-year follow-up. J Nerv Ment Dis. 1988;176:176–181. doi: 10.1097/00005053-198803000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Magura S, Rosenblum A, Rodriguez EM. Changes in HIV risk behaviors among cocaine-using methadone patients. J Addict Dis. 1998;17:71–90. doi: 10.1300/J069v17n04_07. [DOI] [PubMed] [Google Scholar]
- 11.Magura S, Rosenblum A, Fong C, Villano C, Richman B. Treating cocaine-using methadone patients: predictors of outcomes in a psychosocial clinical trial. Subst Use Misuse. 2002;37:1927–1955. doi: 10.1081/ja-120016225. [DOI] [PubMed] [Google Scholar]
- 12.Black JL, Dolan MP, Penk WE, Robinowitz R, DeFord HA. The effect of increased cocaine use on drug treatment. Addict Behav. 1987;12:289–292. doi: 10.1016/0306-4603(87)90042-6. [DOI] [PubMed] [Google Scholar]
- 13.Dunteman GH, Condelli WS, Fairbank JA. Predicting cocaine use among methadone patients: analysis of findings from a national study. Hosp Community Psychiatry. 1992;43:608–611. doi: 10.1176/ps.43.6.608. [DOI] [PubMed] [Google Scholar]
- 14.Lima MS, Reisser AA, Soares BG, Farrell M. Antidepressants for cocaine dependence. Cochrane Database Syst Rev. 2003:CD002950. doi: 10.1002/14651858.CD002950. [DOI] [PubMed] [Google Scholar]
- 15.Soares B, Lima Reisser AA, Farrell M, Silva de Lima M. WITHDRAWN: Dopamine agonists for cocaine dependence. Cochrane Database Syst Rev. 2010:CD003352. doi: 10.1002/14651858.CD003352.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Lima AR, Lima MS, Soares BG, Farrell M. Carbamazepine for cocaine dependence. Cochrane Database Syst Rev. 2002:CD002023. doi: 10.1002/14651858.CD002023. [DOI] [PubMed] [Google Scholar]
- 17.Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, et al. Efficacy of Disulfiram and Cognitive Behavior Therapy in Cocaine-Dependent Outpatients: A Randomized Placebo-Controlled Trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- 19.Carroll KM. Psychotherapeutic treatment of cocaine abuse: models for its evaluation alone and in combination with pharmacotherapy. NIDA Res Monogr. 1993;135:116–132. [PubMed] [Google Scholar]
- 20.George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- 21.Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- 22.Laje G, McMahon FJ. The pharmacogenetics of major depression: past, present, and future. Biol Psychiatry. 2007;62:1205–1207. doi: 10.1016/j.biopsych.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003;160:1830–1835. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–796. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 25.deLeon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 and CYP450 2C19. Psychosomatics. 2006;47:75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- 26.Rogers JF, Nafziger AN, Bertino JSJ. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am J Med. 2002;113:746–750. doi: 10.1016/s0002-9343(02)01363-3. [DOI] [PubMed] [Google Scholar]
- 27.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 28.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 29.Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98:901–911. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 30.Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 2002;59:538–565. doi: 10.1001/archpsyc.59.6.538. [DOI] [PubMed] [Google Scholar]
- 31.Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Investig Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- 32.Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol. 1996;139:102–108. doi: 10.1006/taap.1996.0147. [DOI] [PubMed] [Google Scholar]
- 33.Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, et al. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 34.Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, et al. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 35.Ross SB, Wetterberg L, Myrhed M. Genetic control of plasma dopamine-beta-hydroxylase. Life Sci. 1973;12:529–532. doi: 10.1016/0024-3205(73)90056-8. [DOI] [PubMed] [Google Scholar]
- 36.Weinshilboum RM, Raymond FA, Elveback LR, Weidman WH. Serum dopamine-beta-hydroxylase activity: sibling-sibling correlation. Science. 1973;181:943–945. doi: 10.1126/science.181.4103.943. [DOI] [PubMed] [Google Scholar]
- 37.Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals--a genetic study. J Psychiatr Res. 1986;20:19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- 38.Wei J, Ramchand CN, Hemmings GP. Possible control of dopamine beta-hydroxylase via a codominant mechanism associated with the polymorphic (GT)n repeat at its gene locus in healthy individuals. Hum Genet. 1997;99:52–55. doi: 10.1007/s004390050310. [DOI] [PubMed] [Google Scholar]
- 39.Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, et al. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- 40.Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O'Connor DT, Price LH, et al. Dopamine beta-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- 41.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zabetian CP, Buxbaum SG, Elston RC, Kohnke MD, Anderson GM, Gelernter J, et al. The structure of linkage disequilibrium at the DBH locus strongly influences the magnitude of association between diallelic markers and plasma dopamine beta-hydroxylase activity. Am J Genet. 2003;72:1389–1400. doi: 10.1086/375499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohnke MD, Zabetian CP, Anderson GM, Kolb W, Gaertner I, Buchkremer G, et al. A genotype-controlled analysis of plasma dopamine beta-hydroxylase in healthy and alcoholic subjects: evidence for alcohol-related differences in noradrenergic function. Biol Psychiatry. 2002;52:1151–1158. doi: 10.1016/s0006-3223(02)01427-0. [DOI] [PubMed] [Google Scholar]
- 44.Bhaduri N, Mukhopadhyay K. Correlation of plasma dopamine beta-hydroxylase activity with polymorphisms in DBH gene: A study on eastern Indian populaion. Cell Mol Neurobiol. 2008;28:343–350. doi: 10.1007/s10571-007-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deinum J, Steenbergen-Spanjers GC, Jansen M, Boomsma F, Lenders JW, van Ittersum FJ, et al. DBH gene variants that cause low plasma dopamine beta hydroxylase with or without a severe orthostatic syndrome.A. J Med Genet. 2004;41:e38. doi: 10.1136/jmg.2003.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J, Ramchand CN, Hemmings GP. TaqI polymorphic sites at the human dopamine beta-hydroxylase gene possibly associated with biochemical alterations of the catecholamine pathway in schizophrenia. Psychiatr Genet. 1998;8:19–24. doi: 10.1097/00041444-199800810-00003. [DOI] [PubMed] [Google Scholar]
- 47.Rogeness GA, Hernandez JM, Macedo CA, Amrung SA, Hoppe SK. Near-zero plasma dopamine-beta-hydroxylase and conduct disorder in emotionally disturbed boys. J Am Acad Child Psychiatry. 1986;25:521–527. doi: 10.1016/s0002-7138(10)60012-x. [DOI] [PubMed] [Google Scholar]
- 48.Rogeness GA, Hernandez JM, Macedo CA, Mitchell EL, Amrung SA, Harris WR. Clinical characteristics of emotionally disturbed boys with very low activities of dopamine-beta-hydroxylase. J Am Acad Child Psychiatry. 1984;23:203–208. doi: 10.1097/00004583-198403000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Rogeness GA, Javors MA, Maas JW, Macedo CA, Fischer C. Plasma dopamine-beta-hydroxylase, HVA, MHPG, and conduct disorder in emotionally disturbed boys. Biol Psychiatry. 1987;22:1158–1162. doi: 10.1016/0006-3223(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 50.Rogeness GA, Maas JW, Javors MA, Macedo CA, Harris WR, Hoppe SK. Diagnoses, catecholamine metabolism, and plasma dopamine-beta-hydroxylase. J Am Acad Child Adolesc Psychiatry. 1988;27:121–125. doi: 10.1097/00004583-198801000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Galvin M, Shekhar A, Simon J, Stilwell B, Ten Eyck R, Laite G, et al. Low dopamine-beta-hydroxylase: a biological sequela of abuse and neglect? Psychiatry Res. 1991;39:1–11. doi: 10.1016/0165-1781(91)90002-7. [DOI] [PubMed] [Google Scholar]
- 52.Jonsson EG, Bah J, Melke J, Abou Jamra R, Schumacher J, Westberg L, et al. Monoamine related functional gene variants and relationships to monoamine metabolite concentrations in CSF of healthy volunteers. BMC Psychiatry. 2004;4:4. doi: 10.1186/1471-244X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Carroll KM. Manual-guided psychosocial treatment. A new virtual requirement for pharmacotherapy trials? Arch Gen Psychiatry. 1997;54:923–928. doi: 10.1001/archpsyc.1997.01830220041007. [DOI] [PubMed] [Google Scholar]
- 55.Plaseski T, Noveski P, Trivodalieva S, Efremov GD, Plaseska-Karanfilska D. Quantitative fluorescent-PCR detection of sex chromosome aneuploidies and AZF deletions/duplications. Genet Test. 2008;12:595–605. doi: 10.1089/gte.2008.0068. [DOI] [PubMed] [Google Scholar]
- 56.Vienna, editor. R_Development_Core_Team. R: A language and environment for statistical computing. 2.9.1. Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 57.Lao O, van Duijn K, Kersbergen P, de Knijff P, Kayser M. Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. Am J Hum Genet. 2006;78:680–690. doi: 10.1086/501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 62.Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci U S A. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, et al. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- 65.McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 66.McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry. 1998;43:540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- 67.Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- 68.Meltzer HY, Cho HW, Carroll BJ, Russo P. Serum dopamine-beta-hydroxylase activity in the affective psychoses and schizophrenia. Decreased activity in unipolar psychotically depressed patients. Arch Gen Psychiatry. 1976;33:585–591. doi: 10.1001/archpsyc.1976.01770050047007. [DOI] [PubMed] [Google Scholar]
- 69.Mod L, Rihmer Z, Magyar I, Arato M, Alfoldi A, Bagdy G. Serum DBH activity in psychotic vs. nonpsychotic unipolar and bipolar depression. Psychiatry Res. 1986;19:331–333. doi: 10.1016/0165-1781(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 70.Sapru MK, Rao BS, Channabasavanna SM. Serum dopamine-beta-hydroxylase activity in clinical subtypes of depression. Acta Psychiatr Scand. 1989;80:474–478. doi: 10.1111/j.1600-0447.1989.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 71.van Kammen DP, Kelley ME, Gilbertson MW, Gurklis J, O'Connor DT. CSF dopamine beta-hydroxylase in schizophrenia: associations with premorbid functioning and brain computerized tomography scan measures. Am J Psychiatry. 1994;151:372–378. doi: 10.1176/ajp.151.3.372. [DOI] [PubMed] [Google Scholar]
- 72.Sternberg DE, van Kammen DP, Lerner P, Ballenger JC, Marder SR, Post RM, et al. CSF dopamine beta-hydroxylase in schizophrenia. Arch Gen Psychiatry. 1983;40:743–747. doi: 10.1001/archpsyc.1983.01790060041005. [DOI] [PubMed] [Google Scholar]
- 73.Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, et al. Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry. 2002;51:358–364. doi: 10.1016/s0006-3223(01)01349-x. [DOI] [PubMed] [Google Scholar]
- 74.Yao L, Fan P, Arolfo M, Jiang Z, Olive MF, Zablocki J, et al. Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP a cocaine use-dependent inhibitor of dopamine synthesis. Nat Med. 2010;16:1024–1028. doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, et al. Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br J Pharmacol. 1997;121:1803–1809. doi: 10.1038/sj.bjp.0701315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, et al. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cunningham KA, Carbone CL, Anastasio NC, Harper TA, Moeller FG, Ware DL, et al. Dopamine ® hydroxylase inhibitor SYN117 decreases subjective effects of cocaine; CPDD 72nd Annual Meeting; Arizona: Scottsdale; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.