Abstract
The ubiquitous inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) Ca2+ release channel plays a central role in the generation and modulation of intracellular Ca2+ signals, and is intricately regulated by multiple mechanisms including cytoplasmic ligand (InsP3, free Ca2+, free ATP4−) binding, posttranslational modifications, and interactions with cytoplasmic and endoplasmic reticulum (ER) luminal proteins. However, regulation of InsP3R channel activity by free Ca2+ in the ER lumen ([Ca2+]ER) remains poorly understood because of limitations of Ca2+ flux measurements and imaging techniques. Here, we used nuclear patch-clamp experiments in excised luminal-side-out configuration with perfusion solution exchange to study the effects of [Ca2+]ER on homotetrameric rat type 3 InsP3R channel activity. In optimal [Ca2+]i and subsaturating [InsP3], jumps of [Ca2+]ER from 70 nM to 300 µM reduced channel activity significantly. This inhibition was abrogated by saturating InsP3 but restored when [Ca2+]ER was raised to 1.1 mM. In suboptimal [Ca2+]i, jumps of [Ca2+]ER (70 nM to 300 µM) enhanced channel activity. Thus, [Ca2+]ER effects on channel activity exhibited a biphasic dependence on [Ca2+]i. In addition, the effect of high [Ca2+]ER was attenuated when a voltage was applied to oppose Ca2+ flux through the channel. These observations can be accounted for by Ca2+ flux driven through the open InsP3R channel by [Ca2+]ER, raising local [Ca2+]i around the channel to regulate its activity through its cytoplasmic regulatory Ca2+-binding sites. Importantly, [Ca2+]ER regulation of InsP3R channel activity depended on cytoplasmic Ca2+-buffering conditions: it was more pronounced when [Ca2+]i was weakly buffered but completely abolished in strong Ca2+-buffering conditions. With strong cytoplasmic buffering and Ca2+ flux sufficiently reduced by applied voltage, both activation and inhibition of InsP3R channel gating by physiological levels of [Ca2+]ER were completely abolished. Collectively, these results rule out Ca2+ regulation of channel activity by direct binding to the luminal aspect of the channel.
INTRODUCTION
Modulating cytoplasmic free Ca2+ concentration ([Ca2+]i) is a universal intracellular signaling pathway that regulates numerous cellular physiological processes including apoptosis, gene expression, bioenergetics, secretion, immune responses, fertilization, muscle contraction, and synaptic transmission (Clapham, 1995; Marks, 1997; Berridge, 1998, 2003; Berridge et al., 2000; Bootman et al., 2001; Orrenius et al., 2003; Braet et al., 2004; Randriamampita and Trautmann, 2004; Cárdenas et al., 2010). Ubiquitous ER-localized inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) Ca2+ release channels (Foskett et al., 2007) play a central role in this pathway in many cells (Taylor and Richardson, 1991; Putney and Bird, 1993; Bezprozvanny and Ehrlich, 1995; Furuichi and Mikoshiba, 1995; Patterson et al., 2004; Foskett et al., 2007; Joseph and Hajnóczky, 2007; Cárdenas et al., 2010; Foskett, 2010). InsP3 generated in the cytoplasm in response to extracellular stimuli (Berridge, 1993) binds to and activates InsP3R channels to release Ca2+ stored in the ER lumen into the cytoplasm, generating diverse local and global [Ca2+]i signals (Berridge, 1993, 1997; Hagar and Ehrlich, 2000; Thrower et al., 2001; Taylor and Laude, 2002; Foskett et al., 2007). Whereas much is known regarding the intricate regulation of InsP3R channel gating by multiple processes—binding of cytoplasmic ligands (Ca2+, InsP3, and ATP4−), posttranslational modifications, interactions with proteins, clustering, differential localization (Joseph, 1996; MacKrill, 1999; Patel et al., 1999; Johenning and Ehrlich, 2002; Foskett et al., 2007; Betzenhauser et al., 2008; Kang et al., 2008; Wagner et al., 2008; Li et al., 2009; Taufiq-Ur-Rahman et al., 2009)—the regulation of InsP3R channel activity by free Ca2+ in the lumen of the ER ([Ca2+]ER) remains poorly understood and controversial (Irvine, 1990; Tregear et al., 1991; Ferris et al., 1992; Swillens, 1992; Kindman and Meyer, 1993; Bezprozvanny and Ehrlich, 1994; Bootman, 1994a,b; Swillens et al., 1994; Shuttleworth, 1995; Dupont and Swillens, 1996; Missiaen et al., 1996; Parys et al., 1996; Beecroft and Taylor, 1997; Caroppo et al., 2003; Dawson et al., 2003; Fraiman and Dawson, 2004; Foskett et al., 2007; McCarron et al., 2008; Yamasaki-Mann and Parker, 2011).
The main techniques for studying possible [Ca2+]ER modulation of InsP3R channel gating have been 45Ca2+ flux measurements (Nunn and Taylor, 1991, 1992; Tregear et al., 1991; Missiaen et al., 1992a,b; Parys et al., 1993; Beecroft and Taylor, 1997) and fluorescence Ca2+ imaging (Combettes et al., 1992, 1996; Missiaen et al., 1992c; Shuttleworth, 1992; Renard-Rooney et al., 1993; Short et al., 1993; Steenbergen and Fay, 1996; Tanimura and Turner, 1996; Tanimura et al., 1998; Caroppo et al., 2003; Higo et al., 2005; McCarron et al., 2008; Yamasaki-Mann and Parker, 2011). Both approaches rely on changes in [Ca2+]i or [Ca2+]ER to infer channel activity and therefore cannot rigorously control both [Ca2+]ER and [Ca2+]i simultaneously during experiments. This has made it difficult to differentiate direct effects of [Ca2+]ER on the luminal aspect of the InsP3R from feed-through effects caused by Ca2+ flux through the open channel.
Electrophysiological recordings of single InsP3R channels allow InsP3R channel activity to be determined from currents carried by K+ through open channels (Foskett et al., 2007) and therefore can be performed under rigorously controlled and defined [Ca2+]i and [Ca2+]ER. However, only two electrophysiological studies of the effects of [Ca2+]ER on InsP3R channel activity have been reported (Bezprozvanny and Ehrlich, 1994; Thrower et al., 2000). Both used InsP3R reconstituted in lipid bilayers and explored only a limited set of [Ca2+]ER, [Ca2+]i, and [InsP3]. Insights about [Ca2+]ER regulation of InsP3R channels from these studies were limited by insufficient buffering of [Ca2+]i and the use of nonphysiological concentrations of divalent cations in the luminal solutions in Bezprozvanny and Ehrlich (1994), or inappropriate Ca2+ buffering and nonphysiological [KCl] used in Thrower et al. (2000).
Here, we studied systematically the effects of [Ca2+]ER on the activity of single homotetrameric channels of recombinant rat type 3 InsP3R (r-InsP3R-3) expressed in cells with no endogenous InsP3R expression (Sugawara et al., 1997; Mak et al., 2005), using nuclear patch-clamp techniques (Mak et al., 2005; Vais et al., 2010a) that record activities of the InsP3R channel in its native membrane milieu (Foskett et al., 2007) with ionic conditions on both sides of the channel, especially [Ca2+]i and [Ca2+]ER, rigorously controlled. By comparing activities (open probability [Po]) of type 3 InsP3R channels in excised luminal-side-out (lum-out) nuclear membrane patches exposed to different [Ca2+]ER by rapid perfusion solution exchange (Vais et al., 2010a), we found that high [Ca2+]ER modulated InsP3R channel activity. However, our experiments ruled out [Ca2+]ER modulation of InsP3R channel activity through intrinsic functional Ca2+-binding sites on the luminal side of the channel. Instead, the experimental results were consistent with [Ca2+]ER affecting InsP3R channel gating solely through the rise in local [Ca2+]i in the vicinity of the open channel generated by the [Ca2+]ER-driven Ca2+ flux through the open channel itself, which modulates InsP3R channel activity through functional cytoplasmic Ca2+-binding sites of the channel.
MATERIALS AND METHODS
Nucleus isolation and nuclear patch-clamp electrophysiology
Generation and maintenance of DT40-KO-r-InsP3R-3 cells (mutant cells derived from chicken B cells with the endogenous genes for all three InsP3R isoforms knocked out and then stably transfected to express recombinant r-InsP3R-3) were described in Mak et al. (2005). Nuclear patch-clamp experiments were performed using nuclei isolated from DT40-KO-r-InsP3R-3 cells as described previously (Mak et al., 2005). Experiments investigating InsP3R activity under constant ligand conditions were performed in the on-nucleus configuration (Mak et al., 2007). Excised nuclear membrane patches in the lum-out configuration were obtained from isolated nuclei (Mak et al., 2007) using protocols analogous to those used to obtain inside-out excised patches in plasma membrane patch-clamp experiments. The solution around the excised nuclear membrane patch was rapidly switched multiple times using a solution-switching setup described in Mak et al. (2007).
InsP3R channel current traces were acquired at room temperature as described previously (Mak et al., 1998), digitized at 5 kHz, and anti-aliasing filtered at 1 kHz. All applied potentials (Vapp) were measured relative to the bath electrode. All on-nucleus experiments were performed at Vapp = −40 mV. All lum-out experiments were performed at Vapp = −30 mV unless stated otherwise.
Experimental solution composition
All experimental solutions contained 140 mM KCl and 10 mM HEPES, pH to 7.3 with KOH. Because physiological levels of free Mg2+ (0–3 mM) have no effects on channel activities (Mak et al., 1999), and to avoid the complicating effects of Mg2+ on InsP3R channel conductance (Mak and Foskett, 1998) and free [ATP] in experimental solutions, Mg2+ was not added to any of the solutions used.
All experiments were performed using the same bath solution with free Ca2+ concentration ([Ca2+]f) of 70 nM buffered by 0.5 mM BAPTA (1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid). All pipette solutions contained 0.5 mM Na2ATP.
Pipette solutions used in on-nucleus patch-clamp experiments contained various [Ca2+]f buffered by 0.5 mM Ca2+ chelator and [InsP3] as specified. BAPTA was used for 20 nM < [Ca2+]f < 600 nM, diBrBAPTA (5,5′-dibromo BAPTA) for 600 nM ≤ [Ca2+]f ≤ 4 µM, and hydroxyethylethylenediaminetriacetic acid (HEDTA) for [Ca2+]f > 4 µM. ATP contributed to Ca2+ buffering in solutions with [Ca2+]f > 30 µM (Bers et al., 2010).
Pipette solutions used in lum-out patch-clamp experiments had [Ca2+]f of either 2 µM buffered by various concentrations of diBrBAPTA or HEDTA as specified, or 55 nM buffered by various concentrations of BAPTA as specified. Four perfusion solutions were used in these experiments: one had [Ca2+]f of 70 nM buffered by 0.5 mM BAPTA with no ATP; one contained 1.3 mM CaCl2 and 1.5 mM Na2ATP, so that [Ca2+]f was buffered to 300 µM according to Max Chelator freeware; and two with no Ca2+ chelator, with one containing 1 mM CaCl2 and the other containing 2 mM CaCl2, giving [Ca2+]f of 550 µM and 1.1 mM, respectively, according to activity coefficient calculations (Butler, 1968; Vais et al., 2010a).
[Ca2+]f in all solutions (<100 µM) was confirmed by Ca2+-sensitive dye fluorimetry.
Data analysis
Because there is no Ca2+ flux through open InsP3R channels in steady-state on-nucleus experiments using bath solutions containing only 70 nM [Ca2+]f, there was no possibility of cross talk between channels. Furthermore, we detected no effect of channel clustering on gating of InsP3R channels expressed in DT40-KO-r-InsP3R-3 cells (Vais et al., 2011). Thus, multi-channel and single-channel current traces were selected for channel Po and dwell-time analysis using QuB software (Qin et al., 2000a,b). However, because substantial Ca2+ flux can be driven through open InsP3R channels when some perfusion solutions were used in excised lum-out nuclear patch-clamp experiments, only single-channel current traces from such experiments were selected for analysis to avoid complications arising from Ca2+ flux through one InsP3R channel affecting gating behaviors of neighboring active channel(s). We accepted only current records long enough to allow the number of active channels observed to be accurately determined (with >99% confidence) for data analysis (Mak et al., 2001b; Ionescu et al., 2006; Vais et al., 2010b). Only single-channel current traces >15 s were used for channel Po > 0.02. Longer traces were required for lower Po, so only single-channel traces >1.5 min were used for Po ≈ 0.005. Least-square fitting and statistical analysis of data were done with IGOR Pro software (WaveMetrics).
Modeling of the [Ca2+]i profile in the vicinity of an InsP3R channel
The [Ca2+]i profile around an open InsP3R channel was calculated by considering the open channel as a circular aqueous pore with a diameter of 2.5 nm (Jiang et al., 2002; Serysheva et al., 2003; Wolfram et al., 2010). Magnitudes of the Ca2+ current through an open channel (iCa) in the presence of 140 mM KCl and various [Ca2+]i, [Ca2+]ER, and Vapp were evaluated using the general Goldman–Hodgkin–Katz current equation (Lewis, 1979; Hille, 2001):
| (1) |
where (= 2) is the valence of Ca2+, F is the Faraday constant, R is the gas constant, T is the absolute temperature, and PCa is the effective channel permeability for Ca2+ through the InsP3R channel measured experimentally in buffers containing 140 mM KCl (Vais et al., 2010a).
Although the analytical equation obtained by using the approximation in Neher (1986, 1998), Smith (1996), and Naraghi and Neher (1997) can provide a reasonable estimation of the steady-state [Ca2+]i profile around a Ca2+ channel generated by Ca2+ flux through the channel buffered by mobile Ca2+-binding chelators (Liu et al., 2010; Vais et al., 2010a), it says nothing about the time scale of the evolution of the [Ca2+]i profile to reach the steady state. These dynamics then affect the dissipation of the Ca2+ profile after the channel closes. Once a channel closes, the profile collapses first to a level that is above basal very quickly but then dissipates toward basal on a slow time scale. Therefore, to follow the time-dependent evolution of the [Ca2+]i profiles around the channel as it gates, numerical modeling of Ca2+ diffusion around the open InsP3R channel was used to calculate the [Ca2+]i profiles in the vicinity of the channel under various [Ca2+]i, [Ca2+]ER, and Vapp combinations.
In the simulations, [Ca2+]i is controlled by spatial diffusion, with the rate equation for [Ca2+]i at distance r from the channel at time t after the channel opens, C(r,t), given as
| (2) |
where J is the Ca2+ flux passing through the channel from the ER lumen, DC is the diffusion coefficient of Ca2+ in the medium (= 800 µm2s−1 [Cussler, 1997] for aqueous medium), and b is the free Ca2+ buffer concentration.
For mobile Ca2+ buffers, is given as
| (3) |
where B is the total Ca2+ buffer concentration, and kon and koff are the rates of Ca2+ binding to and dissociating from the buffer, respectively, so that koff/kon = Kd (dissociation constant) of the buffer. Db is the diffusion coefficient of the mobile buffer.
Kd for diBrBAPTA, HEDTA, and BAPTA is 1.6 µM, 4.7 µM, and 180 nM, respectively. kon for diBrBAPTA, HEDTA, and BAPTA is 450, 4.5, and 450 µM−1s−1, respectively (Tsien, 1980; Naraghi, 1997). Db for diBrBAPTA, HEDTA, and BAPTA is 296, 319, and 390 µm2s−1, respectively, estimated from where Mb and MC are the molecular weights of the buffer and Ca2+, respectively.
iCa in Eq. 1 is converted into J in Eq. 2 by
| (4) |
where rch is the radius of the channel pore. For simplicity, the channel is considered to be embedded in an infinite membrane, opening into a semi-infinite cytoplasmic volume. Then, δV is the volume of a hemisphere with radius rch over the channel. Propagation of Ca2+ and mobile buffer (if present) was simulated throughout a homogeneous semi-infinite 3-D cytosolic space.
With spherical symmetry around the channel, the Laplacian of C and b in spherical coordinates is
| (5) |
where X = C or b.
The differential Eqs. 2 and 3 were solved implicitly with a spatial grid size of 0.625 nm using the Tridiagonal Matrix Solver software in a hemispherical volume of a large radius of 10 µm, so that the volume was effectively semi-infinite. The channel opened at t = 0 and remained open for the duration of the simulation. Calcium profiles were evolved with 0.1-µs time steps.
To simulate the collapse of the [Ca2+]i profile after channel closure, the [Ca2+]i profile was allowed to evolve for 50 ms after the channel opened. Then, J was set equal to 0 for all r at t = 0 as the channel closed. The [Ca2+]i profile was then evolved using the same software with the same time steps for various durations.
RESULTS
Dependence of steady-state InsP3R-3 channel activity on cytoplasmic ligands
To investigate the effects of ER luminal Ca2+ concentration ([Ca2+]ER) on InsP3R-3 channel gating, we first performed on-nucleus patch-clamp experiments on nuclei isolated from DT40-KO-r-InsP3R-3 cells to characterize the gating behaviors of single recombinant homotetrameric r-InsP3R-3 channels over a wide range of [InsP3] and [Ca2+]i in the presence of a physiological level (5 mM) of free ATP, which supports InsP3R channel gating (Mak et al., 1999, 2001a). In a bath solution with [Ca2+]f = 70 nM and no MgATP to support activity of the SERCA in the outer nuclear membrane, [Ca2+]f in the perinuclear space of the isolated nuclei (equivalent to the ER lumen topologically) equilibrated with that of the bath solution so that [Ca2+]ER = 70 nM in these experiments. The channel with large conductance (∼550 pS in 140 mM KCl) observed in the outer nuclear membrane of nuclei isolated from DT40-KO-r-InsP3R-3 cells (Fig. 1) was identified as recombinant InsP3R-3 channel by its requirement of InsP3 for activation (Cheung et al., 2008; Vais et al., 2010a).
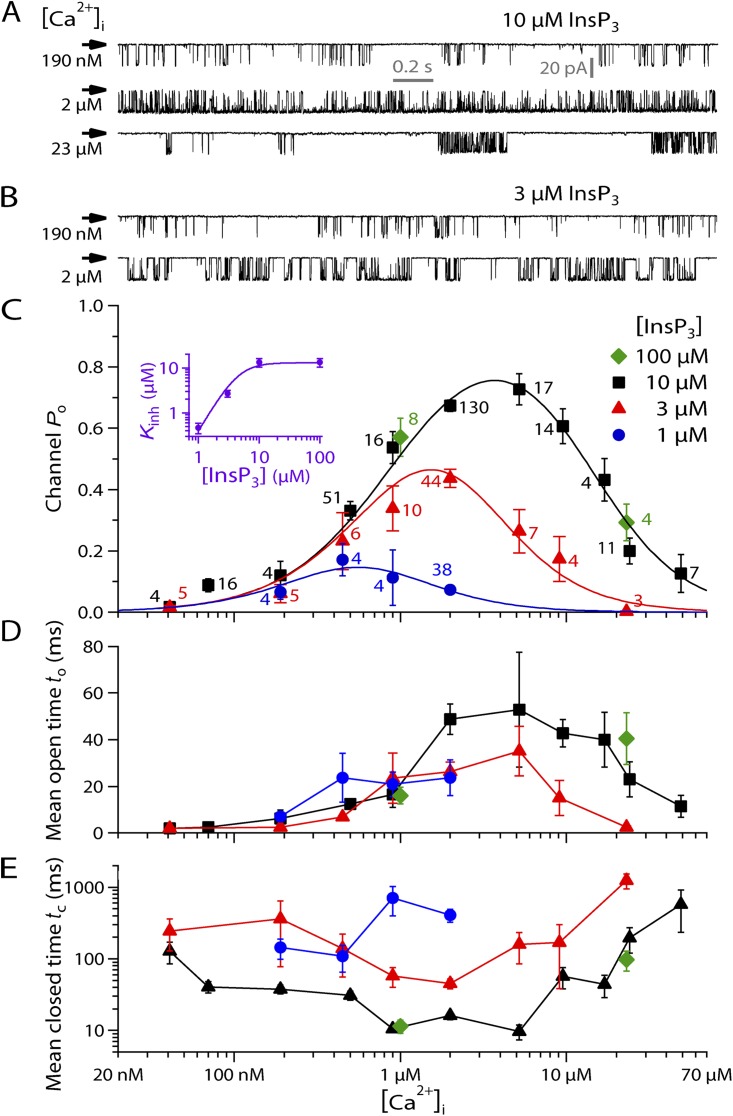
Figure 1.
Ligand dependence of gating of single r-InsP3R-3 channels. Vapp = −40 mV. (A) Typical single-channel on-nucleus patch-clamp current traces of InsP3R-3 channels in suboptimal (190 nM), optimal (2 µM), and inhibitory (23 µM) [Ca2+]i in the presence of saturating (10 µM) [InsP3], demonstrating biphasic [Ca2+]i dependence of InsP3R-3 channel activity. Arrow indicates closed-channel baseline current level for these and all subsequent current traces. (B) Typical single-channel on-nucleus patch-clamp current traces in the presence of subsaturating (3 µM) [InsP3] showing that [InsP3] reduction has little effect on channel activity at suboptimal (190 nM) [Ca2+]i but increases channel sensitivity to Ca2+ inhibition, so channel activity is substantially decreased at [Ca2+]i = 2 µM. (C) [Ca2+]i dependence of mean channel Po in various [InsP3] as tabulated. Error bars show the SEM in this and all subsequent figures unless stated otherwise. The number of current traces analyzed for each data point is tabulated next to the data point in the same color. Curves are empirical biphasic Hill equation fits to mean Po data points for various [InsP3] with the same Pmax, Kact, Hact, and Hinh. The purple inset shows the dependence of the Kinh on [InsP3]. Error bars here show the estimates of fitting errors of Kinh derived from the biphasic Hill equation fits. The curve is the empirical simple Hill equation fit of the [InsP3] dependence. (D and E) [Ca2+]i dependence of mean open and closed durations of InsP3R channel in various [InsP3], derived from the same experimental data used in C. Data points in the same [InsP3] are connected with lines for clearer presentation.
In the presence of high (10 µM) [InsP3], the InsP3R-3 channel exhibited biphasic dependence on [Ca2+]i, with channel Po increasing as [Ca2+]i increased until a maximum Po was reached at [Ca2+]i of ∼2–6 µM. Further increases in [Ca2+]i inhibited channel Po (Fig. 1, A and C).
A further increase in [InsP3] (100 µM) did not change the gating of InsP3R-3 appreciably, indicating that the channel was saturated by 10 µM InsP3 in [Ca2+]i between 1 and 20 µM (Fig. 1 C). Reduction in [InsP3] below 10 µM did not affect Ca2+ activation of the channel significantly but substantially enhanced the sensitivity of the channel to [Ca2+]i inhibition so the channel was inhibited at lower [Ca2+]i (Fig. 1, B and C). This reduced both the maximum channel Po observed and the range of [Ca2+]i over which the channel gated appreciably in low [InsP3] (notably for [InsP3] = 1 µM in Fig. 1 C).
The [Ca2+]i dependence of InsP3R-3 channel Po in all [InsP3] can be well fitted simultaneously for all [InsP3] investigated by the biphasic Hill equation (Foskett et al., 2007) (Fig. 1 C):
| (7) |
with four of the five parameters retaining the same values: Pmax = 1, Kact = 940 nM, Hact (Hill coefficient for Ca2+ activation) = 1.3, and Hinh (Hill coefficient for Ca2+ inhibition) = 1.6. Only Kinh varies with [InsP3] (inset in Fig. 1 C). Pmax from the Hill equation fit of the Po data is significantly greater than the maximum Po (0.78) observed in saturating [InsP3]. This indicates that even in saturating [InsP3], the recombinant InsP3R-3 channel in DT40-KO-r-InsP3R-3 cells is not fully activated by [Ca2+]i before it begins to be inhibited by [Ca2+]i. Because the [Ca2+]i dependence of the InsP3R-3 channel does not exhibit a clear plateau with channel Po staying at Pmax over a broad range of [Ca2+]i, Kact and Kinh are not uniquely defined by the observed [Ca2+]i dependence (Foskett et al., 2007). Nevertheless, because Pmax must be ≤1, the large observed maximum Po (≈0.78) indicates that the values of Kact and Kinh derived from the biphasic Hill equation fit are reasonable indications of the apparent affinities of the activating and inhibitory cytoplasmic Ca2+-binding sites of the channel. Thus, the biphasic Hill equation fitting result that only Kinh depends on [InsP3] indicates that InsP3 modulates InsP3R-3 gating solely by changing the sensitivity of the channel to Ca2+ inhibition (Mak et al., 1998; Foskett et al., 2007). Both Hill coefficients for Ca2+ activation and inhibition are moderately >1, suggesting that both Ca2+ activation and inhibition are cooperative but not strongly so.
The dependence of Kinh on [InsP3] is well described by a simple activating Hill equation (inset in Fig. 1 C):
with (Kinh in saturating [InsP3]) ≈ 13 µM and KInsP3 (half-maximal [InsP3]) ≈ 4.5 µM. HInsP3 (Hill coefficient for modulation of Kinh by [InsP3]) is ∼2.3. This suggests that InsP3 modulation of InsP3R-3 channel activity is strongly cooperative.
These main features of ligand regulation of the activity of homotetrameric recombinant r-InsP3R-3 channel in DT40-KO-r-InsP3R-3 cells are highly reminiscent of those of a variety of InsP3R channels in different cell systems examined using the same approach: endogenous Xenopus laevis type 1 InsP3R (InsP3R-1) channel in oocytes, recombinant r-InsP3R-3 channel expressed in Xenopus oocytes, and endogenous insect InsP3R channel in Sf9 cells (Foskett et al., 2007). However, the r-InsP3R-3 channel in DT40-KO-r-InsP3R-3 cells is significantly less sensitive to [Ca2+]i and [InsP3] activation than the other channels, with significantly higher Kact and KInsP3. Furthermore, the efficacy of InsP3 to activate the channel by reducing its sensitivity to inhibition by Ca2+i is also lowest for r-InsP3R-3 channel in DT40-KO-r-InsP3R-3 cells among InsP3R channels studied (Foskett et al., 2007), as indicated by its low .
Regulation of r-InsP3R-3 channel gating kinetics by cytoplasmic ligands
The [Ca2+]i dependence of the mean open duration (to) of r-InsP3R-3 channels expressed in DT40-KO-r-InsP3R-3 cells loosely mirrors that of channel Po, with to continuously increasing as [Ca2+]i was increased from 40 nM to ≈1 µM. Within this [Ca2+]i range, to was similar in the same [Ca2+]i for all [InsP3]. to then remained high for a range of [Ca2+]i extending beyond the point where channel Po started to be reduced by higher [Ca2+]i. Beyond a threshold [Ca2+]i, to started to be reduced by higher [Ca2+]i. The threshold [Ca2+]i was ≈20 µM in 10 µM InsP3 and ≈6 µM in 3 µM InsP3. Thus, the threshold [Ca2+]i decreased as [InsP3] was reduced (Fig. 1 D).
Ligand regulation of the mean channel closed duration (tc) is more complex. In saturating [InsP3], tc decreased continuously as [Ca2+]i was increased up to 1 µM, when it reached a minimum of ∼10 ms at [Ca2+]i ≈ 900 nM, before Po attained its maximum value. tc remained at the minimum value as [Ca2+]i was increased to 6 µM. Then, tc increased when Po started to be reduced by rising [Ca2+]i. Unlike to, tc was increased in all [Ca2+]i as [InsP3] was reduced below saturating levels, except at very low [Ca2+]i (∼40 nM). As [Ca2+]i was increased, tc followed a trend that is the inverse of that of Po, decreasing until tc reached its minimum at the [Ca2+]i at which Po was maximal, and then increasing as Po decreased (Fig. 1 E).
These ligand dependencies of the gating characteristics (to and tc) of r-InsP3R-3 in DT40-KO-r-InsP3R-3 cells are markedly more complex than those of other InsP3R channels examined (Mak et al., 1998, 2001b; Ionescu et al., 2006). For the other channels, to remained effectively constant over most of the ranges of [InsP3] and [Ca2+]i examined, so that once a channel opens, the duration for which it remains open is largely independent of the local [Ca2+]i and [InsP3]. Ligand modulations of Po of those channels therefore result mostly from ligand modulations of tc.
Effects of physiological levels of [Ca2+]ER on InsP3R-3 channel gating
To study possible modulation of InsP3R-3 channel gating by [Ca2+]ER, nuclear patch-clamp experiments in the excised lum-out configuration were performed on nuclei from DT40-KO-r-InsP3R-3 cells. The luminal side of InsP3R channels in the isolated nuclear membrane patches was exposed rapidly and repeatedly to solutions containing different [Ca2+]f using rapid perfusion solution exchange (Vais et al., 2010a,b). To avoid the complication of Ca2+ moving through one active InsP3R channel affecting the activity of neighboring active channels by raising local [Ca2+]i, only single-channel current traces obtained in these lum-out nuclear patch-clamp experiments were used for analysis.
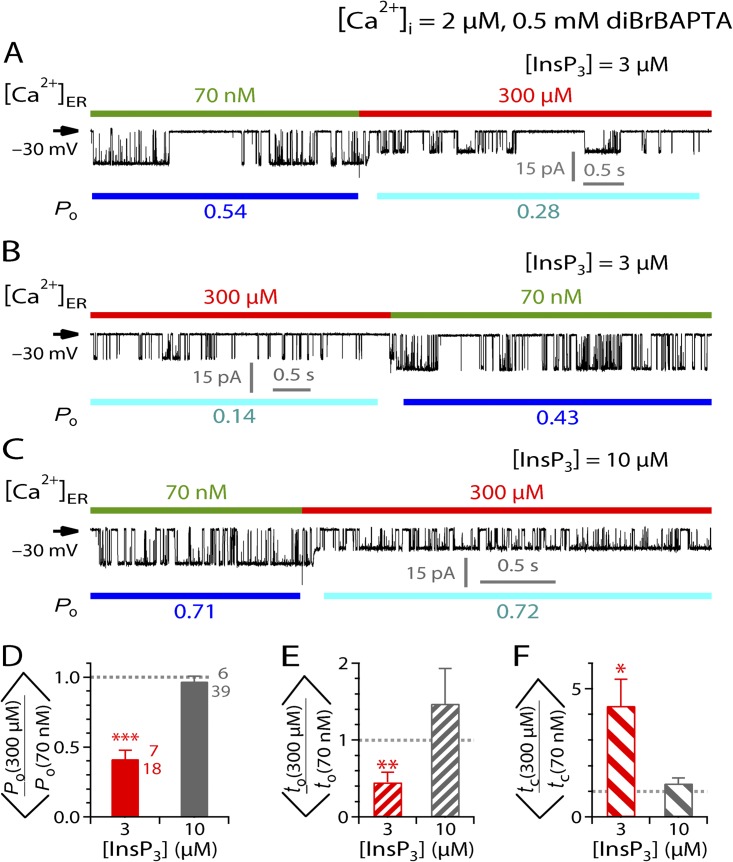
To detect possible activating or inhibitory effects of [Ca2+]ER on InsP3R channel gating, we first used a pipette solution containing subsaturating (3 µM) [InsP3] and optimal (2 µM) [Ca2+]i buffered by 0.5 mM diBrBAPTA. Active InsP3R-3 channels were exposed alternately to [Ca2+]ER of 70 nM, a sub-physiological [Ca2+]ER in which no Ca2+ flux flowed from the luminal side of the channels to the cytoplasmic side, and 300 µM, a physiological [Ca2+]ER that drives substantial Ca2+ flux through the channels (Vais et al., 2010a). Increasing [Ca2+]ER from 70 nM to 300 µM caused a reduction in the magnitude of the current passing through the active InsP3R-3 channels (Fig. 2 A) because Ca2+ acts as permeant channel blocker, reducing K+ conductance of the channel (Vais et al., 2010a). Returning [Ca2+]ER to 70 nM restored the channel current magnitude (Fig. 2 B). Such changes in channel current size were used in all experiments to mark the time when the perfusion solution exchange was completed at the luminal side of the active InsP3R channels.
Figure 2.
Effects of [Ca2+]ER on InsP3R-3 channel activity in various [InsP3]. (A–C) Typical single-channel current traces from excised lum-out nuclear membrane patches recorded during a rapid switch of [Ca2+]ER by perfusion solution exchange. For clarity, compositions of pipette solutions ([Ca2+]i, concentration and nature of Ca2+ chelator used, [InsP3]) common to all experiments presented in this figure (current traces and bar graphs) are tabulated at the top of the figure. Pipette solution composition(s) specific to each current trace is tabulated at the top of the corresponding current trace. In each current trace, Vapp used is tabulated at left, color bars at the top indicate [Ca2+]ER in the perfusion solutions, and blue bars at the bottom indicate segments used for evaluating the channel Po tabulated below. In these figures, short current segments were plotted to show the InsP3R channel gating more clearly. The mean Po, to, and tc ratios (discussed in Results and Discussion, and shown in bar graphs) were derived from current segments that are significantly longer to ensure that only single-channel nuclear patch current traces were used (see Materials and methods). (D–F) Bar graphs of mean ratios of channel Po, to, and tc, respectively, observed before and after [Ca2+]ER switching between 70 nM and 300 µM for different [InsP3]. Numbers beside the bars are the number of experiments (top) and perfusion solution switches (bottom) analyzed. ***, **, and * mark significant deviation of a ratio from unity (P < 0.005, 0.01, and 0.05, respectively; paired t test). These symbols and conventions are also used for Figs. 3–6, and 8 and 9. For clarity, bars of the same color in these figures correspond to the same set of data obtained under the same experimental conditions.
Besides the reduction in channel conductance, the jump in [Ca2+]ER from 70 nM to 300 µM caused a significant decrease in channel Po (Fig. 2 A), which was reversed when [Ca2+]ER was returned to 70 nM (Fig. 2 B). This effect of [Ca2+]ER on channel Po was quantified by the ratio of the Po observed when [Ca2+]ER = 300 µM (Po(300 µM)) to that when [Ca2+]ER = 70 nM (Po(70 nM)), evaluated immediately before and after each perfusion solution switch (Fig. 2, A and B). With [InsP3] = 3 µM and [Ca2+]i = 2 µM, the InsP3R channel was significantly less active in [Ca2+]ER = 300 µM than in [Ca2+]ER = 70 nM, so the mean Po ratio ( the angle brackets are used to emphasize that this is the mean of the Po ratios, not the ratio of the mean Po’s at different [Ca2+]ER) was significantly lower than unity (Fig. 2 D, red bar). The change in [Ca2+]ER also caused significant changes in both to and tc, so the mean to and tc ratios ( and , respectively) are both significantly different from unity (Fig. 2, E and F, red bars).
The observed inhibition of InsP3R gating by elevated [Ca2+]ER can be caused by luminal free Ca2+ (Ca2+ER) binding to an inhibitory site on the luminal side of the channel. Alternatively, it could be caused by Ca2+ binding to cytoplasmic sites on the InsP3R channel because of local [Ca2+]i in the vicinity of the pore elevated by Ca2+ flux driven through the channel by the high [Ca2+]ER. Because [Ca2+]i in the pipette solution used (2 µM) optimally activates InsP3R channels in 3 µM InsP3, a rise in local [Ca2+]i near the channel is predicted to reduce Po caused by Ca2+ binding to the inhibitory Ca2+-binding sites on the cytoplasmic side of the channel (Foskett et al., 2007).
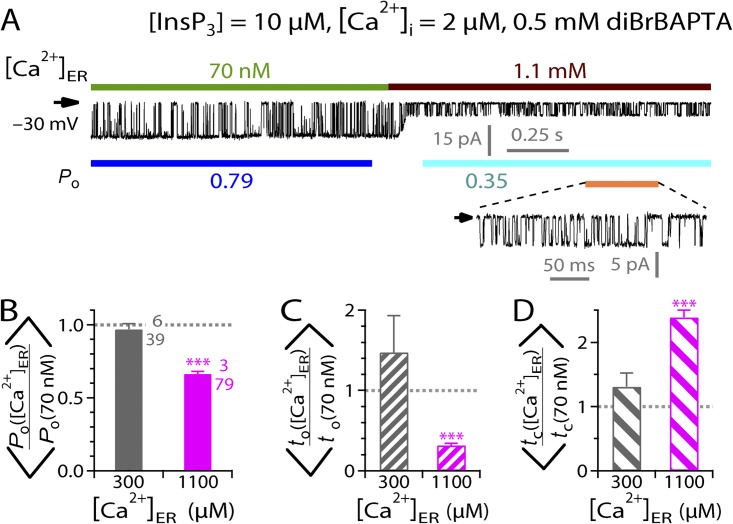
[Ca2+]ER inhibition of InsP3R channel activity is abolished by saturating [InsP3] but restored by higher [Ca2+]ER
To better characterize the observed [Ca2+]ER regulation of InsP3R channel activity, we investigated the [InsP3] dependence of the effect of [Ca2+]ER by using pipette solutions containing different [InsP3] in our lum-out nuclear patch-clamp experiments. Inhibition of InsP3R channel gating by raising [Ca2+]ER from 70 nM to 300 µM was abrogated in the presence of saturating [InsP3] (10 µM) (Fig. 2 C), so the mean Po, to, and tc ratios observed were not significantly different from unity (Fig. 2, D–F, gray bars). In contrast, even in saturating [InsP3], InsP3R channel activity was still substantially inhibited when [Ca2+]ER was raised to higher (1.1 mM) levels (Fig. 3 A), with mean Po ratio significantly less than unity (Fig. 3 B) because of both longer tc and shorter to (Fig. 3, C and D). This is similar to the suppression of channel gating by [Ca2+]ER jumping from 70 nM to 300 µM in subsaturating (3 µM) [InsP3] (Fig. 2). Accordingly, if the inhibitory effect of high [Ca2+]ER on channel Po is mediated by some luminal Ca2+-binding site(s) on the channel, the site must be allosterically coupled to the InsP3-binding sites on the cytoplasmic side of the channel so that channel activation by InsP3 binding to its cytoplasmic site and channel inhibition by Ca2+ binding to the luminal site are mutually antagonistic. Alternatively, if the [Ca2+]ER effect is mediated by the Ca2+ flux through the channel, the lack of effect on channel gating of [Ca2+]ER jump from 70 nM to 300 µM in the presence of saturating [InsP3] can be accounted for by the InsP3-induced reduction in sensitivity of InsP3R to [Ca2+]i inhibition. In this scenario, a rise in local [Ca2+]i caused by Ca2+ flux driven through the channel by [Ca2+]ER of 300 µM that inhibits InsP3R channel gating in subsaturating [InsP3] is not sufficient to affect channel gating, as saturating [InsP3] reduces the sensitivity of the channel to [Ca2+]i inhibition. However, the higher rise in local [Ca2+]i driven by a higher [Ca2+]ER of 1.1 mM can still cause suppression of channel activity, even in the presence of saturating [InsP3].
Figure 3.
Modulation of InsP3R channel activity by various [Ca2+]ER in saturating [InsP3]. (A) A typical single-channel current trace recorded during a switch of [Ca2+]ER from 70 nM to 1.1 mM. Note the substantially smaller channel current when [Ca2+]ER = 1.1 mM as a result of the blocking of the channel by permeant Ca2+. A part of the current trace (indicated by an orange line) is shown with larger current and time scales in the inset to show the details of channel gating in [Ca2+]ER = 1.1 mM. (B–D) Bar graphs of mean ratios of channel Po, to, and tc observed before and after [Ca2+]ER switches between 70 nM and 300 µM or 1.1 mM, as indicated.
[Ca2+]i dependence of the modulation of InsP3R channel Po by [Ca2+]ER
To identify the mechanisms underlying the regulation of InsP3R channel activity by [Ca2+]ER, we investigated the [Ca2+]i dependence of the effect. If the observed [Ca2+]ER modulation of InsP3R channel activity is mediated by cytoplasmic Ca2+-binding sites of the channel, [Ca2+]ER modulation should be consistent with the biphasic [Ca2+]i regulation of InsP3R channel Po (Fig. 1 C). Accordingly, when a pipette solution containing suboptimal low [Ca2+]i is used instead of one with optimal [Ca2+]i, the rise in local [Ca2+]i caused by the Ca2+ flux driven through the open channel by high [Ca2+]ER is expected to activate instead of inhibit channel gating. Indeed, when excised lum-out experiments were performed with saturating (10 µM) [InsP3] and suboptimal low (55 nM) [Ca2+]i in the pipette solution, the activity of the InsP3R channel was significantly enhanced when [Ca2+]ER was switched from 70 nM to 300 µM (Fig. 4 A), as reflected in a mean Po ratio significantly larger than unity (Fig. 4 B) mainly caused by increase in to (Fig. 4 C) while tc remained unaltered (Fig. 4 D). This observation is difficult to account for with the hypothesis of a luminal Ca2+-binding site on the InsP3R channel regulating its activity.
Figure 4.
Effects of [Ca2+]i on [Ca2+]ER modulation of InsP3R channel activity. (A) A typical single-channel lum-out patch-clamp current trace recorded during a switch of [Ca2+]ER from 70 nM to 300 µM by perfusion solution exchange in suboptimal (55 nM) [Ca2+]i. (B–D) Bar graphs of mean ratios of channel Po, to, and tc, respectively, observed before and after [Ca2+]ER switches between 70 nM and 300 µM for different [Ca2+]i. [Ca2+]i was buffered to 2 µM by 0.5 mM diBrBAPTA and to 55 nM by 0.5 mM BAPTA.
[Ca2+]ER modulation of InsP3R channel Po depends on the magnitude of the Ca2+ flux
To further confirm that rise in local [Ca2+]i in the vicinity of the channel pore caused by feed-through Ca2+ flux driven by high [Ca2+]ER modulates InsP3R channel significantly by Ca2+ binding to cytoplasmic sites on the channel, we investigated whether channel activity would be affected if the magnitude of the Ca2+ flux through the InsP3R channel was altered by changing Vapp only, with [Ca2+]ER kept the same. In all previous experiments, Vapp = −30 mV. This Vapp drove Ca2+ from the bath solution through the channel to the pipette solution, in the same direction as the [Ca2+]f gradient when the perfusion solution contained [Ca2+]ER of 300 µM. When the polarity of Vapp is reversed (Vapp = +30 mV), the applied Vapp opposes the [Ca2+]f gradient. Although this change in Vapp polarity is insufficient to reverse the direction of the Ca2+ flux through the channel, it reduces the magnitude of the flux and therefore diminishes the rise in local [Ca2+]i around the channel pore. In excised lum-out nuclear patch-clamp experiments with saturating [InsP3] (10 µM) and suboptimal [Ca2+]i (70 nM), jumps of [Ca2+]ER from 70 nM to 300 µM with Vapp = +30 mV still enhanced InsP3R channel activity (Fig. 5 A) in a qualitatively similar way as in Vapp = −30 mV, giving a mean Po ratio significantly larger than unity (Fig. 5 B) solely by prolonging to (Fig. 5, C and D). However, the increases in channel Po and to were substantially less in Vapp = +30 mV than those observed with Vapp = −30 mV (Fig. 5, B and C).
Figure 5.
[Ca2+]ER modulation of InsP3R-3 channel activity depends on magnitude of Ca2+ flux through the open-channel pore. (A) A typical single-channel lum-out nuclear patch-clamp current trace recorded during a switch of [Ca2+]ER from 70 nM to 300 µM in Vapp = +30 mV. Note that the change in channel current size as the result of the change in [Ca2+]ER was smaller. This is because of the reduction in Ca2+ flux through the channel by the positive Vapp. (B–D) Bar graphs of mean ratios of channel Po, to, and tc observed before and after [Ca2+]ER switches between 70 nM and 300 µM in Vapp = ±30 mV. ooo indicates statistically significant difference between the two ratios connected by the bracket (P < 0.005; unpaired t test).
[Ca2+]ER modulation of InsP3R channel Po depends on cytoplasmic Ca2+-buffering conditions
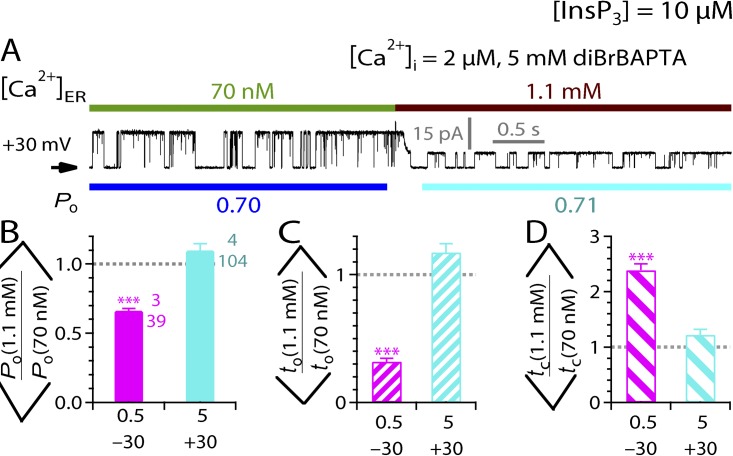
Our experiments so far have demonstrated convincingly that most of the effects of [Ca2+]ER on InsP3R channel activity are mediated by the Ca2+ flux driven by [Ca2+]ER to raise local [Ca2+]i to modify InsP3R channel activity through the cytoplasmic-activating and inhibitory Ca2+-binding sites on the channel. We next performed experiments to examine more closely the existence of an intrinsic functional regulatory Ca2+-binding site on the luminal side of the channel. We investigated the effects of cytoplasmic Ca2+-buffering conditions on [Ca2+]ER modulation of channel activity in optimal [Ca2+]i (2 µM) and subsaturating [InsP3] (3 µM). In all previous experiments involving [Ca2+]i = 2 µM, [Ca2+]i in the pipette (cytoplasmic) solution was buffered by 0.5 mM of the fast Ca2+ chelator diBrBAPTA (Ca2+ binding rate kon of ∼450 µM−1s−1; Naraghi, 1997). In weaker buffering conditions in which [Ca2+]i was buffered to 2 µM by a low concentration (0.1 mM) of the slower Ca2+ chelator HEDTA (kon of ∼4.5 µM−1s−1) (Naraghi, 1997), suppression of channel activity when [Ca2+]ER was raised from 70 nM to 300 µM was more profound than that observed in 0.5 mM diBrBAPTA (Fig. 6 A). Accordingly, the mean Po ratio was significantly lower under weak cytoplasmic Ca2+-buffering conditions than under the normally used buffering conditions, even though [Ca2+]i was kept at 2 µM (Fig. 6 C). This is solely because of a significant increase in tc (Fig. 6, D and E). In contrast, the inhibitory effects of the same [Ca2+]ER jump were completely abolished when cytoplasmic Ca2+ was strongly buffered at 2 µM with a high concentration (5 mM) of diBrBAPTA (Fig. 6 B), giving mean Po, to, and tc ratios not significantly different from unity (Fig. 6, C–E). Control experiments demonstrated that different Ca2+-buffering conditions themselves had no significant effect on Po in the absence of Ca2+ flux through the active channels ([Ca2+]ER = 70 nM) in both on-nucleus and excised lum-out configurations (Fig. 7).
Figure 6.
Effects of cytoplasmic Ca2+-buffering conditions on [Ca2+]ER modulation of InsP3R channel activity. (A and B) Typical single-channel lum-out nuclear patch-clamp current traces recorded during switches of [Ca2+]ER from 70 nM to 300 µM in different cytoplasmic Ca2+-buffering conditions. (C–E) Bar graphs of mean ratios of Po, to, and tc, respectively, observed before and after [Ca2+]ER switches between 70 nM and 300 µM for different cytoplasmic Ca2+-buffering conditions. oo indicates statistically significant difference between the two ratios connected by the bracket (P < 0.01; unpaired t test). Note the logarithmic scale used for the tc axis (in red) in E.
Figure 7.
InsP3R channel Po is independent of cytoplasmic Ca2+-buffering conditions in the absence of Ca2+ flux through the channel. (A–C) Typical single-channel on-nucleus patch-clamp current traces with [Ca2+]i in pipette solutions buffered to 2 µM by 5 mM diBrBAPTA (A), 0.5 mM diBrBAPTA (B), or 0.1 mM HEDTA (C). [InsP3] = 3 µM and Vapp = −40 mV. Bath solution contained [Ca2+]ER = 70 nM. (D) Mean InsP3R channel Po for [InsP3] = 3 µM and [Ca2+]i = 2 µM in various cytoplasmic Ca2+-buffering conditions for on-nucleus (closed bars) and excised lum-out (open bars) patch-clamp experiments. Excised lum-out patches were perfused with solution containing [Ca2+]ER = 70 nM. H stands for HEDTA, and dB stands for diBrBAPTA. No statistically significant difference exists between Po in any two of the three Ca2+-buffering conditions plotted for on-nucleus or lum-out experiments (ANOVA).
If [Ca2+]ER modulation of channel Po is mediated by luminal Ca2+-binding site(s), it is difficult to conceive how such a luminal site(s) could be sensitive to Ca2+ buffering on the cytoplasmic side by artificial chemical Ca2+ chelators that are not naturally found in vivo. Furthermore, the complete abolition of the effect of physiological [Ca2+]ER (300 µM) on channel activity by sufficiently strong cytoplasmic Ca2+ buffering suggests that there is no luminal Ca2+-binding site intrinsic to the InsP3R, activating or inhibitory, that is sensitive to 300 µM [Ca2+]ER.
Complete abrogation of modulatory effects of [Ca2+]ER in the physiological range on InsP3R channel activity
To determine if [Ca2+]ER in the upper limits of the physiological range could possibly inhibit InsP3R channel activity through a luminal Ca2+-binding site on the channel, we looked for inhibitory effects on InsP3R channel activity by [Ca2+]ER jumps from 70 nM to 1.1 mM (higher than most observed [Ca2+]ER; Button and Eidsath, 1996; Bygrave and Benedetti, 1996; Meldolesi and Pozzan, 1998; Yu and Hinkle, 2000) that were independent of the rise in [Ca2+]i resulting from Ca2+ flux passing through the open InsP3R channel. In excised lum-out nuclear patch-clamp experiments with Vapp = +30 mV (to reduce the magnitude of the Ca2+ flux through the open channel) and pipette solution containing saturating (10 µM) [InsP3] and optimal (2 µM) [Ca2+]i buffered by 5 mM diBrBAPTA (to reduce the rise in [Ca2+]i caused by the Ca2+ flux), [Ca2+]ER jumps from 70 nM to 1.1 mM did not affect InsP3R channel gating (Fig. 8 A), so that the mean Po, to, and tc ratios observed were not significantly different from unity (Fig. 8, B–D).
Figure 8.
Abrogation of inhibition of InsP3R-3 channel activity by physiological levels of [Ca2+]ER. (A) A typical single-channel current trace recorded during a switch of [Ca2+]ER from 70 nM to 1.1 mM. (B–D) Bar graphs of mean ratios of channel Po, to, and tc observed before and after [Ca2+]ER switches between 70 nM and 1.1 mM, showing the abrogation of inhibitory effects of 1.1 mM [Ca2+]ER on InsP3R channel activity in the presence of stronger cytoplasmic Ca2+ buffering (5 mM BAPTA) and smaller Ca2+ flux through the channel as a result of positive Vapp. Labels on the x axes indicate [diBrBAPTA] in mM (top) and Vapp in mV (bottom).
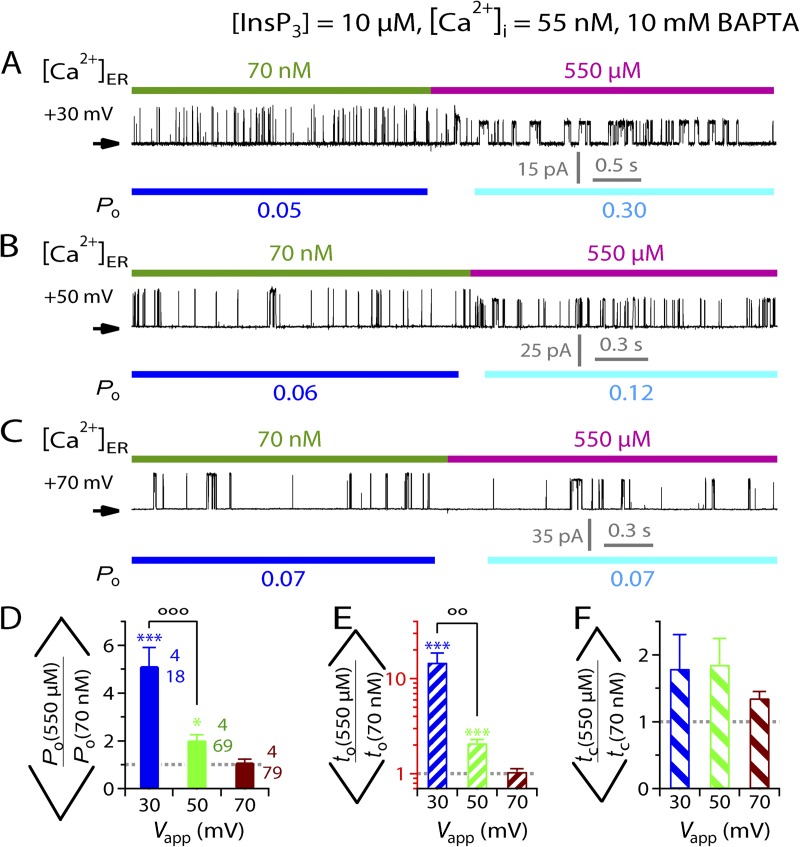
We also checked whether the activating effects of [Ca2+]ER on InsP3R channel activity observed in suboptimal (55 nM) [Ca2+]i (Fig. 4) could be completely abrogated by a combination of positive Vapp and strong cytoplasmic Ca2+ buffering. Unlike the inhibitory effects, [Ca2+]ER jumps from 70 nM to 550 µM (maximal [Ca2+]ER observed in many cell types; Button and Eidsath, 1996; Bygrave and Benedetti, 1996; Meldolesi and Pozzan, 1998; Yu and Hinkle, 2000) still enhanced InsP3R channel activity in excised lum-out nuclear patch-clamp experiments with Vapp = +30 mV and pipette solution containing saturating (10 µM) [InsP3] and suboptimal (55 nM) [Ca2+]i buffered by 10 mM BAPTA (Fig. 9 A), so that the mean Po observed was significantly higher than unity (Fig. 9 D), mostly because of longer to (Fig. 9, E and F). However, increasing Vapp further to +50 mV substantially diminished the activating effects of the [Ca2+]ER jumps (Fig. 9 B), with a mean Po ratio that is much lower although still significantly larger than unity (Fig. 9 D), which is caused by the to ratio that is significantly greater than unity (Fig. 9, E and F). At Vapp = +70 mV, the activating effects of the [Ca2+]ER jumps were completely abrogated so that the mean Po, to, and tc ratios were not different from unity (Fig. 9, D–F).
Figure 9.
Abrogation of activation of InsP3R-3 channel activity by physiological levels of [Ca2+]ER. (A–C) Typical single-current traces recorded during a switch of [Ca2+]ER from 70 nM to 550 µM with different Vapp used. (D–F) Bar graphs of mean ratios of channel Po, to, and tc observed before and after [Ca2+]ER switches between 70 nM and 550 µM, showing the abrogation of activating effects of 550 µM [Ca2+]ER on InsP3R channel activity by increasing positive Vapp and strong cytoplasmic Ca2+ buffering used. ooo and oo indicate statistically significant difference between the two ratios connected by the bracket (P < 0.005 and 0.01, respectively; unpaired t test). Note the logarithmic scale used for the to axis (in red) in E.
In the experiments investigating the abrogation of activating effects of [Ca2+]ER changes, [Ca2+]ER was increased to 550 µM rather than 1.1 mM because using high concentrations of Ca2+ chelator in the pipette solution did not reduce the activating effects of [Ca2+]ER effectively. This is probably because the cytoplasmic activating Ca2+-binding site is located close to the channel pore (see Discussion). To abrogate the effects of [Ca2+]ER jumps to 1.1 mM as in other experiments would require using Vapp of >70 mV, which severely compromised the integrity of the gigaohm seal between the isolated nuclear membrane patch and the patch-clamp microelectrode.
Collectively, the total abrogation of both the inhibitory effects (in optimal [Ca2+]i = 2 µM) and the activating effects (in suboptimal [Ca2+]i = 55 nM) of physiological levels of [Ca2+]ER in conditions that only affected the magnitude of the Ca2+ flux through the channel and the changes in [Ca2+]i demonstrates that there is no regulatory Ca2+-binding site sensitive to physiological [Ca2+]ER on the luminal side of the InsP3R channel.
DISCUSSION
This study is the first investigation of the effects of [Ca2+]ER on single-channel activity of InsP3R using the nuclear patch-clamp approach. Previously, two electrophysiological studies explored the effects of [Ca2+]ER using reconstituted cerebellar type 1 InsP3R channels in planar lipid bilayers. In the first study (Bezprozvanny and Ehrlich, 1994), the cytoplasmic solution contained no permeant ion, with 55 mM Ba2+, Mg2+, Sr2+, or a combination of Ca2+ and Sr2+ in the luminal solutions used as the main charge carrier. Channel Po was significantly reduced as [Ca2+]ER was increased from 300 µM to 44 mM. It was suggested that feed-through effects of [Ca2+]ER-driven Ca2+ flux through the channel contributed to the inhibition of InsP3R channel activity, but the existence of a luminal inhibitory Ca2+ site could not be conclusively ruled out because of insufficient [Ca2+]i buffering by only 1 mM EGTA. The relevance of this study was further diminished by the nonphysiological ionic compositions used, which rendered it questionable whether the magnitude of Ca2+ flux and therefore the resulting changes in [Ca2+]i resembled those in physiological ionic conditions. In the second study (Thrower et al., 2000), 500 mM K+ was present in all solutions with [Ca2+]i buffered to 0.2–0.3 µM by either 10 mM HEDTA or 1.7 mM BAPTA. The conclusion of the study that Ca2+ER affects InsP3R channel gating through direct interaction with the luminal face of the channel was critically undermined by technical difficulties (brief and inconsistent channel activities, multiple conductance substates), a mostly qualitative description of channel activity (no quantitative Po or to-tc analysis), and the inappropriate use of Ca2+ buffers (HEDTA cannot effectively buffer [Ca2+]i at 0.2–0.3 µM despite the high concentrations used because of its low Ca2+ affinity; BAPTA has the right Ca2+ affinity, but only a low concentration was used).
In this study, modulation of InsP3R channel activity by [Ca2+]ER was examined under rigorously controlled [Ca2+]i, [Ca2+]ER, [InsP3], and cytoplasmic Ca2+-buffering conditions in excised lum-out nuclear patch-clamp experiments with perfusion solution exchange. The observed dependencies of [Ca2+]ER modulation of channel activity on [InsP3] (Fig. 2), [Ca2+]ER (Fig. 3), [Ca2+]i (Fig. 4), Vapp (Fig. 5), and cytoplasmic Ca2+-buffering conditions (Fig. 6), and the total abrogation of the effects of [Ca2+]ER on channel activity by conditions that affect the rise in local [Ca2+]i caused by the Ca2+ flux but not [Ca2+]ER itself (Figs. 6, 8, and 9), together demonstrate that InsP3R channel activity is regulated solely by the feed-through effects of the [Ca2+]ER-driven Ca2+ flux through the open channel, raising [Ca2+]i in the microdomain around the channel to alter Po of the channel through its cytoplasmic activating and inhibitory Ca2+-binding sites, and not by direct binding of Ca2+ to the luminal side of the InsP3R channel.
The observed modulation of channel activity by [Ca2+]ER-driven Ca2+ flux through the channel itself provides insights regarding the kinetics of [Ca2+]i regulation of InsP3R channel gating. From these insights and others from previous studies of ligand regulation of InsP3R channel gating, we develop below the concept of the effective time-averaged [Ca2+]i profile around the channel caused by Ca2+ flux through the channel itself when it is gating. With that concept, and using channel Po observed in the presence of various [Ca2+]ER-driven Ca2+ fluxes under various [Ca2+]i, [Ca2+]ER, and [InsP3], we then estimate the distances between the channel pore and the cytoplasmic regulatory Ca2+ sites.
Kinetics of fluctuations of local [Ca2+]i in the vicinity of the channel pore caused by Ca2+ flux through the pore
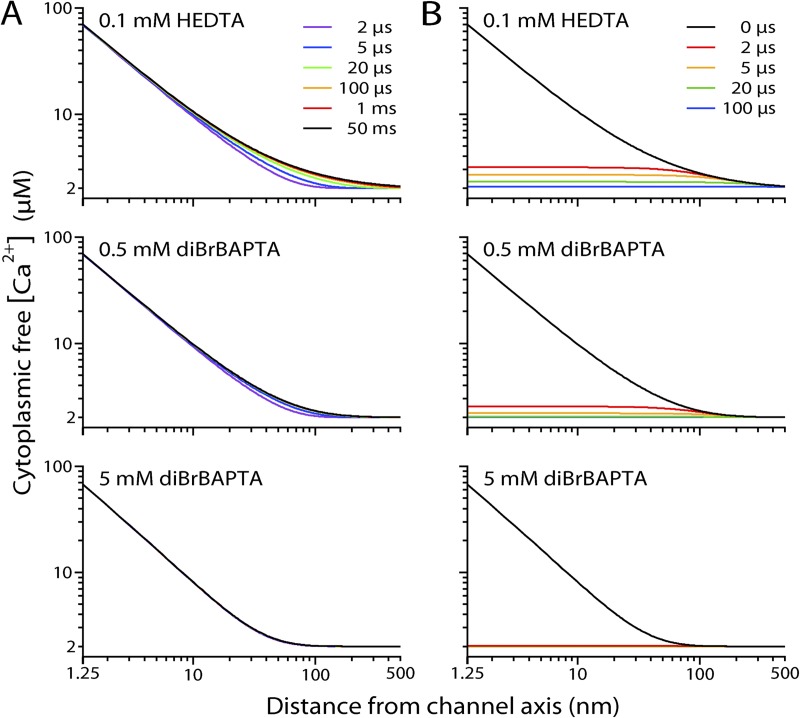
In a previous study (Vais et al., 2010a), we determined that physiological levels of [Ca2+]ER can drive substantial Ca2+ fluxes through an open InsP3R-3 channel. Numeric simulations allow us to follow the changes in the [Ca2+]i profile around the Ca2+-permeable InsP3R channel during its gating. The simulations indicate that under our experimental Ca2+-buffering conditions, the [Ca2+]i profile around an open InsP3R channel achieves steady-state levels within 100 µs after the channel opens (Fig. 10 A). Furthermore, after a channel closes, the elevated [Ca2+]i around the channel collapses rapidly and returns to the basal level within 1 ms (Fig. 10 B). Thus, the [Ca2+]i profile around an InsP3R channel fluctuates abruptly between the steady-state open- and closed-channel levels in a quasi-binary manner, with kinetics rigidly dictated by the opening and closing of the channel. This has significant implications for the kinetic properties of [Ca2+]i regulation of the channel and how [Ca2+]ER-driven Ca2+ flux through the InsP3R channel pore can modulate the activity of the channel itself.
Figure 10.
Simulated [Ca2+]i profiles around an InsP3R channel (cytoplasmic free [Ca2+] at various distances from the channel pore axis) under various Ca2+-buffering conditions. Buffering conditions are indicated for the profiles shown. [Ca2+]ER = 300 µM. Bulk [Ca2+]i (far from the channel) = 2 µM. (A) [Ca2+]i profiles at different t after the channel opens, plotted in different colors as indicated. (B) The channel opened continuously for 50 ms before it closed. Profiles at different t after the channel closed are plotted in different colors as indicated. Even with the weakest buffering (0.1 mM HEDTA), [Ca2+]i profiles reach steady-state level within 1 ms of the channel opening, and [Ca2+]i around the channel returns within 100 µs to essentially the level that existed before the channel opened.
Kinetics of cytoplasmic Ca2+ activation of InsP3R channel deduced from the observed enhancement of channel activity by [Ca2+]ER-driven Ca2+ flux
To derive insights into the kinetics of cytoplasmic Ca2+ activation of InsP3R channel from the observed channel response to [Ca2+]ER-driven Ca2+ flux through the channel itself, we first consider a hypothetical, extreme kind of response of a Ca2+i-activated channel to the increase in local [Ca2+]i at the single activating cytoplasmic Ca2+-binding site of the channel. In this extreme case, the kinetics of channel gating are rigidly dictated by the local [Ca2+]i at its Ca2+ site. To generate this kind of response, the activating latency (interval between local [Ca2+]i increasing beyond Kact and the first resulting opening of the channel, τact) and the deactivating latency (interval between local [Ca2+]i decreasing below Kact and the last closing of the channel, τdeact) must both be much less than the time scale of channel gating (to and tc), so that the channel opens/closes practically instantaneously after the rise/drop in local [Ca2+]i at its activating site. Moreover, the channel must remain open as long as its single activating Ca2+ site is occupied, and it must remain closed whenever the activating site is vacant, i.e., the gating status (open or closed) of the channel is deterministically dependent on the occupancy of the activating Ca2+ site. Given that the [Ca2+]i profile around a Ca2+-permeable channel is rigidly dictated by the gating of the channel under our experimental conditions (as discussed in the previous section), a lone Ca2+i-activated, Ca2+-permeable channel in the ER with its gating rigidly dictated by [Ca2+]i at its activating Ca2+ site as described above would not be expected to be activated by Ca2+ flux through the channel itself. This is simply because the activating site on the channel must be already occupied when the channel is open and therefore cannot further bind Ca2+. Consequently, the channel cannot be affected by the local [Ca2+]i elevated by the [Ca2+]ER-driven Ca2+ flux. Conversely, when its Ca2+-activating site is vacant and available to bind Ca2+, the channel must be closed so that there is no [Ca2+]ER-driven Ca2+ flux through that channel to activate the channel. Thus, the channel would behave as if there is no Ca2+ flux through the open channel.
However, in lum-out nuclear patch-clamp experiments with the pipette solution containing [Ca2+]f = 55 nM and [InsP3] = 10 µM, we observed significant sustained activation of the channel in the presence of high [Ca2+]ER (Figs. 4, A and B, and 5, A and B) that can be completely accounted for by Ca2+ flux through the channel raising local [Ca2+]i at its cytoplasmic activating Ca2+ site to enhance its Po (Fig. 5 A). This observation therefore has nontrivial implications for the kinetics of cytoplasmic Ca2+ activation of InsP3R channel. Most obviously, this indicates that local [Ca2+]i at the cytoplasmic site does not rigidly dictate the gating of the channel. Different mechanisms can contribute to uncoupling of the gating kinetics of the channel from changes in [Ca2+]i at the activating Ca2+ site. Most importantly, Ca2+ regulates InsP3R channel activity stochastically, so Ca2+ binding to the activating site only induces the channel to adopt a more active kinetic conformation with a higher Po, but does not always result in channel opening. The channel can open and close with no change in its Ca2+-binding status (Mak et al., 2003). Furthermore, the tetrameric structure of the channel (Foskett et al., 2007) indicates that it has multiple activating Ca2+ sites. Thus, the channel can open even when there are vacant activating Ca2+ sites on the channel available to detect the elevated local [Ca2+]i and further enhance channel activity. Another significant factor determining the degree of coupling between local [Ca2+]i at the activating Ca2+ site and the gating of the channel is the kinetics of Ca2+i activation of the channel, i.e., τact and τdeact. Previous cytoplasmic-side-out nuclear patch-clamp experiments with rapid perfusion exchange studying endogenous InsP3R channels from insect Sf9 cells (Mak et al., 2007) revealed that in saturating (10 µM) [InsP3], InsP3R channels can respond relatively slowly to abrupt changes in [Ca2+]i, with long latencies (τact of approximately tens of milliseconds and τdeact of approximately a few hundreds of milliseconds). With long response latencies (relative to channel gating to and tc), the channel can open and close, and local [Ca2+]i around the channel jumps up and down, multiple times during the time the channel takes to respond to one change in [Ca2+]i, thereby effectively uncoupling the [Ca2+]i at the activating sites from the gating of the channel.
In the other extreme case, in which the gating of the channel is completely uncoupled from the local [Ca2+]i at the activation sites, instead of responding to the instantaneous [Ca2+]i resulting from individual channel opening and closing events, the gating of the channel depends only on the time-averaged [Ca2+]i at the sites. Over a long period T (>>τact, τdeact, to, tc), a vacant activating Ca2+ site on the channel will on average be exposed to the steady-state open-channel [Ca2+]i (because of the open-channel Ca2+ current, iCa, driven by the electrochemical gradient across the channel) for a period of PoT, and to the steady-state closed-channel [Ca2+]i for a period of (1–Po)T. Thus, assuming first-order Ca2+ binding to the activating sites, the channel will exhibit steady-state gating kinetics similar to that of a channel with activating sites constantly exposed to a local [Ca2+]i equivalent to that generated by a Ca2+ current of magnitude Po iCa passing through the pore.
In reality, the coupling between InsP3R channel gating and changes of local [Ca2+]i is partial, lying between the two extremes of total rigid dictation of gating by [Ca2+]i at the activating sites and complete decoupling with gating unrelated to instantaneous [Ca2+]i at the sites. Therefore, the activating sites are effectively exposed to a [Ca2+]i that is equivalent to a time-averaged Ca2+ current of magnitude between 0 and Po iCa.
Besides deducing that InsP3R channel gating is not rigidly dictated by [Ca2+]i at the activating sites, other insights about the kinetics of Ca2+i activation of InsP3R channel can be derived from the observed activating effects of Ca2+-driven Ca2+ flux on channel gating. In the present study, in the absence of Ca2+ flux with low [Ca2+]ER (70 nM), the InsP3R-3 channel was observed to exhibit low Po (∼0.02–0.05) with short to (∼2 ms) and long tc (∼50 ms) in constant suboptimal [Ca2+]i (55 nM), even in saturating [InsP3] (10 µM) (Figs. 1 C, 4 A, and 9 A). Nevertheless, abrupt and sustained increases in channel Po and to were observed (Figs. 4 A and 9 A) in response to the onset of Ca2+ flux through the channel to the cytoplasmic side resulting from [Ca2+]ER being raised to physiological levels. This rapid response indicates that vacant cytoplasmic activating Ca2+ sites of the channel were able to capture Ca2+ during the first couple of brief channel-opening events after the [Ca2+]ER jump, when the local [Ca2+]i at the sites was raised by the Ca2+ flux and before the channel closed and terminated the Ca2+ flux. Thus, the rate of Ca2+ binding to the activating sites must be high, suggesting that the Ca2+ flux can raise the local [Ca2+]i at the activating sites to a high level, and therefore the sites are probably located close to the channel pore (see further discussion below).
Another feature of the activating effects of [Ca2+]ER on InsP3R-3 channel gating is that the increase in channel Po as a result of [Ca2+]ER jumps from 70 nM to physiological levels was mostly achieved by prolonging to, with no significant change in tc (Figs. 4 D, 5 E, and 9 F). It has been suggested that because cytoplasmic regulatory Ca2+ sites are inaccessible to [Ca2+]ER when a Ca2+-permeable, Ca2+i-regulated channel is closed, the absence of luminal Ca2+ site on the channel means that tc of the channel should not depend on [Ca2+]ER (Laver, 2007a,b). According to this statement, our observation that tc was not significantly affected by [Ca2+]ER is consistent with a conclusion that the InsP3R channel has no luminal regulatory Ca2+ site. However, the statement is only true for a Ca2+i-regulated, Ca2+-permeable channel whose gating is strongly dictated by [Ca2+]i at its cytoplasmic regulatory Ca2+ sites. Because gating of the InsP3R channel is not rigidly dictated by [Ca2+]i at its cytoplasmic Ca2+-activating sites, the observation is better interpreted as an indication that under the experimental conditions used in Figs. 4 A, 5 A, and 9 (A and B), the conformations assumed by the channel in the presence and absence of Ca2+ flux have similar tc.
Cytoplasmic inhibitory Ca2+ sites also experience an effective time-averaged local [Ca2+]i due to [Ca2+]ER-driven Ca2+ flux through the channel
In the extreme case where gating is rigidly dictated by [Ca2+]i at the regulatory sites, the situation for Ca2+ inhibition is different from that for Ca2+ activation for a Ca2+i-regulated, Ca2+-permeable channel. Whereas activating sites can never experience the flux through the channel (discussed above), the inhibitory sites will be vacant when the channel opens and occupied when the channel is closed. Thus, effectively, the vacant sites will always be exposed to the elevated open-channel local [Ca2+]i caused by the Ca2+ current of magnitude iCa through the pore.
However, this cannot be true for the InsP3R-3 channel. Even though our experimental observations clearly demonstrated that the channel has no luminal regulatory Ca2+-binding site (Fig. 8), and the rapid collapse of the [Ca2+]i profile around the channel pore after the channel closes (Fig. 10) means that the cytoplasmic inhibitory Ca2+ sites are effectively inaccessible to [Ca2+]ER when the channel is closed, channel tc still exhibited clear dependence on [Ca2+]ER when the channel was inhibited by rise in [Ca2+]i caused by Ca2+ flux through the open channel (Figs. 2 F, 3 D, and 6 E). Therefore, the gating of the channel cannot be rigidly dictated by [Ca2+]i at the inhibitory sites. Rather, the observations suggest the presence of uncoupling mechanisms: the tetrameric InsP3R channel having multiple inhibitory sites that regulate channel Po stochastically, with Ca2+ inhibition latency and the latency of channel recovery from Ca2+ inhibition both significantly longer than the time scale of channel gating (to and tc). Such mechanisms can allow the modulation of InsP3R channel gating by the high local [Ca2+]i at the inhibitory sites established during a channel opening to extend beyond the termination of that opening and the subsequent rapid collapse of the local [Ca2+]i rise.
At the other extreme, with the gating of the channel completely decoupled from the fluctuations of local [Ca2+]i caused by the openings and closings of the channel, the channel should gate with kinetics similar to those associated with one of the inhibitory sites exposed to a steady local [Ca2+]i equivalent to that generated by a Ca2+ current of magnitude Po iCa passing through the pore, the same as the situation for the activating site. For realistic partial coupling between the two extremes, the inhibitory sites are effectively exposed to a time-averaged local [Ca2+]i caused by a Ca2+ current of magnitude between Po iCa and iCa.
In summary, the observed sustained activation and inhibition of gating by Ca2+ flux through an InsP3R channel indicate that channel gating is not deterministically regulated by [Ca2+]i, and that a channel can respond to the Ca2+ flux through itself because its activation and inhibition kinetics enable it to sense an effective steady-state local flux-driven [Ca2+]i.
Estimates of the locations of functional cytoplasmic Ca2+-binding sites from Ca2+ flux–mediated modulation of InsP3R channel activity
Our experiments demonstrate that [Ca2+]ER modulates the activity of r-InsP3R-3 channels in DT40-KO-r-InsP3R-3 cells solely via the Ca2+ flux it drives through the channel that raises the local [Ca2+]i at the cytoplasmic regulatory Ca2+-binding sites. Using the steady-state [Ca2+]i dependence of the channel Po (Fig. 1), the effects of [Ca2+]ER on channel activity can provide estimates of the effective time-averaged local [Ca2+]i at the cytoplasmic activating or inhibitory Ca2+-binding sites of the channel (Table 1). [Ca2+]i profiles ([Ca2+]i at various distances from the channel pore) were numerically generated (Materials and methods) for the different Ca2+-buffering conditions, Ca2+ electrochemical gradients, and cytoplasmic ligand concentrations used in our experiments (Table 1). Checking the estimates of the effective time-averaged local [Ca2+]i at the regulatory sites against the appropriate simulated [Ca2+]i profiles, estimates can be made of the locations of the regulatory Ca2+-binding sites relative to the channel pore, which is situated at the center of the channel based on the structural symmetry of the tetrameric InsP3R channel (Foskett et al., 2007).
Table 1.
Estimations of the distances between cytoplasmic regulatory Ca2+-binding sites and the channel pore axis
| Experimental conditions | ||||||||||||
| Representative current trace | [InsP3] | [Ca2+]ERa | Bulk [Ca2+]ib | Vapp | Cytoplasmic Ca2+ buffering | Mean Po | iCa | iCa Po | [Ca2+]i profiles | Regulatory Ca2+ site | [Ca2+]i at site | Range of distance from pore |
| µM | mV | fA | fA | µM | nm | |||||||
| Fig. 2 (A and B) | 3 | 300 µM | 2 µM | −30 | 0.5 mM diBrBAPTA | 0.22 | 230 | 51 | Fig. 11 A | Inhibitory | 5.7 µM | 12 < x < 39 |
| Fig. 2 C | 10 | 300 µM | 2 µM | −30 | 0.5 mM diBrBAPTA | 0.7 | 230 | 161 | Fig. 11 B | Inhibitory | 7.2 µM | 23 < x < 31 |
| Fig. 3 A | 10 | 1.1 mM | 2 µM | −30 | 0.5 mM diBrBAPTA | 0.46 | 830 | 380 | Fig. 11 C | Inhibitory | 14.3 µM | 25 < x < 42 |
| Fig. 6 A | 3 | 300 µM | 2 µM | −30 | 0.1 mM HEDTA | 0.09 | 230 | 21 | Fig. 11 D | Inhibitory | 12 µM | 2 < x < 21 |
| Fig. 6 B | 3 | 300 µM | 2 µM | −30 | 5 mM diBrBAPTA | 0.4 | 230 | 92 | Fig. 11 E | Inhibitory | 3.1 µM | 29 < x < 44 |
| Fig. 8 A | 10 | 1.1 mM | 2 µM | +30 | 5 mM diBrBAPTA | 0.7 | 75 | 53 | Fig. 11 F | Inhibitory | 3.1 µM | 22 < x < 27 |
| Fig. 4 A | 10 | 300 µM | 55 nM | −30 | 0.5 mM BAPTA | 0.29 | 230 | 64 | Fig. 11 G | Activating | 500 nM | x < 62 |
| Fig. 5 A | 10 | 300 µM | 55 nM | +30 | 0.5 mM BAPTA | 0.12 | 20 | 2.5 | Fig. 11 H | Activating | 150 nM | x < 22 |
| Fig. 9 A | 10 | 550 µM | 55 nM | +30 | 10 mM BAPTA | 0.25 | 38 | 10 | Fig. 11 I | Activating | 410 nM | x < 13 |
| Fig. 9 B | 10 | 550 µM | 55 nM | +50 | 10 mM BAPTA | 0.08 | 12 | 1 | Fig. 11 J | Activating | 100 nM | x < 14 |
| Fig. 9 C | 10 | 550 µM | 55 nM | +70 | 10 mM BAPTA | 0.05 | 3 | 0.15 | Fig. 11 K | Activating | 80 nM | x < 9 |
[Ca2+]ER = free [Ca2+] in perfusion solution.
Bulk [Ca2+]i = [Ca2+]i at large distance from the channel pore = [Ca2+]r →∞ = free [Ca2+] in pipette solution.
For the inhibitory Ca2+ sites, depending on the degree of coupling between the gating of the channel and the fluctuations in local [Ca2+]i associated with each opening and closing event, the [Ca2+]i profile suitable for estimating the location of the sites lies between the [Ca2+]i profile generated for Ca2+ current = iCa derived from the Goldman–Hodgkin–Katz current equation (Eq. 1) (for deterministic coupling between channel gating and the local [Ca2+]i at the inhibitory Ca2+ site), and the profile generated for Ca2+ current = Po iCa (for completely uncoupled channel gating and local [Ca2+]i at the inhibitory site). Because the exact degree of coupling between channel gating and local [Ca2+]i fluctuations for the experimental conditions used are not known, we use the two [Ca2+]i profiles for currents = iCa and Po iCa to derive upper and lower limits for the distance of the inhibitory Ca2+ sites from the channel pore.
In this study, we made six independent measurements of the inhibitory effects on channel gating of raising local [Ca2+]i around the r-InsP3R-3 channel beyond 2 µM (optimal [Ca2+]i) (shown in Figs. 2, A and C, 3 A, 6, A and B, and 8 A), each of which provides an independent estimate of the range for the distance of the inhibitory Ca2+-binding site from the channel pore (Fig. 11, A–F, and Table 1). The estimated upper limits of this distance range between 21 and 44 nm (Table 1), with an average of 34 ± 4 nm. The estimated lower limits range between 2 and 29 nm (Table 1), with an average of 19 ± 4 nm. These suggest that the distance from the channel pore to the inhibitory site is ∼20–30 nm.
Figure 11.
Estimated effective time-averaged [Ca2+]i determining InsP3R channel gating activity, as sensed by cytoplasmic regulatory Ca2+-binding sites at various distances from the channel pore in various lum-out experiments. Pipette solution [Ca2+]f ([Ca2+]r→∞), perfusion solution [Ca2+]f ([Ca2+]ER), Vapp, [InsP3], and cytoplasmic Ca2+-buffering conditions used in each set of experiment are tabulated in each corresponding graph. A–F are related to experiments investigating the effect of Ca2+ flux mediated by the cytoplasmic inhibitory Ca2+-binding site(s), whereas G–K are related to experiments investigating the effect mediated by the cytoplasmic activating site(s). The effective [Ca2+]i that produced the observed channel Po are marked by dotted lines and tabulated (in red for inhibitory Ca2+ site and in blue for activating Ca2+ site). Black curves are effective [Ca2+]i profiles derived from Ca2+ flux of magnitude = Po iCa. The limits for the distances between the regulatory Ca2+-binding site and the channel pore derived from these [Ca2+]i profiles are marked by black dotted lines and tabulated in black. Green curves in A–F are effective [Ca2+]i profiles derived from Ca2+ flux of magnitude = iCa. The upper limits for the distances between the inhibitory Ca2+ site to the channel pore derived from these [Ca2+]i profiles are marked by green dotted lines and tabulated in green.
Using image reconstructions based on electron cryomicroscopy or electron microscopy with negative staining, 3-D structures of single tetrameric InsP3R channel have been determined (Jiang et al., 2002; da Fonseca et al., 2003; Hamada et al., 2003; Serysheva et al., 2003; Sato et al., 2004; Wolfram et al., 2010; Ludtke et al., 2011). Although the details differ significantly, they generally show a large structure on the cytoplasmic side with maximum radius (r) from the channel pore axis between 10.5 and 14.2 nm, and height above the ER membrane (h) between 13.5 and 18.3 nm. Simple geometric consideration for a 3-D structure suggests that the maximum distance between the channel pore and a Ca2+-binding site on the channel is ∼24–32.5 nm (r + h). Thus, our estimate of the inhibitory site being 20–30 nm from the pore of the channel is not in conflict with the 3-D structures reported and suggests that the inhibitory site may be located in a part of the channel furthest from the pore.
For the activating Ca2+ sites, an upper limit for the distance to the channel pore can be derived from the [Ca2+]i profile for current with magnitude = Po iCa, which corresponds to the extreme case when the channel gating is completely decoupled from local [Ca2+]i at the activating sites during channel openings and closings. However, no lower limit can be deduced for the pore-to-activating-site distance from the extreme case with channel gating rigidly dictated by local [Ca2+]i at the activating sites. This is because in this case, the channel cannot be activated by [Ca2+]ER-driven Ca2+ flux through the pore, no matter where the activating Ca2+ sites are.
We made five independent measurements of the activating effects on channel gating of raising local [Ca2+]i around the channel above 70 nM (resting [Ca2+]i) (Figs. 4 A, 5 A, and 9, A–C). These provided five independent estimates of the upper limits for the activating site to channel pore distance (Fig. 11, G–K, and Table 1), suggesting that the activating site is less than ∼9–62 nm from the channel pore, which is also consistent with the 3-D structures of the channel. Moreover, 5 mM diBrBAPTA can effectively buffer the local [Ca2+]i at the inhibitory Ca2+-binding sites to abolish the inhibiting effect of Ca2+ flux driven through the channel by 1.1 mM [Ca2+]ER at Vapp = +30 mV (Fig. 8 A), whereas even 10 mM BAPTA cannot sufficiently buffer the local [Ca2+]i at the activating Ca2+-binding sites to abolish the activating effect of Ca2+ flux driven by 0.55 mM [Ca2+]ER at the same Vapp (Fig. 9 A). These observations strongly suggest that the activating Ca2+ sites are closer to the channel pore than the inhibitory sites.
It should be pointed out that these estimates of the locations of the cytoplasmic regulatory Ca2+ sites relative to the channel pore are very rough because the [Ca2+]i profiles around the channel were simulated without taking into consideration factors that can affect the distribution of Ca2+ around the channel but are not known in any detail, like the 3-D surface topology and charge distribution of the channel.
A very similar approach to that used here was applied to estimate the locations of activating and inhibitory Ca2+-binding sites in the RyR intracellular Ca2+ release channel that also exhibits [Ca2+]ER-driven Ca2+ flux modulation of channel activity (Liu et al., 2010). However, in that study, the couplings between channel gating and the local [Ca2+]i fluctuations at the regulatory sites during channel openings and closings were not taken into consideration. The distances derived from the effective time-averaged [Ca2+]i profile generated from Ca2+ current of magnitude = Po iCa was assumed to be estimates of the actual distances between the regulatory sites and the channel pore, instead of limiting values of those distances. This led to their conclusion that the inhibitory Ca2+ site was 1.2 ± 0.16 nm from the channel pore. This is probably an underestimation because that was actually the lower limit of the distance. The activating Ca2+ site to channel pore distance was calculated to be 1.7 µm, which led to a conclusion that the activating site on the open channel was shielded from the channel’s own Ca2+ flux. However, this value should be the upper limit of the activating Ca2+ site to pore distance. Accordingly, their derivation does not provide strong support for the notion that the activating site is shielded from feed-through effects of the channel’s own Ca2+ flux.
Limitation of the excised lum-out nuclear patch-clamp experiments
Using excised-patch lum-out nuclear patch clamping, we have demonstrated that all modulation by [Ca2+]ER of the gating activity of the r-InsP3R-3 channel can be attributed to feed-through effects causing a rise in local [Ca2+]i at cytoplasmic regulatory Ca2+-binding sites of the channel. We found no modulatory effects on InsP3R channel gating involving luminal Ca2+-binding site(s) on the channel. However, it must be noted that the luminal side of the excised nuclear membrane patches was perfused with various solutions to change [Ca2+]ER in our experiments. It is possible that a luminal Ca2+-binding factor(s) that could mediate effects of [Ca2+]ER on InsP3R channel activity was washed off by the perfusion. The possible existence of [Ca2+]ER regulation of InsP3R channel activity mediated by factor(s) in the ER lumen loosely associated with the channel should be investigated in the future using nuclear patch-clamp experiments in which the ER luminal milieu is preserved.
Acknowledgments
This work was supported by National Institutes of Health grants R01 MH059937 (to J.K. Foskett) and 5R01 GM065830 (to J.E. Pearson, D.-O.D. Mak, and J.K. Foskett).
Sharona E. Gordon served as editor.
Footnotes
Abbreviations used in this paper:
- HEDTA
- hydroxyethylethylenediaminetriacetic acid
- InsP3
- inositol 1,4,5-trisphosphate
- InsP3R
- InsP3 receptor
- lum-out
- luminal-side-out
- Po
- open probability
- r-InsP3R-3
- rat type 3 InsP3R
References
- Beecroft M.D., Taylor C.W. 1997. Incremental Ca2+ mobilization by inositol trisphosphate receptors is unlikely to be mediated by their desensitization or regulation by luminal or cytosolic Ca2+. Biochem. J. 326:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. 1997. Elementary and global aspects of calcium signalling. J. Physiol. 499:291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. 1998. Neuronal calcium signaling. Neuron. 21:13–26 10.1016/S0896-6273(00)80510-3 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. 2003. Cardiac calcium signalling. Biochem. Soc. Trans. 31:930–933 10.1042/BST0310930 [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bers D.M., Patton C.W., Nuccitelli R. 2010. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 99:1–26 10.1016/B978-0-12-374841-6.00001-3 [DOI] [PubMed] [Google Scholar]
- Betzenhauser M.J., Wagner L.E., II, Iwai M., Michikawa T., Mikoshiba K., Yule D.I. 2008. ATP modulation of Ca2+ release by type-2 and type-3 inositol (1, 4, 5)-triphosphate receptors. Differing ATP sensitivities and molecular determinants of action. J. Biol. Chem. 283:21579–21587 10.1074/jbc.M801680200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. 1994. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 104:821–856 10.1085/jgp.104.5.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. 1995. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145:205–216 [DOI] [PubMed] [Google Scholar]
- Bootman M. 1994a. Intracellular calcium. Questions about quantal Ca2+ release. Curr. Biol. 4:169–172 10.1016/S0960-9822(94)00041-2 [DOI] [PubMed] [Google Scholar]
- Bootman M.D. 1994b. Quantal Ca2+ release from InsP3-sensitive intracellular Ca2+ stores. Mol. Cell. Endocrinol. 98:157–166 10.1016/0303-7207(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Collins T.J., Peppiatt C.M., Prothero L.S., MacKenzie L., De Smet P., Travers M., Tovey S.C., Seo J.T., Berridge M.J., et al. 2001. Calcium signalling—an overview. Semin. Cell Dev. Biol. 12:3–10 10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- Braet K., Cabooter L., Paemeleire K., Leybaert L. 2004. Calcium signal communication in the central nervous system. Biol. Cell. 96:79–91 10.1016/j.biolcel.2003.10.007 [DOI] [PubMed] [Google Scholar]
- Butler J.N. 1968. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys. J. 8:1426–1433 10.1016/S0006-3495(68)86564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D., Eidsath A. 1996. Aequorin targeted to the endoplasmic reticulum reveals heterogeneity in luminal Ca++ concentration and reports agonist- or IP3-induced release of Ca++. Mol. Biol. Cell. 7:419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F.L., Benedetti A. 1996. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium. 19:547–551 10.1016/S0143-4160(96)90064-0 [DOI] [PubMed] [Google Scholar]
- Cárdenas C., Miller R.A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.H., Yang J., Parker I., et al. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 142:270–283 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo R., Colella M., Colasuonno A., DeLuisi A., Debellis L., Curci S., Hofer A.M. 2003. A reassessment of the effects of luminal [Ca2+] on inositol 1,4,5-trisphosphate-induced Ca2+ release from internal stores. J. Biol. Chem. 278:39503–39508 10.1074/jbc.M305823200 [DOI] [PubMed] [Google Scholar]
- Cheung K.H., Shineman D., Müller M., Cárdenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V.M., Foskett J.K. 2008. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 58:871–883 10.1016/j.neuron.2008.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. 1995. Calcium signaling. Cell. 80:259–268 10.1016/0092-8674(95)90408-5 [DOI] [PubMed] [Google Scholar]
- Combettes L., Claret M., Champeil P. 1992. Do submaximal InsP3 concentrations only induce the partial discharge of permeabilized hepatocyte calcium pools because of the concomitant reduction of intraluminal Ca2+ concentration? FEBS Lett. 301:287–290 10.1016/0014-5793(92)80258-I [DOI] [PubMed] [Google Scholar]
- Combettes L., Cheek T.R., Taylor C.W. 1996. Regulation of inositol trisphosphate receptors by luminal Ca2+ contributes to quantal Ca2+ mobilization. EMBO J. 15:2086–2093 [PMC free article] [PubMed] [Google Scholar]
- Cussler E.L. 1997. Diffusion: Mass Transfer in Fluid Systems. Second edition Cambridge University Press, Cambridge: 580 pp [Google Scholar]
- da Fonseca P.C., Morris S.A., Nerou E.P., Taylor C.W., Morris E.P. 2003. Domain organization of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis. Proc. Natl. Acad. Sci. USA. 100:3936–3941 10.1073/pnas.0536251100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A.P., Lea E.J., Irvine R.F. 2003. Kinetic model of the inositol trisphosphate receptor that shows both steady-state and quantal patterns of Ca2+ release from intracellular stores. Biochem. J. 370:621–629 10.1042/BJ20021289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Swillens S. 1996. Quantal release, incremental detection, and long-period Ca2+ oscillations in a model based on regulatory Ca2+-binding sites along the permeation pathway. Biophys. J. 71:1714–1722 10.1016/S0006-3495(96)79373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.D., Cameron A.M., Huganir R.L., Snyder S.H. 1992. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 356:350–352 10.1038/356350a0 [DOI] [PubMed] [Google Scholar]
- Foskett J.K. 2010. Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflugers Arch. 460:481–494 10.1007/s00424-010-0826-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J.K., White C., Cheung K.H., Mak D.-O.D. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87:593–658 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiman D., Dawson S.P. 2004. A model of IP3 receptor with a luminal calcium binding site: stochastic simulations and analysis. Cell Calcium. 35:403–413 10.1016/j.ceca.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Furuichi T., Mikoshiba K. 1995. Inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 64:953–960 10.1046/j.1471-4159.1995.64030953.x [DOI] [PubMed] [Google Scholar]
- Hagar R.E., Ehrlich B.E. 2000. Regulation of the type III InsP3 receptor and its role in β cell function. Cell. Mol. Life Sci. 57:1938–1949 10.1007/PL00000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Terauchi A., Mikoshiba K. 2003. Three-dimensional rearrangements within inositol 1,4,5-trisphosphate receptor by calcium. J. Biol. Chem. 278:52881–52889 10.1074/jbc.M309743200 [DOI] [PubMed] [Google Scholar]
- Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 120:85–98 10.1016/j.cell.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ionic Channels of Excitable Membranes. Third edition Sinauer Associates, Inc., Sunderland, MA: 814 pp [Google Scholar]
- Ionescu L., Cheung K.H., Vais H., Mak D.-O.D., White C., Foskett J.K. 2006. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. 573:645–662 10.1113/jphysiol.2006.109504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F. 1990. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates—a possible mechanism. FEBS Lett. 263:5–9 10.1016/0014-5793(90)80692-C [DOI] [PubMed] [Google Scholar]
- Jiang Q.X., Thrower E.C., Chester D.W., Ehrlich B.E., Sigworth F.J. 2002. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J. 21:3575–3581 10.1093/emboj/cdf380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johenning F.W., Ehrlich B.E. 2002. Signaling microdomains: InsP3 receptor localization takes on new meaning. Neuron. 34:173–175 10.1016/S0896-6273(02)00665-7 [DOI] [PubMed] [Google Scholar]
- Joseph S.K. 1996. The inositol triphosphate receptor family. Cell. Signal. 8:1–7 10.1016/0898-6568(95)02012-8 [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Hajnóczky G. 2007. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 12:951–968 10.1007/s10495-007-0719-7 [DOI] [PubMed] [Google Scholar]
- Kang S., Kang J., Kwon H., Frueh D., Yoo S.H., Wagner G., Park S. 2008. Effects of redox potential and Ca2+ on the inositol 1,4,5-trisphosphate receptor L3-1 loop region: implications for receptor regulation. J. Biol. Chem. 283:25567–25575 10.1074/jbc.M803321200 [DOI] [PubMed] [Google Scholar]
- Kindman L.A., Meyer T. 1993. Use of intracellular Ca2+ stores from rat basophilic leukemia cells to study the molecular mechanism leading to quantal Ca2+ release by inositol 1,4,5-trisphosphate. Biochemistry. 32:1270–1277 10.1021/bi00056a011 [DOI] [PubMed] [Google Scholar]
- Laver D.R. 2007a. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys. J. 92:3541–3555 10.1529/biophysj.106.099028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R. 2007b. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin. Exp. Pharmacol. Physiol. 34:889–896 10.1111/j.1440-1681.2007.04708.x [DOI] [PubMed] [Google Scholar]
- Lewis C.A. 1979. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J. Physiol. 286:417–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Mongillo M., Chin K.T., Harding H., Ron D., Marks A.R., Tabas I. 2009. Role of ERO1-α–mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 186:783–792 10.1083/jcb.200904060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Porta M., Qin J., Ramos J., Nani A., Shannon T.R., Fill M. 2010. Flux regulation of cardiac ryanodine receptor channels. J. Gen. Physiol. 135:15–27 10.1085/jgp.200910273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke S.J., Tran T.P., Ngo Q.T., Moiseenkova-Bell V.Y., Chiu W., Serysheva I.I. 2011. Flexible architecture of IP3R1 by Cryo-EM. Structure. 19:1192–1199 10.1016/j.str.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKrill J.J. 1999. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem. J. 337:345–361 10.1042/0264-6021:3370345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. 1998. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 275:C179–C188 [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 1998. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 95:15821–15825 10.1073/pnas.95.26.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 1999. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 274:22231–22237 10.1074/jbc.274.32.22231 [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 2001a. ATP-dependent adenophostin activation of inositol 1,4,5-trisphosphate receptor channel gating: kinetic implications for the durations of calcium puffs in cells. J. Gen. Physiol. 117:299–314 10.1085/jgp.117.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 2001b. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 Insp3 receptors. J. Gen. Physiol. 117:435–446 10.1085/jgp.117.5.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S.M., Foskett J.K. 2003. Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating. J. Gen. Physiol. 122:583–603 10.1085/jgp.200308809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., White C., Ionescu L., Foskett J.K. 2005. Nuclear patch clamp electrophysiology of inositol trisphosphate receptor Ca2+ release channels. In Calcium Signaling. Second edition Putney J.W., Jr editor. CRC Press, Boca Raton, FL: 203–229 [Google Scholar]
- Mak D.-O.D., Pearson J.E., Loong K.P.C., Datta S., Fernández-Mongil M., Foskett J.K. 2007. Rapid ligand-regulated gating kinetics of single inositol 1,4,5-trisphosphate receptor Ca2+ release channels. EMBO Rep. 8:1044–1051 10.1038/sj.embor.7401087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A.R. 1997. Intracellular calcium-release channels: regulators of cell life and death. Am. J. Physiol. 272:H597–H605 [DOI] [PubMed] [Google Scholar]
- McCarron J.G., Chalmers S., Muir T.C. 2008. “Quantal” Ca2+ release at the cytoplasmic aspect of the Ins(1,4,5)P3R channel in smooth muscle. J. Cell Sci. 121:86–98 10.1242/jcs.017541 [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Pozzan T. 1998. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci. 23:10–14 10.1016/S0968-0004(97)01143-2 [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. 1992a. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 357:599–602 10.1038/357599a0 [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. 1992b. Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+. J. Biol. Chem. 267:22961–22966 [PubMed] [Google Scholar]
- Missiaen L., Taylor C.W., Berridge M.J. 1992c. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J. Physiol. 455:623–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Parys J.B., Sienaert I., Vanlingen S., Casteels R. 1996. Effects of luminal Ca2+ on inositol trisphosphate-induced Ca2+ release: facts or artifacts? Cell Calcium. 19:91–93 10.1016/S0143-4160(96)90016-0 [DOI] [PubMed] [Google Scholar]
- Naraghi M. 1997. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium. 22:255–268 10.1016/S0143-4160(97)90064-6 [DOI] [PubMed] [Google Scholar]
- Naraghi M., Neher E. 1997. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 17:6961–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. 1986. Concentrations profiles of intracellular calcium in the presence of a diffusible chelator. In Experimental Brain Research. Volume 14: Calcium Electrogenesis and Neuronal Functioning. Heinemann U., Klee M., Neher E., Singer W., editors. Springer, Heidelberg: 80–96 [Google Scholar]
- Neher E. 1998. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 24:345–357 10.1016/S0143-4160(98)90058-6 [DOI] [PubMed] [Google Scholar]
- Nunn D.L., Taylor C.W. 1991. Regulation of Ca2+ mobilization by Ins (1,4,5)P3 and intraluminal Ca2+ in permeabilized hepatocytes. Biochem. Soc. Trans. 19:206S. [DOI] [PubMed] [Google Scholar]
- Nunn D.L., Taylor C.W. 1992. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol. Pharmacol. 41:115–119 [PubMed] [Google Scholar]
- Orrenius S., Zhivotovsky B., Nicotera P. 2003. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4:552–565 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- Parys J.B., Missiaen L., De Smedt H., Casteels R. 1993. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J. Biol. Chem. 268:25206–25212 [PubMed] [Google Scholar]
- Parys J.B., Missiaen L., Smedt H.D., Sienaert I., Casteels R. 1996. Mechanisms responsible for quantal Ca2+ release from inositol trisphosphate-sensitive calcium stores. Pflugers Arch. 432:359–367 10.1007/s004240050145 [DOI] [PubMed] [Google Scholar]
- Patel S., Joseph S.K., Thomas A.P. 1999. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 25:247–264 10.1054/ceca.1999.0021 [DOI] [PubMed] [Google Scholar]
- Patterson R.L., Boehning D., Snyder S.H. 2004. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 73:437–465 10.1146/annurev.biochem.73.071403.161303 [DOI] [PubMed] [Google Scholar]
- Putney J.W., Jr, Bird G.S. 1993. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr. Rev. 14:610–631 [DOI] [PubMed] [Google Scholar]
- Qin F., Auerbach A., Sachs F. 2000a. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys. J. 79:1915–1927 10.1016/S0006-3495(00)76441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Auerbach A., Sachs F. 2000b. Hidden Markov modeling for single channel kinetics with filtering and correlated noise. Biophys. J. 79:1928–1944 10.1016/S0006-3495(00)76442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. 2004. Ca2+ signals and T lymphocytes; “New mechanisms and functions in Ca2+ signalling”. Biol. Cell. 96:69–78 10.1016/j.biolcel.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Renard-Rooney D.C., Hajnóczky G., Seitz M.B., Schneider T.G., Thomas A.P. 1993. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J. Biol. Chem. 268:23601–23610 [PubMed] [Google Scholar]
- Sato C., Hamada K., Ogura T., Miyazawa A., Iwasaki K., Hiroaki Y., Tani K., Terauchi A., Fujiyoshi Y., Mikoshiba K. 2004. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J. Mol. Biol. 336:155–164 10.1016/j.jmb.2003.11.024 [DOI] [PubMed] [Google Scholar]
- Serysheva I.I., Bare D.J., Ludtke S.J., Kettlun C.S., Chiu W., Mignery G.A. 2003. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by electron cryomicroscopy. J. Biol. Chem. 278:21319–21322 10.1074/jbc.C300148200 [DOI] [PubMed] [Google Scholar]
- Short A.D., Klein M.G., Schneider M.F., Gill D.L. 1993. Inositol 1,4,5-trisphosphate-mediated quantal Ca2+ release measured by high resolution imaging of Ca2+ within organelles. J. Biol. Chem. 268:25887–25893 [PubMed] [Google Scholar]
- Shuttleworth T.J. 1992. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal Ca2+. J. Biol. Chem. 267:3573–3576 [PubMed] [Google Scholar]
- Shuttleworth T.J. 1995. A re-evaluation of the apparent effects of luminal Ca2+ on inositol 1,4,5-trisphosphate-induced Ca2+ release. Cell Calcium. 17:393–398 10.1016/0143-4160(95)90085-3 [DOI] [PubMed] [Google Scholar]
- Smith G.D. 1996. Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys. J. 71:3064–3072 10.1016/S0006-3495(96)79500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen J.M., Fay F.S. 1996. The quantal nature of calcium release to caffeine in single smooth muscle cells results from activation of the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 271:7398–7403 10.1074/jbc.271.13.7398 [DOI] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki M., Takata M., Kurosaki T. 1997. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 16:3078–3088 10.1093/emboj/16.11.3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S. 1992. Dynamic control of inositol 1,4,5-trisphosphate-induced Ca2+ release: a theoretical explanation for the quantal release of Ca2+. Mol. Pharmacol. 41:110–114 [PubMed] [Google Scholar]
- Swillens S., Combettes L., Champeil P. 1994. Transient inositol 1,4,5-trisphosphate-induced Ca2+ release: a model based on regulatory Ca2+-binding sites along the permeation pathway. Proc. Natl. Acad. Sci. USA. 91:10074–10078 10.1073/pnas.91.21.10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A., Turner R.J. 1996. Calcium release in HSY cells conforms to a steady-state mechanism involving regulation of the inositol 1,4,5-trisphosphate receptor Ca2+ channel by luminal [Ca2+]. J. Cell Biol. 132:607–616 10.1083/jcb.132.4.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A., Tojyo Y., Matsumoto Y. 1998. Imaging of quantal calcium release in the inositol 1,4,5-trisphosphate-sensitive organelles of permeabilized HSY cells. Cell Struct. Funct. 23:129–135 10.1247/csf.23.129 [DOI] [PubMed] [Google Scholar]
- Taufiq-Ur-Rahman, Skupin A., Falcke M., Taylor C.W. 2009. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+. Nature. 458:655–659 10.1038/nature07763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Laude A.J. 2002. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium. 32:321–334 10.1016/S0143416002001859 [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Richardson A. 1991. Structure and function of inositol trisphosphate receptors. Pharmacol. Ther. 51:97–137 10.1016/0163-7258(91)90043-L [DOI] [PubMed] [Google Scholar]
- Thrower E.C., Mobasheri H., Dargan S., Marius P., Lea E.J.A., Dawson A.P. 2000. Interaction of luminal calcium and cytosolic ATP in the control of type 1 inositol (1,4,5)-trisphosphate receptor channels. J. Biol. Chem. 275:36049–36055 10.1074/jbc.M000970200 [DOI] [PubMed] [Google Scholar]
- Thrower E.C., Hagar R.E., Ehrlich B.E. 2001. Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators. Trends Pharmacol. Sci. 22:580–586 10.1016/S0165-6147(00)01809-5 [DOI] [PubMed] [Google Scholar]
- Tregear R.T., Dawson A.P., Irvine R.F. 1991. Quantal release of Ca2+ from intracellular stores by InsP3: tests of the concept of control of Ca2+ release by intraluminal Ca2+. Proc. Biol. Sci. 243:263–268 10.1098/rspb.1991.0040 [DOI] [PubMed] [Google Scholar]
- Tsien R.Y. 1980. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 19:2396–2404 10.1021/bi00552a018 [DOI] [PubMed] [Google Scholar]
- Vais H., Foskett J.K., Mak D.-O.D. 2010a. Unitary Ca2+ current through recombinant type 3 InsP3 receptor channels under physiological ionic conditions. J. Gen. Physiol. 136:687–700 10.1085/jgp.201010513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H., Siebert A.P., Ma Z., Fernández-Mongil M., Foskett J.K., Mak D.-O.D. 2010b. Redox-regulated heterogeneous thresholds for ligand recruitment among InsP3R Ca2+-release channels. Biophys. J. 99:407–416 10.1016/j.bpj.2010.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H., Foskett J.K., Mak D.-O.D. 2011. InsP3R channel gating altered by clustering? Nature. 478:E1–E2 10.1038/nature10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L.E., II, Joseph S.K., Yule D.I. 2008. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J. Physiol. 586:3577–3596 10.1113/jphysiol.2008.152314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram F., Morris E., Taylor C.W. 2010. Three-dimensional structure of recombinant type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 428:483–489 10.1042/BJ20100143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki-Mann M., Parker I. 2011. Enhanced ER Ca2+ store filling by overexpression of SERCA2b promotes IP3-evoked puffs. Cell Calcium. 50:36–41 10.1016/j.ceca.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Hinkle P.M. 2000. Rapid turnover of calcium in the endoplasmic reticulum during signaling. Studies with cameleon calcium indicators. J. Biol. Chem. 275:23648–23653 10.1074/jbc.M002684200 [DOI] [PubMed] [Google Scholar]