Abstract
Background:
Identification of sleep-disordered breathing (SDB) using questionnaires is critical from a clinical and research perspective. However, which questions to use and how well such questionnaires perform has thus far been fraught with substantial uncertainty. We aimed at delineating the usefulness of a set of questions for identifying pediatric SDB.
Methods:
Random prospective sampling of urban 5- to 9-year-old children from the community and enriched for habitual snoring underwent overnight sleep study. Subjective indicators or questions were evaluated to further characterize and discriminate SDB.
Results:
Of 1,133 subjects, 52.8% were habitual snorers. This sample was analyzed based on a clinical grouping (ie, established apnea-hypopnea index cutoffs). Several statistical steps were performed and indicated that complaints can be ranked according to a severity hierarchy: shake child to breathe, apnea during sleep, struggle breathing when asleep, and breathing concerns while asleep, followed by loudness of snoring and snoring while asleep. With a posteriori cutoff, a predictive score > 2.72 on the severity scale was found (ie, area under the curve, 0.79 ± 0.03; sensitivity, 59.03%; specificity, 82.85%; positive predictive value, 35.4; negative predictive value, 92.7), making this cutoff applicable for confirmatory purposes.
Conclusions:
As a result, the set of six hierarchically arranged questions will aid the screening of children at high risk for SDB but cannot be used as the sole diagnostic approach.
Sleep-disordered breathing (SDB) is a frequent condition in children. In the last 2 decades, associations between SDB and behavioral, neurocognitive, cardiovascular, and metabolic morbidities have been extensively reported, and dose-dependent relationships with certain polysomnographic measures and even sleep-related questions have been suggested.1‐6 However, the eventual impact of SDB on impairments in the quality of life of a developing child7,8 may be best understood in the context of a spectrum of disease, rather than specific clinical categories (eg, mild, moderate, severe). Although the primary symptom of SDB is habitual snoring, which is indicative of the presence of increased upper airway resistance during sleep, the actual perception as to the presence of snoring and associated symptoms is highly subjective.9‐12 Complaints of snoring and somnolence,13 diminished performance, behavioral problems,14 or headaches15 by nonapneic snorers will not reliably discriminate them from those with habitual snoring who suffer from sleep apnea. Consequently, such observations have led to the consistent conclusion that clinical symptoms are unable to identify pediatric patients with clinically relevant SDB.16,17 Accordingly, the apnea-hypopnea index (AHI) has thus far been the key selected discriminator in research and clinical practice of all polysomnographic measures. This approach is fraught with major obstacles in the clinical setting, especially when considering the aforementioned limitations, which have led to < 10% of all children being diagnosed using polysomnography.18
Under such circumstances, reliance on sleep questionnaires is frequent,19,20 with questions about snoring frequency and loudness recurring in nearly every published sleep questionnaire.19,20 However, it is only recently that the psychometric qualities of such sleep questionnaires have begun to be critically examined.19,20 In the literature, several questions on respiratory symptoms are applied in questionnaires.21‐25 Therefore, one of the main objectives of the present study was to identify potential questionnaire-based items within our questionnaire that may allow for useful discrimination of SDB.
Materials and Methods
Data collection was approved by the University of Louisville Human Research Committee (protocol #474.99), and the Institutional Review Boards of Jefferson County Public Schools and Archdiocese of Louisville Catholic Schools. Informed consent was obtained from the legal caregiver of each participant, with assent being obtained from children > 7 years of age.
Sleep Questionnaire and Polysomnographic Assessment
Parents of all children 5 to 9 years of age were invited to complete our sleep questionnaire,1,26 which in addition to demographic information and significant medical history of the child included sleep-related questions (37 items in total) (Table 1 or Reference 1). All sleep-related questions used the Likert-type responses “never” (0), “rarely” (once per week; 1), “occasionally” (twice per week; 2), “frequently” (three to four times per week; 3) and “almost always” (> 4 times per week; 4) for the preceding 6-month time frame.
Table 1.
—The 37 Questions in Our Sleep Questionnaire
| Question |
| On the average, how long does your child sleep at night? |
| At what time does your child go to bed? |
| At what time does your child wake up? |
| Have you seen or heard your child having nightmares that he/she does not remember the next day? |
| Has he/she expressed fear of sleeping in the dark? |
| Is your child easy to wake up in the morning? |
| Does your child go to bed willingly? |
| Is he/she a restless sleeper? |
| Have you seen your child smiling during sleep? |
| Does he/she wake up at night? |
| Have your heard your child talking in his/her sleep? |
| Have you observed him/her sleepwalking? |
| While asleep, does he/she ever sit up in bed? |
| Does he/she grind his/her teeth during sleep? |
| Have you heard your child laugh during sleep? |
| Has your child told you about having a frightening dream? |
| Have you observed repetitive actions such as rocking or head banging during sleep? |
| Does he/she have problems with bed wetting? |
| Have your observed your child having a nightmare during which he/she appeared extremely afraid or terrified? |
| Have you looked in on your child and discovered he/she was crying while asleep? |
| Has he/she told you about having a pleasant dream? |
| Does your child complain about difficulties going to sleep? |
| Does your child get up to go to the bathroom during the night? |
| Does your child stop breathing during sleep? (Apnea during sleep) (Q2) |
| Does your child struggle to breathe while asleep? (Struggle breathing when asleep) (Q3) |
| Does your child fall asleep easily? |
| Do you ever shake your child to make him/her breathe again when asleep? (Shake child to breath) (Q1) |
| Do your child’s lips ever turn blue or purple while asleep? |
| Are you ever concerned about your child’s breathing during sleep? (Breathing concerns while asleep) (Q4) |
| How loud is the snore? (Loudness of snoring) (Q5)a |
| How often does your child snore? (Snoring during sleep) (Q6) |
| How often does your child have a sore throat? |
| Does your child complain of morning headaches? |
| Is your child a daytime mouth breather? |
| Is your child sleepy during the daytime? |
| Does your child fall asleep at school? |
| Does your child fall asleep while watching television? |
Q refers to questions in the Mokken Scale Analyses.
Q5 is scored as 0: mildly quiet; 1: medium loud; 2: loud; 3: very loud; 4: extremely loud.
From the returned questionnaires, children were randomly selected and invited to the Pediatric Sleep Medicine Center for a nocturnal polysomnogram (NPSG) assessment. Children were excluded if they had any known developmental or chronic medical conditions or genetic or craniofacial syndromes. Detailed information on all NPSG-related procedures can be found in Spruyt et al.27
Statistics
The same analytical steps were conducted on the sample divided into routinely used clinical AHI cutoff groups: AHI ≤ 1/h total sleep time (TST) (AHI_G1), 1 > AHI ≤ 2/h TST (AHI_G2), 2 > AHI ≤ 3/h TST (AHI_G3), 3 > AHI ≤ 5/h TST (AHI_G4), 5 > AHI < 10/h TST (AHI_G5), and ≥ 10/h TST (AHI_G6).
Descriptive analyses of the sleep questionnaire items were conducted. Subsequently, data mining was as follows: In step 1, factor analysis (with varimax normalized and Eigenvalue > 1 criteria) and item reliability analyses describing the factor structure as well as the internal consistency of the questions (STATISTICA 8.0; StatSoft, Inc). In step 2, Mokken Scale Analysis (MSA) (MSPwin version 5; iecProGAMMA) assumes the existence of an underlying latent trait, which is represented by ordering a set of questions related to the latent trait. Coefficients (of Loevinger) (H) ≥ 0.30 or higher indicate an acceptable to very good scalability (or ordering) power. The resulting hierarchy of questions (or scale) is, therefore, an ordering of questions (or subjective complaints) by degree of severity (ie, any individual who endorses a particular question will also “agree with,” hence exhibit complaints of, all the questions ranked lower in the severity hierarchy). After establishing the ordering in the total sample, MSA was conducted for the clinical AHI cutoff groups. Of note, since MSA requires a complete set of data points, this analysis included 667 cases. No significant differences were found on sociodemographic or NPSG parameters between these 667 cases and nonincluded cases for step 2. In step 3, sensitivity and specificity of the set of questions was tested via receiver operator curves (ROCs) (SPSS, version 16; IBM). In ROCs, sensitivity stands for the proportion of correct inclusion of cases, and specificity is the proportion of correct exclusion of cases. The area under the curve (AUC) ≥ 0.8 by convention represents a good “test” (hereafter named question or set of questions). Results are printed as mean ± SD unless specified otherwise (ie, for ROC, the AUC is printed as mean ± SE [95% CI]).
Results
We focused on the distributions of the 11 sleep questions that were significantly different across AHI groups: breathing concerns while asleep (Question [Q]4) (Kruskal-Wallis test H[5,N = 1,036] = 128.8, P = .0001), apnea during sleep (Q2) (H[5,N = 994] = 121.2, P < .00001), snoring during sleep (Q6) (H[5,N = 1,051] = 103.1, P < .00001), loudness of snoring (Q5) (H[5,N = 856] = 89.7, P < .00001), struggle breathing when asleep (Q3) (H[5,N = 1,019] = 85.9, P < .00001), shake child to breath (Q1) (H[5,N = 1,045] = 70.9, P < .00001), daytime mouth breathing (H[5,N = 1,018] = 51.9, P < .00001), falls asleep at school (H[5,N = 1,040] = 20.5, P = .0010), falls asleep watching TV (H[5,N = 1,055] = 16.1, P = .0067), sore throat (H[5,N = 1,050] = 18.5, P = .0024), excessive daytime sleepiness (H[5,N = 1,050] = 14.3, P = .0138). Additional descriptive analyses of the sample can be found in e-Appendix 1 (1.1MB, pdf) and e-Tables 1-3 (1.1MB, pdf) .
Step 1: Factor Analysis—Item Reliability Analysis
These 11 sleep questions loaded on three factors explaining 62.2% of variance. The third factor explained < 10% of the variance, and for ease of interpretation we determined a two-factor solution for the 11 questions (explained variance to 53%). Factor 1 explaining 38.7% of the total variance consisted of seven items: apnea during sleep (Q2) (factor loading [FL], 0.83), struggle breathing when asleep (Q3) (FL, 0.84), shake child to breath (Q1) (FL, 0.70), breathing concerns while asleep (Q4) (FL, 0.83), snoring during sleep (Q6) (FL, 0.58), loudness of snoring (Q5) (FL, 0.65), and daytime mouth breathing (FL, 0.51). Factor 2 composed 14.3% of total explained variance, four items: sore throat (FL, 0.40), excessive daytime sleepiness (FL, 0.77), falls asleep at school (FL, 0.73), or falls asleep when watching TV (FL, 0.73). This is a moderate amalgamation of questions with a Cronbach α of 0.83 (average intercorrelation of 0.32).
Step 2: Mokken Scale Analysis
For MSA in the total sample see e-Appendix 1 (1.1MB, pdf) . Briefly, factor 2 had low scalability (H < 0.5), and in factor 1, daytime mouth breathing showed a consistent bad fit and was omitted.
MSA in the AHI Cutoff Groups:
Subsequently, the set of six questions was analyzed within each group (Fig 1, Table 2). When the AHI was > 3/h TST, the hierarchy changed; namely, when higher scores on breathing concerns while asleep (Q4) were marked, it was likely that high scores on loudness of snoring (Q5) were reported. As a result, the switch in order of the items loudness of snoring (Q5) and breathing concerns while asleep (Q4) across the AHI groups suggests a different latent severity. The first three items were constant among groups, indicative that higher scores on struggle breathing when asleep (Q3) were significant; that is, likely the previous items had also high scores. Or, alternatively, a higher mean score of the first three items (ie, < 0.9), one likely belonged to AHI_G1-3, or > 1, one likely belonged to AHI_G4-6, given the severity hierarchy (Fig 1). With respect to the remaining three items, loudness of snoring (Q5) was often the weakest, and snoring during sleep (Q6) always took the last place (except for AHI_G6). In other words, these questions might aid the screening process, but suffer from low discriminative power across the AHI cutoffs, especially those closer to each other. For instance, a high score on snoring during sleep (Q6) was nearly always preceded by somewhat higher scores on the previous items in the hierarchy, but it is difficult to determine to which AHI group the child might belong, such that breathing concerns while asleep (Q4) might be a better alternative. As a marginal note, potential confounders, such as colds or flu symptoms, and also a potential limitation in our questionnaire, whereby parental report of no snoring did not leave an option on the subsequent question regarding the loudness of snoring, could be possible explanations of this. A combined question of loudness of snoring (Q5) and snoring during sleep (Q6) may perhaps yield a better power.28,29 Hence, a higher score on breathing concerns while asleep (Q4) might be indicative: A (overall mean) score > 1.8 was likely suggestive for AHI_G4-6, respectively. Finally, from a clinical standpoint, individual (mean) scores can be compared with the proposed severity hierarchy, and, therefore, they serve as a guideline toward assigning an AHI cutoff group.
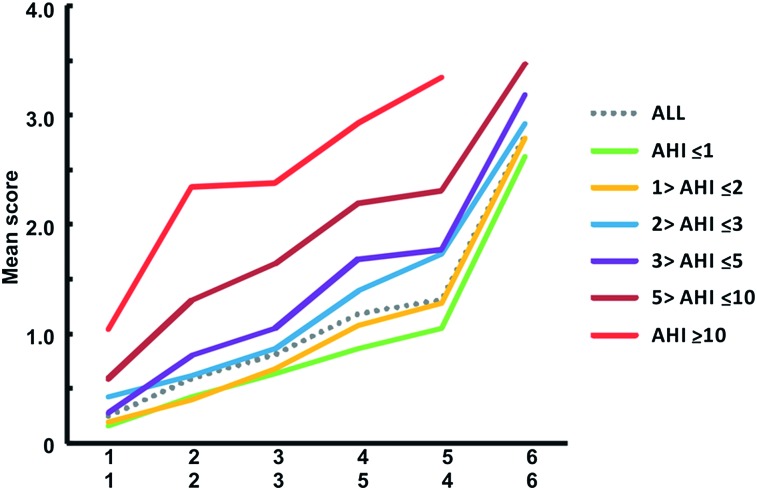
Figure 1.
Severity hierarchy of respiratory complaints in children for the clinical AHI cutoff groups. Q1: shake child to breathe. Q2: apnea during sleep. Q3: struggle breathing when asleep. Q4: breathing concerns while asleep. Q5: loudness of snoring. Q6: snoring during sleep. Daytime mouth breathing (excluded by Mokken Scale Analysis). With a high score on a question it is likely that previous questions within the hierarchy will be scored high as well; also, the higher the mean score the more severe the complaints. The lines depict the mean score per question for each AHI cutoff group when the proposed hierarchy is preserved; hence, they can be a clinical rule of thumb. Visually, the disparities among the lines of severity hierarchy further suggest the closeness and distinctiveness of AHI cutoff groups based on the applied questionnaire. The lower x-axis applies for AHI_G4-6 (or AHI > 3). AHI = apnea-hypopnea index.
Table 2.
—Mokken Scale Analyses in the Clinical AHI Cutoff Groups (Step 2): Severity Hierarchy of Respiratory Complaints
| Clinical AHI Cutoff Group | Question | Mean | H |
| Group 1: AHI ≤ 1; H, 0.60; ρ, 0.86 | |||
| 1 | 0.15 | 0.67 | |
| 2 | 0.42 | 0.58 | |
| 3 | 0.63 | 0.61 | |
| 4 | 0.86 | 0.62 | |
| 5 | 1.05 | 0.52a | |
| 6 | 2.62 | 0.62 | |
| Group 2: 1 > AHI ≤ 2; H, 0.54; ρ, 0.85 | |||
| 1 | 0.19 | 0.55 | |
| 2 | 0.39 | 0.56 | |
| 3 | 0.67 | 0.56 | |
| 4 | 1.07 | 0.56 | |
| 5 | 1.27 | 0.45a | |
| 6 | 2.79 | 0.60 | |
| Group 3: 2 > AHI ≤ 3; H, 0.57; ρ, 0.88 | |||
| 1 | 0.42 | 0.61 | |
| 2 | 0.61 | 0.49a | |
| 3 | 0.86 | 0.56 | |
| 4 | 1.39 | 0.60 | |
| 5 | 1.72 | 0.55 | |
| 6 | 2.92 | 0.56 | |
| Group 4: 3 > AHI ≤ 5; H, 0.54; ρ, 0.84 | |||
| 1 | 0.27 | 0.47 | |
| 2 | 0.80 | 0.45 | |
| 3 | 1.05 | 0.60 | |
| 5 | 1.68 | 0.47a | |
| 4 | 1.77 | 0.61 | |
| 6 | 3.18 | 0.57 | |
| Group 5: 5 > AHI < 10; H, 0.75; ρ, 0.92 | |||
| 1 | 0.58 | 0.71 | |
| 2 | 1.31 | 0.79 | |
| 3 | 1.64 | 0.74 | |
| 5 | 2.19 | 0.73 | |
| 4 | 2.31 | 0.78 | |
| 6 | 3.47 | 0.69a | |
| Group 6: AHI ≥ 10; H, 0.52; ρ, 0.81 | |||
| 1 | 1.03 | 0.61 | |
| 2 | 2.34 | 0.59 | |
| 3 | 2.38 | 0.55 | |
| 5 | 2.93 | 0.30a | |
| 4 | 3.34 | 0.57 | |
| (6) | … | … | |
AHI = apnea-hypopnea index; H = coefficient of Loevinger; ρ = reliability.
Weakest item in terms of scalability assumptions.
Step 3: ROC
The ROCs for each of the 11 questions are presented in e-Table 3 (1.1MB, pdf) , as follows:
For the Severity Hierarchy of Complaints:
Given the hierarchical ordering of the complaints, we generated ROC on the cumulative average score of the hierarchy (Table 3) and compared AHI groups. AHI_G1 could accurately be discriminated from AHI_G5 with a cumulative average score > 1.59. AHI_G3 could be discriminated from AHI_G6 when the cumulative average was > 2.94 on the severity hierarchy, and AHI_G1, AHI_G2, and AHI_G4 discriminated from AHI_G6 with a cumulative average score > 2.91 (Fig 1). However, these criteria were more likely to correctly identify children with AHI ≥ 10 (or AHI_G6) from the severity hierarchy of AHI cutoff groups.
Table 3.
—Receiver Operating Characteristics for the Severity Hierarchy for the Clinical AHI Cutoff Groups
| Group | Criterion | AUC, Mean ± SE (95% CI) | P Value | Sensitivity, Mean (95% CI) | Specificity, Mean (95% CI) | Negative Predictive Value, % | Positive Predictive Value, % |
| Group 1: AHI ≤ 1 vs | |||||||
| Group 2: 1 > AHI ≤ 2 | > 2.00 | 0.561 ± 0.0241 (0.527-0.595) | .0110 | 44.97 (37.7-52.4) | 68.09 (64.4-71.6) | 81.2 | 28.8 |
| Group 3: 2 > AHI ≤ 3 | > 2.03 | 0.626 ± 0.0410 (0.590-0.662) | .0021 | 52.63 (39.0-66.0) | 68.39 (64.7-71.9) | 94.3 | 12.6 |
| Group 4: 3 > AHI ≤ 5 | > 1.50 | 0.734 ± 0.0389 (0.700-0.766) | .0001 | 82.46 (70.1-91.3) | 54.71 (50.8-58.6) | 97.3 | 13.6 |
| Group 5: 5 > AHI < 10 | > 1.59 | 0.776 ± 0.0389 (0.743-0.806) | .0001 | 84.62 (71.9-93.1) | 55.93 (52.0-59.8) | 97.9 | 13.2 |
| Group 6: AHI ≥ 10 | > 2.91 | 0.951 ± 0.0255 (0.932-0.966) | .0001 | 91.43 (76.9-98.2) | 89.06 (86.4-91.3) | 99.5 | 30.8 |
| Group 2: 1 > AHI ≤ 2 vs | |||||||
| Group 3: 2 > AHI ≤ 3 | > 2.69 | 0.570 ± 0.0443 (0.505-0.633) | .1143 | 33.33 (21.4-47.1) | 80.42 (74.0-85.8) | 80.0 | 33.9 |
| Group 4: 3 > AHI ≤ 5 | > 1.5 | 0.682 ± 0.0427 (0.620-0.740) | .0001 | 82.46 (70.1-91.3) | 47.09 (39.8-54.5) | 89.9 | 32.0 |
| Group 5: 5 > AHI < 10 | > 2.84 | 0.734 ± 0.0426 (0.674-0.789) | .0001 | 51.92 (37.6-66.0) | 85.19 (79.3-89.9) | 86.6 | 49.1 |
| Group 6: AHI ≥ 10 | > 2.91 | 0.944 ± 0.0274 (0.906-0.970) | .0001 | 91.43 (76.9-98.2) | 87.30 (81.7-91.7) | 98.2 | 57.1 |
| Group 3: 2 > AHI ≤ 3 vs | |||||||
| Group 4: 3 > AHI ≤ 5 | > 1.50 | 0.621 ± 0.0524 (0.525-0.710) | .0211 | 82.46 (70.1-91.3) | 40.35 (27.6-54.2) | 69.7 | 58.0 |
| Group 5: 5 > AHI < 10 | > 2.94 | 0.680 ± 0.0514 (0.584-0.766) | .0005 | 48.08 (34.0-62.4) | 78.95 (66.1-88.6) | 67.6 | 62.5 |
| Group 6: AHI ≥ 10 | > 2.94 | 0.895 ± 0.0381 (0.813-0.949) | .0001 | 88.57 (73.3-96.8) | 78.95 (66.1-88.6) | 91.8 | 72.1 |
| Group 4: 3 > AHI ≤ 5 vs | |||||||
| Group 5: 5 > AHI < 10 | > 3.63 | 0.574 ± 0.0550 (0.475-0.668) | .1811 | 23.08 (12.5-36.8) | 92.98 (83.0-98.1) | 57.0 | 75.0 |
| Group 6: AHI ≥ 10 | > 2.91 | 0.826 ± 0.0476 (0.733-0.897) | .0001 | 91.43 (76.9-98.2) | 64.91 (51.1-77.1) | 92.5 | 61.5 |
| Group 5: 5 > AHI < 10 vs | |||||||
| Group 6: AHI ≥ 10 | > 2.88 | 0.724 ± 0.0577 (0.618-0.814) | .0001 | 91.18 (76.3-98.1) | 50.94 (36.8-64.9) | 90.0 | 54.4 |
Significant area under curve indicated in boldface. Criterion is the cumulative average score. See Table 2 legend for expansion of abbreviation.
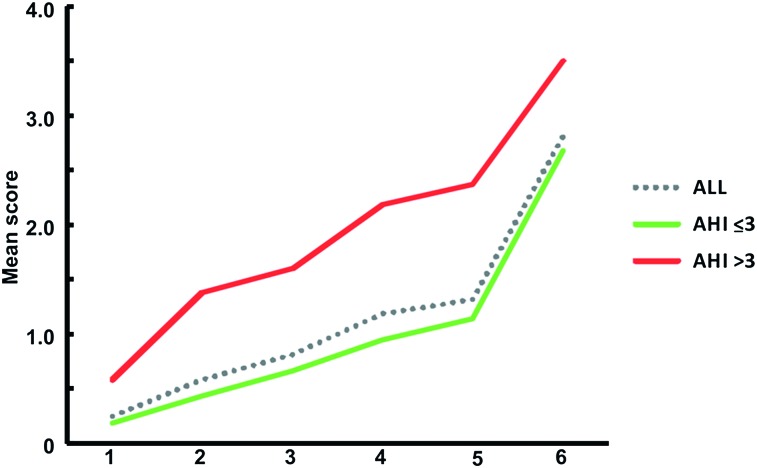
The MSA and ROC findings ultimately and a posteriori led to our final analyses based on only two AHI cutoff groups. More specifically, and corroborating the switch in order of questions in the MSA, findings indicated an AHI ≤ 3/h TST and AHI > 3/h TST division being accurately discriminated, with a cumulative average score > 2.72 on the severity hierarchy: an AUC 0.79 ± 0.03 (95% CI, 0.76-0.81), a sensitivity of 59.03% (95% CI, 50.5%-67.1%), and a specificity of 82.85% (95% CI, 80.2%-85.3%). Its positive predictive value was 35.4, and the negative predictive value was 92.7, making this cutoff applicable for confirmatory purposes (ie, a high negative predictive value suggests that it will rarely misclassify a child with SDB as not having SDB). This can also be visually appreciated in Figure 2 (or Fig 1), where the score > 2.72 potentially identifies the children with more severe AHI score based on their complaint severity. In general, however, misclassifications can be expected since we originally relied on an a priori arbitrary single NPSG cutoff being the clinical AHI groups to start with. Conversely, and to reflect on the clinical applicability of such an unbiased a posteriori cutoff, when our sample was divided based on this severity hierarchy criterion of 2.72, the AUC 0.70 ± 0.02 (95% CI, 0.66-0.72) with a sensitivity of 55% (95% CI, 48.05%-61.4%) and a specificity of 76.92% (73.9%-79.8%) corresponded to an AHI cutoff of 1.2/h TST, which coincides with the clinical practice of AHI > 1 as potentially problematic.
Figure 2.
Severity hierarchy of respiratory complaints in children for the a posteriori AHI ≤ 3 and AHI > 3. Q1: shake child to breathe. Q2: apnea during sleep. Q3: struggle breathing when asleep. Q4: breathing concerns while asleep. Q5: loudness of snoring. Q6: snoring during sleep. Daytime mouth breathing (excluded by Mokken Scale Analysis). The scores on the green and red line may aid the diagnostic process. If the average score on the severity hierarchy is > 2.72, the child likely has SDB.
Finally, as a practical example of our severity hierarchy of complaints, the scoring is the cumulative average score of all six questions, according to the following formula (where Q1 = raw score to question 1, Q2 = raw score to question 2, and so forth): A = (Q1 + Q2)/2; B = (A + Q3)/2; C = (B + Q4)/2; D = (C + Q5)/2; and the score on the Severity Hierarchy of Complaints = (D + Q6)/2.
For a random child, XX, who was chosen from our sample pool, and based on our questionnaire (Table 1) the parental report for XX on the severity hierarchy was:
Shake child to breathe (Q1): rarely, raw score = 1
Apnea during sleep (Q2): occasionally, raw score = 2
Struggle breathing when asleep (Q3): almost always, raw score = 4
Breathing concerns while asleep (Q4): frequently, raw score = 3
Loudness of snoring (Q5): very loud, raw score = 3
Snoring during sleep (Q6): almost always, raw score = 4.
These answers would in current practice suggest that child XX has SDB. On one hand, the high raw scores, especially on loudness of snoring (Q5) and snoring during sleep (Q6), would suggest the presence of SDB, and this approach in fact concurs with common clinical practice. On the other hand, as shown in this article, the order of complaints, namely those in the severity hierarchy, would additionally lead to expectation of high scores on the lower ranked items. Furthermore, the severity hierarchy allows auxiliary specification across groups. The following illustrates that the answer pattern on our severity hierarchy might thus help in elucidating the place of the child across the SDB spectrum. (As in clinical practice, the severity hierarchy requires several “if…then…” reasonings. In contrast to others, our analyses incorporate the severity, which means the answer categories remain intact [no regrouping or collapsing of answers; see references 19 and 30 for more explanation on their importance] in addition to ordering the complaints, and hence expressing a latent complaint.)
For child XX, the cumulative average score of all six questions would be 3.47 (ie, A = [1 + 2]/2, being 1.5; B = [1.5 + 4]/2, being 2.75; C = [2.75 + 3]/2, being 2.88; D = [2.88 + 3]/2, being 2.94; and, thus, the score = [2.94 + 4]/2, being 3.47). Table 3 shows that this score is a positive screener for AHI_G6 but cannot be discriminated from AHI_G5, namely, > 2.91 (AHI_G1 vs AHI_G6, and AHI_G2 vs AHI_G6, AHI_G4 vs AHI_G6) and 2.94 (AHI_G3 vs AHI_G6). This is further confirmed by being > 2.72 (our criterion when a posteriori AHI > 3/h TST, ie, with specificity 82.85% and negative predictive value of 92.7%), thus the child very likely belongs to AHI_G5-AHI_G6 (AHI_G4 could already be discriminated). Figures 1 and 2 concur for AHI_G5 and AHI_G6, or additionally when looking at the raw scores of this child it is comparable to such monotonicity pattern or the cumulative average score is increasing (similar to the mean scores of Table 2).
XX is a special case, though, since breathing concerns while asleep (Q4) and loudness of snoring (Q5) both have raw scores of 3 (so even if we change the order of breathing concerns while asleep [Q4] and loudness of snoring [Q5], the cumulative average remains). The monotonicity pattern would suggest > 5 AHI ≤ 10/h TST. We may further detail based on the valid ROC per question findings by comparing individual scores (e-Appendix 1 (1.1MB, pdf) ). In this example, however, no discriminatory questions are further applicable for the clinical AHI cutoff groups G5 vs G6.
Finally, the illustrative example is now unblended and reveals that child XX is a black, 6.5-year-old girl, and her actual NPSG results are as follows: AHI, 6.6/h TST; apnea index, 0/h TST; obstructive apnea index, 0/h TST; nadir saturation, 80%; spontaneous arousal index, 14.8/h TST; respiratory arousal index, 6.06/h TST; and sleep pressure score, 0.65. Thus, a substantial corroboration of the predictions based on the questionnaire is achieved and is indeed the case for a large proportion of cases, as described here in the validation procedures and ROCs.
Discussion
Based on commonly used subjective respiratory symptoms, a severity hierarchy of parental reported complaints has now been delineated. More specifically, a set of six ordered questions allows for fair discrimination along the SDB spectrum. Snoring and loudness of snoring are potentially valuable screening items; however, their specificity remains low to moderate across the spectrum. A high score on breathing concerns while asleep appears to be discriminative, affording a high probability of agreement on subsequent polysomnography.
A major strength of this study relies on the delineation of an SDB spectrum model based on community-based children who were studied over the course of several years and underwent an NPSG. Another advantage consisted in the modeling being conducted using a compilation of data over several years, such that both questionnaire-based responses and the scoring of polysomnographic parameters would not suffer from seasonal skewness or scorer dependency. We should point out that sleep questionnaires vary substantially across clinical and research settings and that there is a need for a more unified instrument.19,30 Here, we identified six questions in a 5- to 9-year-old sample that could potentially detect the presence of SDB and its severity. However, the format of our questionnaire should be taken into account, and either used in the future as such, or, alternatively, validation will be required if using another format.
The SDB spectrum exhibited a severity hierarchy in respiratory complaints, which was determined based on a single NPSG parameter, namely, AHI severity. A summary of published estimates has reported that snoring per se affects 7.45% of children, and that apneic events occur in 0.2% to 4% of children, whereas prevalence of OSA ranges between 0.1% to 13%.20 Such published prevalence rates have been questioned because of the variety of methodological approaches that pertain to measurement and definition, and the exclusion of cases falling between “primary snoring” and OSA.20 The authors further reported the increased reliance on snoring as a discriminant symptom rather than the AHI.20 The latter was also discussed in our previous unbiased analysis of NPSG data,27 and current analyses are suggestive of the existence of a severity hierarchy of complaints, with breathing concerns being more discriminatory.
Our approach is a clear example of item analyses that go beyond the commonly applied factor analyses and item reliability screening, or even ROC, and constitute an important step of a sleep tool.19,30 Yet, applicability of the proposed set of questions should be restricted to screening, and they should not be used as a surrogate diagnostic tool, at least at the current stage. Indeed, the questions on loud snoring and frequent snoring in our analyses likely identify extremes within the spectrum. Even though addition of loudness of snoring improved specificity, neither of these questions is powerful enough to discriminate across the SDB spectrum. Our severity hierarchy of complaints clearly shows that when endorsing a high score on snoring, it incorporates higher degrees of the other complaints. Thus, it further lends support to the critiques on the limitations of assessing patients’ complaints of snoring as sufficient in the process of discriminating apneic vs nonapneic snorers,16,17,31 an approach that is nevertheless heavily endorsed by many professionals.32,33 Given that it is often used as a single question, in addition to the intrinsic differences in posing the question and scoring the answer, the statistical characteristics found in this study on its use should provide further opportunities for critical discussion. Independently, the psychometric properties of posing such questions will affect agreement with objective measurements such as NPSG.1
A set of questions is likely to provide a better and more reliable approach if one wants to screen across a spectrum of SDB, and attempts to find such a set are encountered in the literature.1,34‐39 Despite their differences, those results are comparable to those presented here, which in addition were generated in a large community sample, and indicate that a set of ordered questions or the severity hierarchy of complaints is reliable for screening purposes. More specifically, a criterion of > 2.72 was found and can be applied to confirm the severity hierarchy as well as providing reasonable sensitivity (about 60%) and specificity (about 82%). Our results are also similar to others with respect to the types of breathing complaints included, namely shake child to breathe, apnea during sleep, struggle breathing when asleep, breathing concerns while asleep, and further suggestive for a severity hierarchy of complaints (hence, order).
The AHI group division showed that not all groups could be accurately discriminated from each other, such that overreliance on any one index (eg, AHI) or even question (eg, snoring) will potentially increase misclassifications. The criteria or cumulative average scores on the severity hierarchy of AHI cutoff groups principally identified children with AHI ≥ 10/h TST (or AHI_G6). As shown in this article, in a community sample, the proposed cumulative average scores (or hierarchy) perform adequately at excluding disease when such is absent and moderately well at confirming disease when present. Finally, our sensitivity and specificity findings, either per question or per the severity hierarchy, may potentially aid prospective epidemiologic studies or guide toward more uniformity in the field.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Spruyt: contributed to performing the analyses of the database and drafting the manuscript and approved the final version of the manuscript.
Dr Gozal: contributed to conception and design, securing the funding for the project, analysis and interpretation of data, drafting the manuscript, and revising it critically for important intellectual content, and gave final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank G. Verleye, PhD, Department of Political and Social Sciences, University of Ghent, Belgium, for providing access to MSPwin software. This work was performed at the University of Louisville, Kentucky.
Additional information: The e-Appendix and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- AHI
apnea-hypopnea index
- AUC
area under curve
- FL
factor loading
- MSA
Mokken Scale Analysis
- NPSG
nocturnal polysomnogram
- Q
question
- ROC
receiver operator curve
- SDB
sleep-disordered breathing
- TST
total sleep time
Footnotes
Funding/Support: This study was supported by the National Institutes of Health [Grant HL65270].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 200427(1):87-94 [DOI] [PubMed] [Google Scholar]
- 2.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004114(1):44-49 [DOI] [PubMed] [Google Scholar]
- 3.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 200413(2):165-172 [DOI] [PubMed] [Google Scholar]
- 4.Montgomery-Downs HE, Jones VF, Molfese VJ, Gozal D. Snoring in preschoolers: associations with sleepiness, ethnicity, and learning. Clin Pediatr (Phila). 200342(8):719-726 [DOI] [PubMed] [Google Scholar]
- 5.Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 20078(1):18-30 [DOI] [PubMed] [Google Scholar]
- 6.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 200528(7):885-890 [DOI] [PubMed] [Google Scholar]
- 7.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002109(4):704-712 [DOI] [PubMed] [Google Scholar]
- 8.Rosen CL, Palermo TM, Larkin EK, Redline S. Health-related quality of life and sleep-disordered breathing in children. Sleep. 200225(6):657-666 [PubMed] [Google Scholar]
- 9.Lamm C, Mandeli J, Kattan M. Evaluation of home audiotapes as an abbreviated test for obstructive sleep apnea syndrome (OSAS) in children. Pediatr Pulmonol. 199927(4):267-272 [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992146(5 pt 1):1235-1239 [DOI] [PubMed] [Google Scholar]
- 11.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006117(3):741-753 [DOI] [PubMed] [Google Scholar]
- 12.Chau KW, Ng DK, Kwok KL, et al. Application of videotape in the screening of obstructive sleep apnea in children. Sleep Med. 20089(4):442-445 [DOI] [PubMed] [Google Scholar]
- 13.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004114(3):768-775 [DOI] [PubMed] [Google Scholar]
- 14.Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest. 2010138(3):519-527 [DOI] [PubMed] [Google Scholar]
- 15.Isik U, Ersu RH, Ay P, et al. Prevalence of headache and its association with sleep disorders in children. Pediatr Neurol. 200736(3):146-151 [DOI] [PubMed] [Google Scholar]
- 16.Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg. 2004131(6):827-832 [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Cheuk DK, Lee SL. Clinical evaluation in predicting childhood obstructive sleep apnea. Chest. 2006130(6):1765-1771 [DOI] [PubMed] [Google Scholar]
- 18.Weatherly RA, Mai EF, Ruzicka DL, Chervin RD. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: a survey of practice patterns. Sleep Med. 20034(4):297-307 [DOI] [PubMed] [Google Scholar]
- 19.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 201115(1):19-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 20085(2):242-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 20001(1):21-32 [DOI] [PubMed] [Google Scholar]
- 22.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007133(3):216-222 [DOI] [PubMed] [Google Scholar]
- 23.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 200023(8):1043-1051 [PubMed] [Google Scholar]
- 24.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 19965(4):251-261 [DOI] [PubMed] [Google Scholar]
- 25.Brouilette R, Hanson D, David R, et al. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984105(1):10-14 [DOI] [PubMed] [Google Scholar]
- 26.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998102(3 pt 1):616-620 [DOI] [PubMed] [Google Scholar]
- 27.Spruyt K, Verleye G, Gozal D. Unbiased categorical classification of pediatric sleep disordered breathing. Sleep. 201033(10):1341-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spruyt K, Cluydts R, Verleye GB. Pediatric sleep disorders: exploratory modulation of their relationships. Sleep. 200427(3):495-501 [DOI] [PubMed] [Google Scholar]
- 29.Spruyt K, O’Brien LM, Macmillan Coxon AP, Cluydts R, Verleye G, Ferri R. Multidimensional scaling of pediatric sleep breathing problems and bio-behavioral correlates. Sleep Med. 20067(3):269-280 [DOI] [PubMed] [Google Scholar]
- 30.Spruyt K, Gozal D. Development of pediatric sleep questionnaires as diagnostic or epidemiological tools: a brief review of dos and don’ts. Sleep Med Rev. 201115(1):7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sproson EL, Hogan AM, Hill CM. Accuracy of clinical assessment of paediatric obstructive sleep apnoea in two English centres. J Laryngol Otol. 2009123(9):1002-1009 [DOI] [PubMed] [Google Scholar]
- 32.Verhulst SL, Kadatis AG. In reference to “Is polysomnography required prior to tonsillectomy and adenoidectomy for diagnosis of obstructive sleep apnea versus mild sleep-disordered breathing in children?” Laryngoscope. 2011;121(5):1126-1127 [DOI] [PubMed] [Google Scholar]
- 33.Yellon RF. Is polysomnography required prior to tonsillectomy and adenoidectomy for diagnosis of obstructive sleep apnea versus mild sleep disordered breathing in children?. Laryngoscope. 2010120(5):868-869 [DOI] [PubMed] [Google Scholar]
- 34.de Serres LM, Derkay C, Astley S, Deyo RA, Rosenfeld RM, Gates GA. Measuring quality of life in children with obstructive sleep disorders. Arch Otolaryngol Head Neck Surg. 2000126(12):1423-1429 [DOI] [PubMed] [Google Scholar]
- 35.Franco RA, Jr, Rosenfeld RM, Rao M. First place—resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000123(1 pt 1):9-16 [DOI] [PubMed] [Google Scholar]
- 36.Li AM, Cheung A, Chan D, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol. 200641(12):1153-1160 [DOI] [PubMed] [Google Scholar]
- 37.Goodwin JL, Babar SI, Kaemingk KL, et al. ; Tucson Children’s Assessment of Sleep Apnea Study Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Chest. 2003124(1):196-203 [DOI] [PubMed] [Google Scholar]
- 38.Preutthipan A, Chantarojanasiri T, Suwanjutha S, Udomsubpayakul U. Can parents predict the severity of childhood obstructive sleep apnoea?. Acta Paediatr. 200089(6):708-712 [DOI] [PubMed] [Google Scholar]
- 39.Tauman R, O’Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 200427(2):274-278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement