Abstract
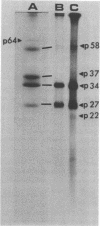
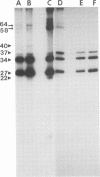
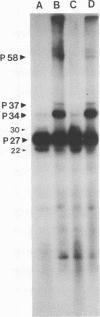
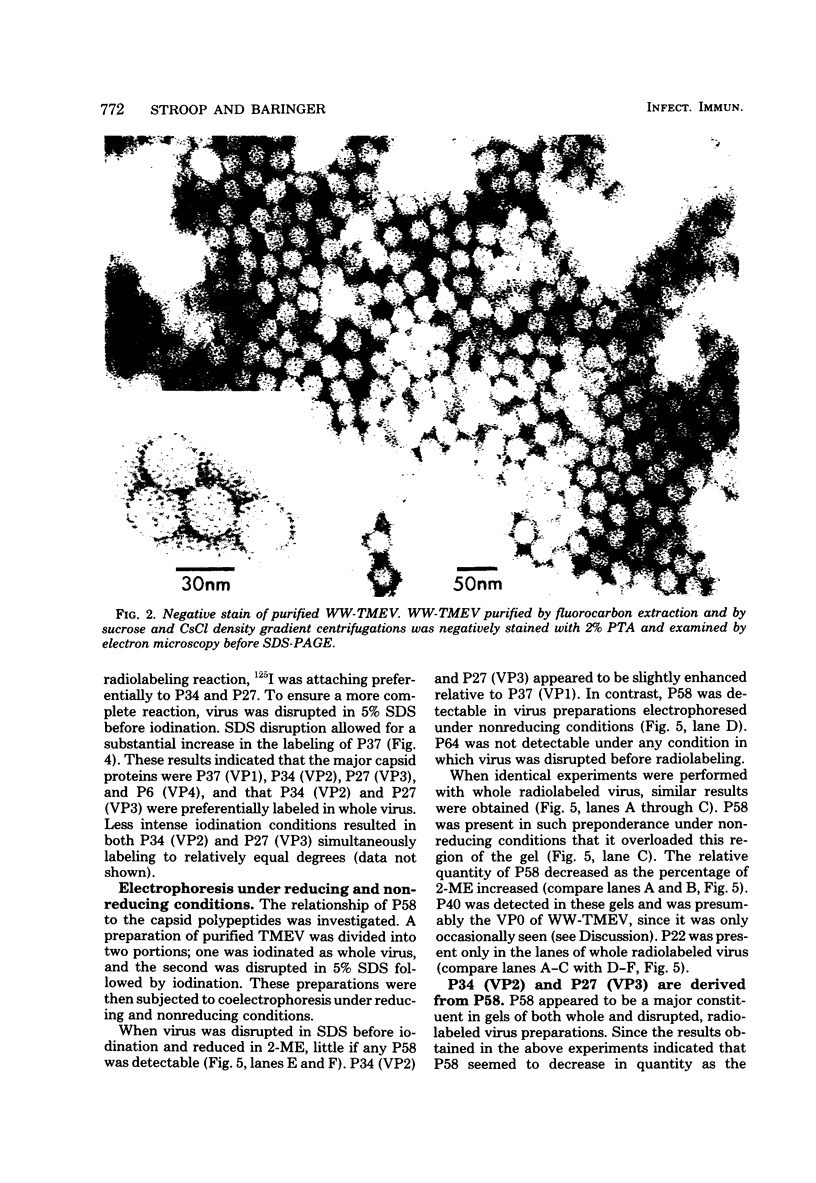
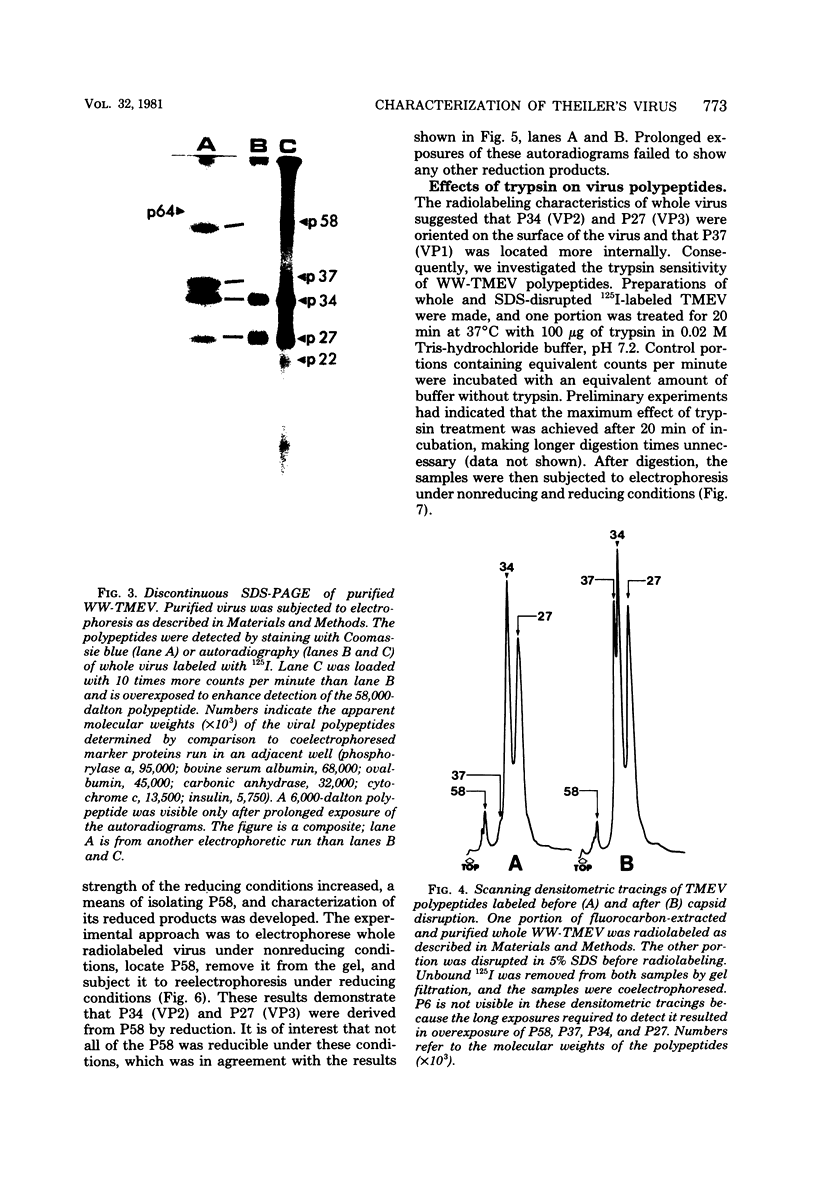
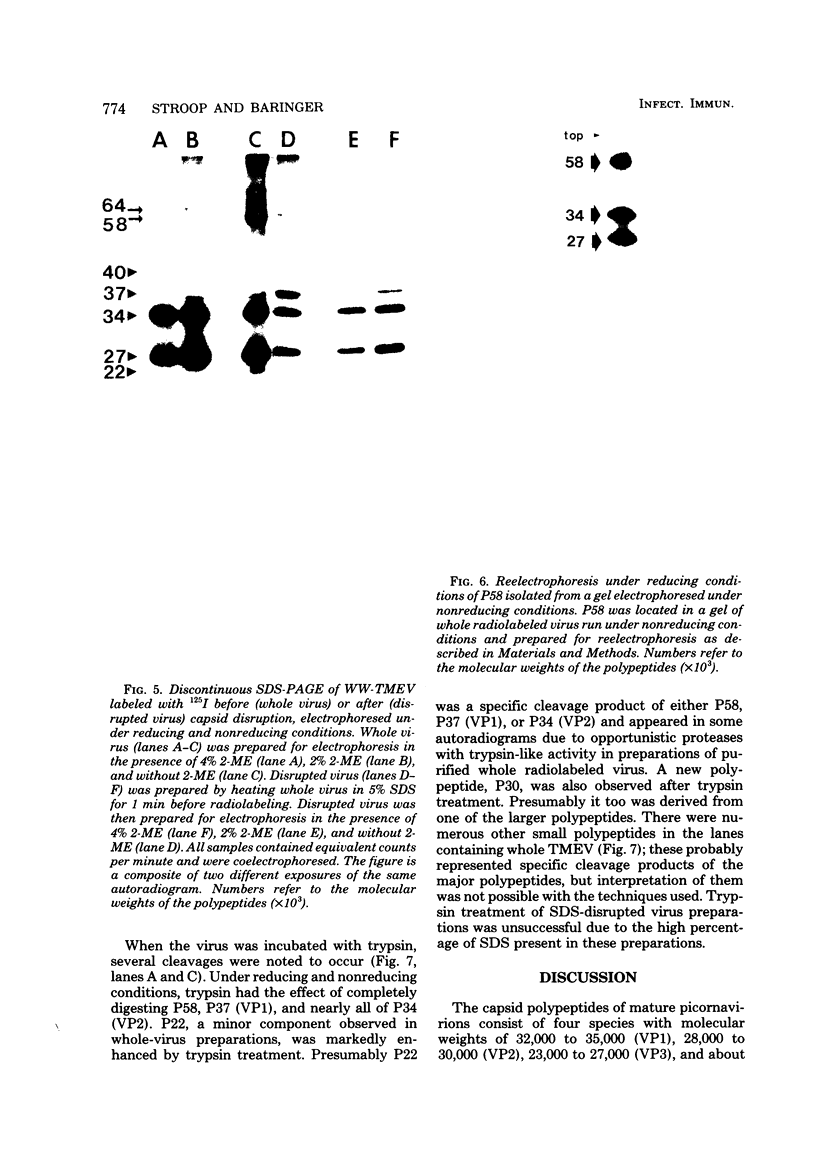
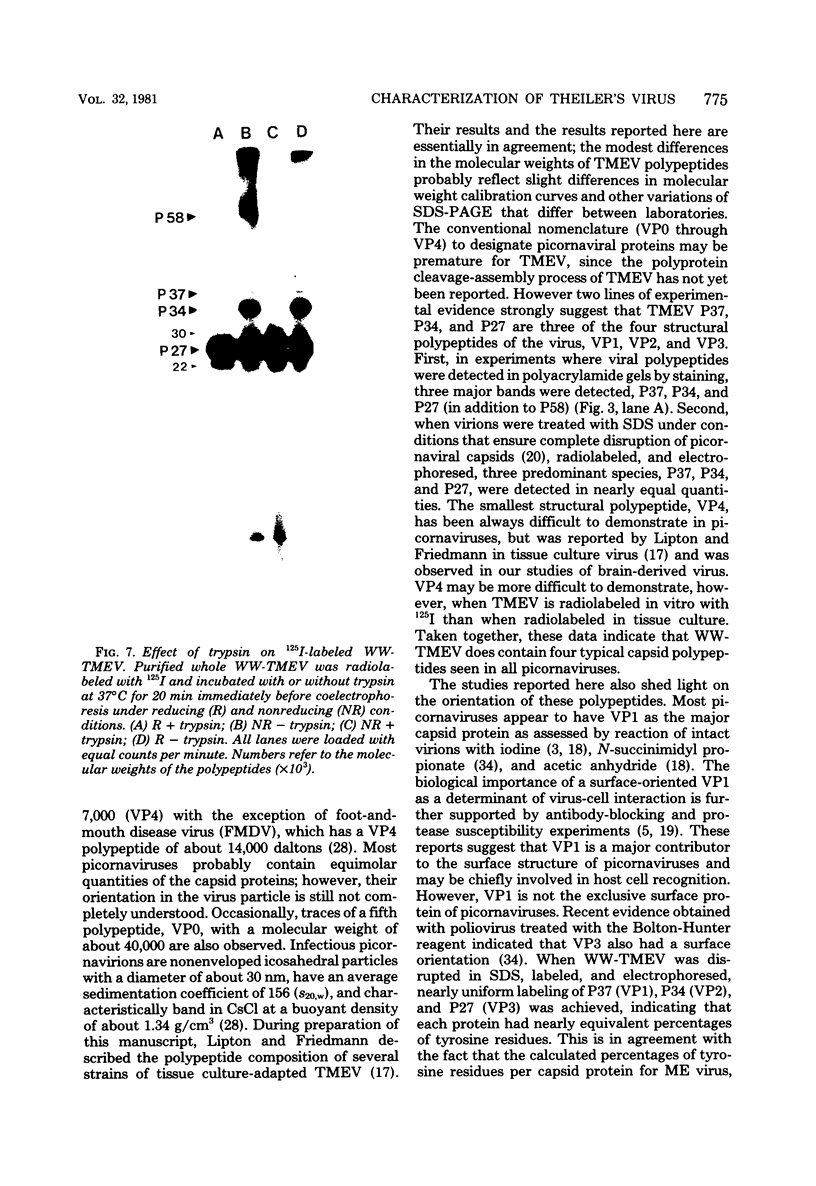
The WW strain of Theiler's murine encephalomyelitis virus (WW-TMEV) was purified from homogenates of acutely infected mouse brain. Infectious WW-TMEV was found to have an estimated sedimentation coefficient of 156 (s20,w) and a density of 1.35 g/cm3 in CsCl. Electron microscopy revealed a homogeneous population of 26-nm nonenveloped particles. Iodination of sodium dodecyl sulfate (SDS)-disrupted virions revealed four major capsid proteins with molecular weights of 58,000, 37,000, 34,000, and 27,000. A 6,000-dalton polypeptide was observed after long exposures of autoradiograms. The 37,000-, 24,000-, 27,000-, and 6,000-dalton polypeptides corresponded to picornaviral VP1, VP2, VP3, and VP4 capsid polypeptides, respectively. Comparison of autoradiograms of virions radiolabeled before and after SDS disruption indicated that the 58,000-dalton protein, VP2, and VP3 preferentially bound 125I under the labeling conditions used. Direct evidence was obtained that VP2 and VP3 were derived from the 58,000-dalton polypeptide by isolation of the 58,000-dalton polypeptide from polyacrylamide gels run under nonreducing conditions and subjecting it to reelectrophoresis under reducing conditions. The effect of trypsin on purified virions and their polypeptides was also investigated. Trypsin-sensitive sites were found in the 58,000-dalton protein, VP1, and VP2. Our results indicate that, in addition to the four typical picornaviral capsid polypeptides, there is a 58,000-dalton polypeptide present in WW-TMEV, which is sensitive to trypsin and can be reduced into two of the capsid proteins, VP2 and VP3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODIAN D. AN ELECTRON-MICROSCOPIC STUDY OF THE MONKEY SPINAL CORD. I. FINE STRUCTURE OF NORMAL MOTOR COLUMN. II. EFFECTS OF RETROGRADE CHROMATOLYSIS. III. CYTOLOGIC EFFECTS OF MILD AND VIRULENT POLIOVIRUS INFECTION. Bull Johns Hopkins Hosp. 1964 Jan;114:13–119. [PubMed] [Google Scholar]
- Beneke T. W., Habermehl K. O., Diefenthal W., Buchholz M. Iodination of poliovirus capsid proteins. J Gen Virol. 1977 Feb;34(2):387–390. doi: 10.1099/0022-1317-34-2-387. [DOI] [PubMed] [Google Scholar]
- CASALS J. IMMUNOLOGICAL CHARACTERIZATION OF VILYUISK HUMAN ENCEPHALOMYELITIS VIRUS. Nature. 1963 Oct 26;200:339–341. doi: 10.1038/200339a0. [DOI] [PubMed] [Google Scholar]
- Carthew P., Martin S. J. The iodination of bovine enterovirus particles. J Gen Virol. 1974 Sep;24(3):525–534. doi: 10.1099/0022-1317-24-3-525. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Sangar D. V., Rowlands D. J., Brown F. Immunogenic and cell attachment sites of FMDV: further evidence for their location in a single capsid polypeptide. J Gen Virol. 1977 Apr;35(1):149–158. doi: 10.1099/0022-1317-35-1-149. [DOI] [PubMed] [Google Scholar]
- Cowan K. M., Graves J. H. A third antigenic component associated with foot-and-mouth disease infection. Virology. 1966 Nov;30(3):528–540. doi: 10.1016/0042-6822(66)90128-0. [DOI] [PubMed] [Google Scholar]
- DANIELS J. B., PAPPENHEIMER A. M., RICHARDSON S. Observations on encephalomyelitis of mice (DA strain). J Exp Med. 1952 Dec;96(6):517–530. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWES D. W., MELNICK J. L., REISSIG M. Sequence of morphological changes in epithelial cell cultures infected with poliovirus. J Exp Med. 1956 Sep 1;104(3):289–304. doi: 10.1084/jem.104.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR J. A. Studies on certain viruses isolated in the tropics of Africa and South America; immunological reactions as determined by cross complement-fixation tests. J Immunol. 1952 Apr;68(4):461–472. [PubMed] [Google Scholar]
- Kew O. M., Pallansch M. A., Omilianowski D. R., Rueckert R. R. Changes in three of the four coat proteins of oral polio vaccine strain derived from type 1 poliovirus. J Virol. 1980 Jan;33(1):256–263. doi: 10.1128/jvi.33.1.256-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Halperen S. Electrophoretic analysis of capsid and non-capsid polypeptides of echovirus 12, and selective inhibtion of the formation of virus particles by actinomycin D. J Gen Virol. 1975 Mar;26(3):239–248. doi: 10.1099/0022-1317-26-3-239. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Characterization of the TO strains of Theiler's mouse encephalomyelitis viruses. Infect Immun. 1978 Jun;20(3):869–872. doi: 10.1128/iai.20.3.869-872.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. The TO strains of Theiler's viruses cause "slow virus-like" infections in mice. Ann Neurol. 1979 Jul;6(1):25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Friedmann A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980 Mar;33(3):1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Ziola B. R., Salmi A., Scraba D. G. Structure of the Mengo virion. V. Distribution of the capsid polypeptides with respect to the surface of the virus particle. Virology. 1977 May 1;78(1):35–44. doi: 10.1016/0042-6822(77)90076-9. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Milstien J. B., Walker J. R., Eron L. J. Correlation of virus polypeptide structure with attenuation of poliovirus type 1. J Virol. 1977 Sep;23(3):811–815. doi: 10.1128/jvi.23.3.811-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatnick J. Isolation of a foot-and-mouth disease polyuridylic acid polymerase and its inhibition by antibody. J Virol. 1980 Feb;33(2):774–779. doi: 10.1128/jvi.33.2.774-779.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS F. C., ENDERS J. F., WELLER T. H. Cytopathogenic effect of poliomyelitis viruses in vitro on, human embryonic tissues. Proc Soc Exp Biol Med. 1950 Nov;75(2):370–374. doi: 10.3181/00379727-75-18202. [DOI] [PubMed] [Google Scholar]
- SPEIR R. W., ALIMINOSA K. V., SOUTHAM C. M. Differences in infectivity of neurotropic viruses incubated in distilled water, isotonic NaCI and hypertonic NaCI. Proc Soc Exp Biol Med. 1962 Jan;109:80–82. doi: 10.3181/00379727-109-27109. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Cavanagh D., Brown F. Characterization of the minor polypeptides in the foot-and-mouth disease particle. J Gen Virol. 1976 Apr;31(1):35–46. doi: 10.1099/0022-1317-31-1-35. [DOI] [PubMed] [Google Scholar]
- Theiler M. SPONTANEOUS ENCEPHALOMYELITIS OF MICE--A NEW VIRUS DISEASE. Science. 1934 Aug 3;80(2066):122–122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J Gen Virol. 1979 Aug;44(2):525–534. doi: 10.1099/0022-1317-44-2-525. [DOI] [PubMed] [Google Scholar]
- Wroblewska Z., Gilden D. H., Wellish M., Rorke L. B., Warren K. G., Wolinsky J. S. Virus-specific intracytoplasmic inclusions in mouse brain produced by a newly isolated strain of Theiler virus. I. Virologic and morphologic studies. Lab Invest. 1977 Dec;37(6):595–602. [PubMed] [Google Scholar]