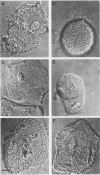
Abstract
The binding to mammalian cells of piliated enteric bacteria and the inhibition of the binding by antibodies to purified pili were studied. The target cells were epithelial cells from human bucca and human and rat urinary tracts, erythrocytes from various species, and Saccharomyces cerevisiae cells. The strains were selected to represent the two main agglutination patterns of enteric bacteria: mannose-resistant agglutination of human and other erythrocytes and mannose-sensitive agglutination of guinea pig and other erythrocytes. Escherichia coli 3669 caused only mannose-resistant agglutination, E. coli 6013 caused only mannose-sensitive agglutination, and E. coli 3048 caused both types of agglutination simultaneously. Salmonella typhimurium SH6749 exhibited only mannose-sensitive hemagglutination and was included to allow comparison of its pili with those of E. coli strains. The range of epithelial cells to which the bacteria adhered was related to their agglutination patterns. All four strains attached to human buccal cells. Only E. coli strains 3669 and 3048, which caused mannose-resistant agglutination, adhered to human urinary tract epithelial cells, and only those strains that caused mannose-sensitive agglutination adhered to rat urinary tract epithelial cells. The binding of S. typhimurium SH6749, but not of the other strains with mannose-sensitive agglutination, was significantly inhibited by d-mannose. Globotetraosylceramide, a glycolipid present in the human urinary tract epithelium, inhibited attachment to human uroepithelial cells of the two strains with mannose-resistant hemagglutination. As tested by the enzyme-linked immunosorbent assay, cross-reactions between type 1 pili of the E. coli strains were strong, but those between S. typhimurium and E. coli mannose-sensitive pili were weak. The two pili that induced mannose-resistant hemagglutination on E. coli did not cross-react. Significant inhibition of adhesion of all four strains was obtained with the homologous anti-pilus antiserum. The binding of bacteria to mammalian cells may thus be mediated by several types of bacterial pili reacting with different receptors on mammalian cells.
Full text
PDF

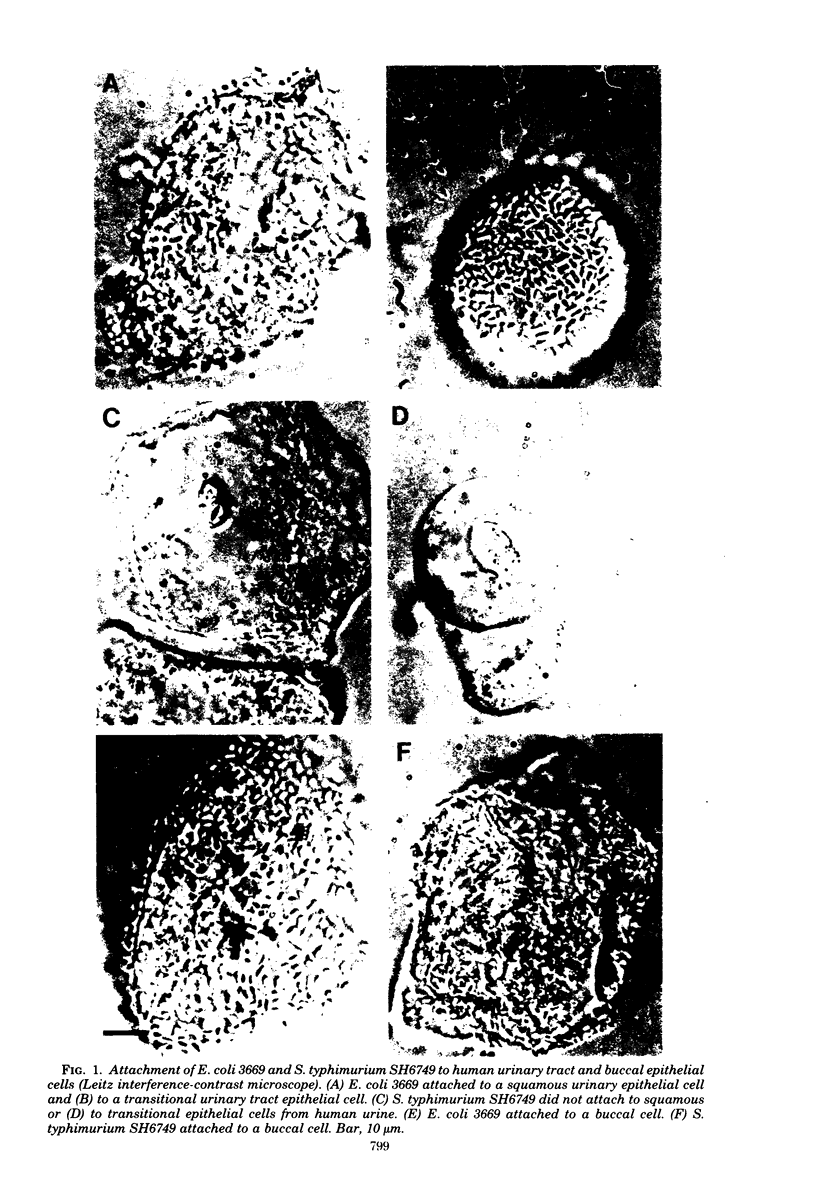




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Campbell I. Antigens of the type-1 fimbriae of salmonellae and other enterobacteria. J Med Microbiol. 1969 Nov 4;2(4):535–553. doi: 10.1099/00222615-2-4-535. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Duguid J. P. The function of bacterial fimbriae. Arch Immunol Ther Exp (Warsz) 1968;16(2):173–188. [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Lounatmaa K., Ranta H., Kuusi N. Characterization of type 1 pili of Salmonella typhimurium LT2. J Bacteriol. 1980 Nov;144(2):800–805. doi: 10.1128/jb.144.2.800-805.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., Kawanami J., Li Y. T., Hakomori S. Structures of ceramide tetrasaccharides from various sources: uniqueness of rat kidney ceramide tetrasaccharide. J Lipid Res. 1972 Sep;13(5):657–662. [PubMed] [Google Scholar]
- Svanborg-Edén C., Svennerholm A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978 Dec;22(3):790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G., Freer J. H. Location of binding sites on common type 1 fimbriae from Escherichia coli. J Gen Microbiol. 1979 Jun;112(2):321–328. doi: 10.1099/00221287-112-2-321. [DOI] [PubMed] [Google Scholar]