Abstract
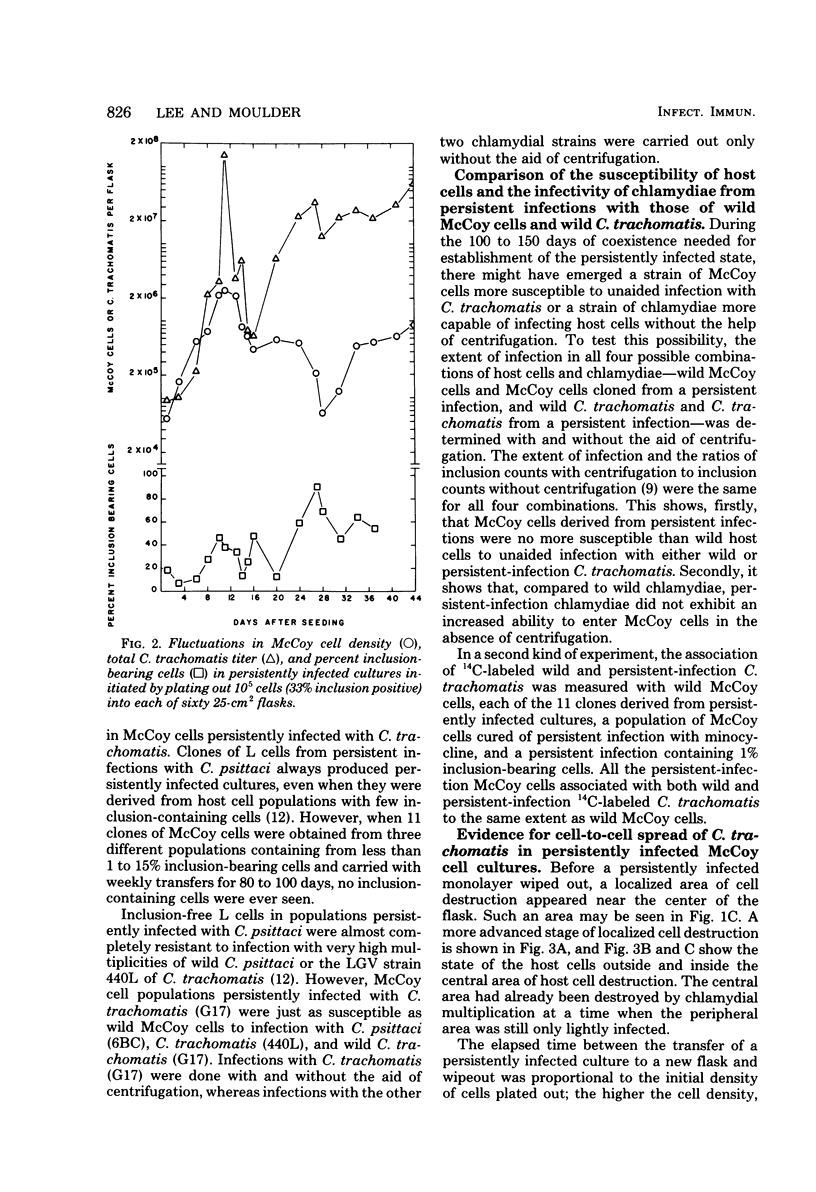
An in vitro model of persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain (G17) of Chlamydia trachomatis has been developed. Persistently infected cultures were established by infecting McCoy cells with high multiplicities of chlamydiae. After the first cycle of chlamydial replication, the host cells multiplied more rapidly than the parasites, so that the fraction of inclusion-bearing cells declined to less than 1%. However, after 100 days, the proportion of inclusion-bearing cells rose dramatically, and the cultures alternated between periods of massive host cell destruction by chlamydiae and periods of host cell proliferation. This cycle continued indefinitely as host cell and parasite densities fluctuated periodically. The chlamydiae in the cycling populations were reidentified as the original serotype. No changes in either host cell susceptibility or chlamydial invasiveness were observed in hosts and parasites recovered from persistently infected populations. All evidence suggests that the parasite maintained itself in McCoy cell populations by cell-to-cell transfer and that an equilibrium between host and parasite multiplication was achieved when the persistently infected cultures fluctuated between periods of host cell destruction and proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. R., Wang S. P., Grayston J. T. Further classification of TRIC agents from ocular trachoma and other sources by the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1469–1478. doi: 10.1016/0002-9394(67)94133-5. [DOI] [PubMed] [Google Scholar]
- Bowie W. R., Lee C. K., Alexander E. R. Prediction of efficacy of antimicrobial agents in treatment of infections due to Chlamydia trachomatis. J Infect Dis. 1978 Nov;138(5):655–659. doi: 10.1093/infdis/138.5.655. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., MANIRE G. P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1961 Apr;86:382–385. [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. ISOLATION OF THE TRACHOMA AGENT IN CELL CULTURE. Proc Soc Exp Biol Med. 1965 Feb;118:354–359. doi: 10.3181/00379727-118-29841. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K. Interaction between a trachoma strain of Chlamydia trachomatis and mouse fibroblasts (McCoy cells) in the absence of centrifugation. Infect Immun. 1981 Feb;31(2):584–591. doi: 10.1128/iai.31.2.584-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIRE G. P., GALASSO G. J. Persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1959 Nov;83:529–533. [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFICER J. E., BROWN A. Serial changes in virus and cells in cultures chronically infected with psittacosis virus. Virology. 1961 May;14:88–99. doi: 10.1016/0042-6822(61)90136-2. [DOI] [PubMed] [Google Scholar]
- SOWA J., COLLIER L. H. Isolation of trachoma virus from patients in West Africa. J Hyg (Lond) 1960 Mar;58:99–108. doi: 10.1017/s0022172400038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




