Abstract
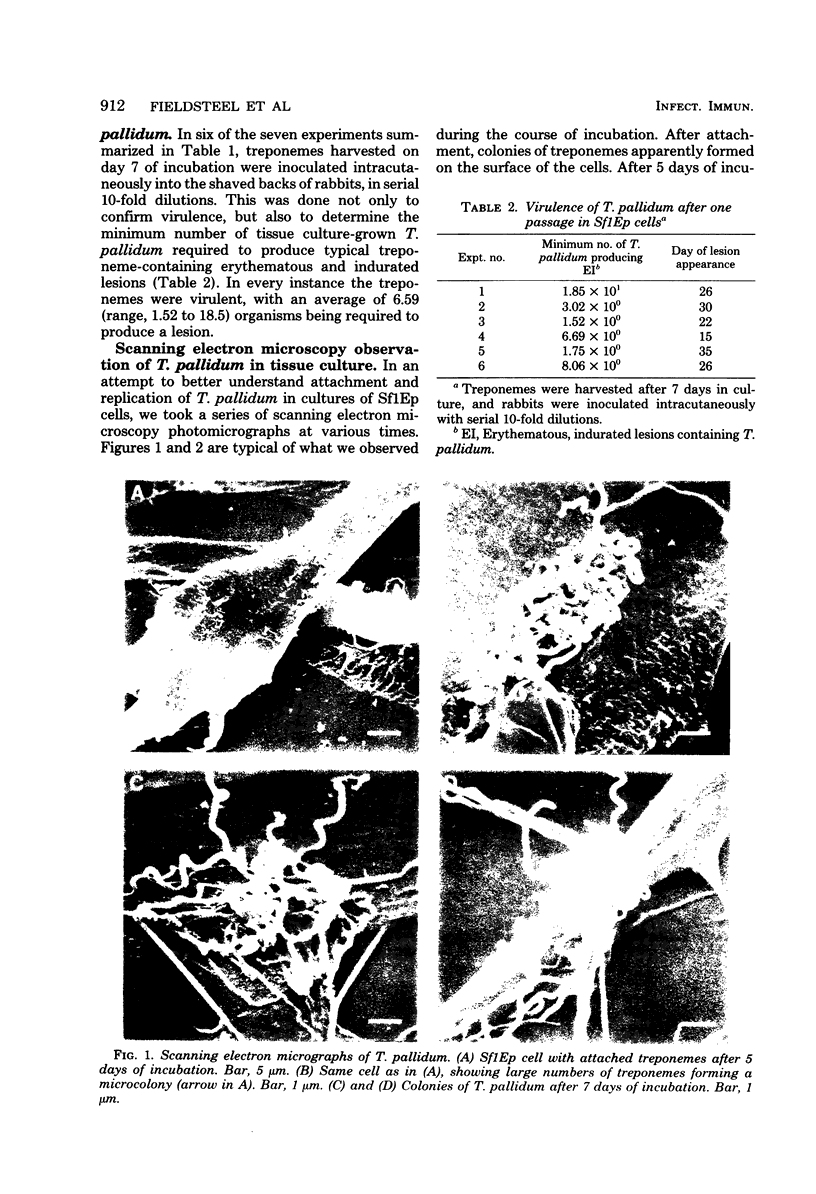
In a series of seven experiments, the virulent Nichols strain of Treponema pallidum was shown to attach and replicate on the surface of tissue culture cells of cottontail rabbit epithelium (Sf1Ep) growing in conventional monolayer cultures under an atmosphere of 1.5% oxygen. Five days after inoculation of 106T. pallidum, the number of treponemes had increased to between 8 × 106 and 2.59 × 107. The viability of harvested organisms ranged from 86 to 97%. The number of T. pallidum continued to increase, generally reaching a plateau between days 9 and 12 of incubation, with increases ranging up to 100-fold and averaging 49-fold. There appeared to be a ceiling of multiplication of about 2 × 108 irrespective of the inoculum, which ranged from 106 to 108T. pallidum. Concurrent deoxyribonucleic acid assays were performed on the cultures containing T. pallidum to obtain further evidence of replication. Significant increases in treponemal deoxyribonucleic acid were observed when the inocula ranged from 106 to 107, with the greatest increases, as might be expected, being in the former group. There was also excellent correlation in the amount of deoxyribonucleic acid per treponeme; the averages for the 106, 2.5 × 106, and 107 groups were 3.46 × 10−14, 3.28 × 10−14, and 2.79 × 10−14 g per treponeme, respectively (3.14 ± 0.72 × 10−14 g per treponeme). In each experiment, organisms were harvested from the group inoculated with 106T. pallidum after 7 days of incubation to test for virulence. In all instances, the organisms were virulent; erythematous, indurated, treponeme-containing lesions were produced from an average of six to seven organisms. Scanning electron microscopy revealed that during the course of replication many microcolonies of treponemes formed on the surface of the cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARK J. W., Jr Loss of virulence in vitro of motile Treponema pallidum. Br J Vener Dis. 1962 Jun;38:78–81. doi: 10.1136/sti.38.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Barber M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 1974 Jul;10(1):123–127. doi: 10.1128/iai.10.1.123-127.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Becker F. A., Stout J. G. Prolonged survival of virulent Treponema pallidum (Nichols strain) in cell-free and tissue culture systems. Infect Immun. 1977 Oct;18(1):173–182. doi: 10.1128/iai.18.1.173-182.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Stout J. G., Becker F. A. Comparative behavior of virulent strains of Treponema pallidum and Treponema pertenue in gradient cultures of various mammalian cells. Infect Immun. 1979 May;24(2):337–345. doi: 10.1128/iai.24.2.337-345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Stout J. G., Becker F. A. Role of serum in survival of Treponema pallidum in tissue culture. In Vitro. 1981 Jan;17(1):28–32. doi: 10.1007/BF02618027. [DOI] [PubMed] [Google Scholar]
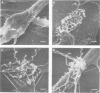
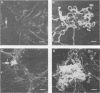
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Sykes J. A., Miller J. N. Interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells: effects of oxygen, reducing agents, serum supplements, and different cell types. Infect Immun. 1977 Feb;15(2):444–452. doi: 10.1128/iai.15.2.444-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Kellogg D. S., Clark J. W., Balows A. The in vitro cultivation of Treponema pallidum. Corroborative studies. Br J Vener Dis. 1977 Dec;53(6):338–339. doi: 10.1136/sti.53.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLIES N. E., ALPER T. The nucleic acid content of Escherichia coli strains B and B/r. Biochim Biophys Acta. 1960 Sep 23;43:182–187. doi: 10.1016/0006-3002(60)90428-5. [DOI] [PubMed] [Google Scholar]
- HODSON P. H., BECK J. V. Origin of deoxyribonucleic acid of the bacterial endospore. J Bacteriol. 1960 May;79:661–665. doi: 10.1128/jb.79.5.661-665.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Finn M. A., Thomas J. J., Folger C. Growth and subculture of pathogenic T. pallidum (Nichols strain) in BHK-21 cultured tissue cells. Br J Vener Dis. 1976 Feb;52(1):18–23. doi: 10.1136/sti.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lysko P. G., Cox C. D. Terminal electron transport in Treponema pallidum. Infect Immun. 1977 Jun;16(3):885–890. doi: 10.1128/iai.16.3.885-890.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M., Matthews H. M., Roberts M. S. Unsustained multiplication of treponema pallidum (nichols virulent strain) in vitro in the presence of oxygen. Infect Immun. 1978 Feb;19(2):421–429. doi: 10.1128/iai.19.2.421-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Knight S. T., Jenkin H. M. Examination of various cell culture techniques for co-incubation of virulent Treponema pallidum (Nichols I strain) under anaerobic conditions. J Clin Microbiol. 1976 Oct;4(4):360–371. doi: 10.1128/jcm.4.4.360-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER M. M. Factors influencing the in vitro survival of Treponema pallidum. Am J Hyg. 1960 May;71:401–417. doi: 10.1093/oxfordjournals.aje.a120123. [DOI] [PubMed] [Google Scholar]