Abstract
The Réunion grey white-eye (Zosterops borbonicus) is a single-island endemic passerine bird that exhibits striking geographically structured melanic polymorphism at a very small spatial scale. We investigated the genetic basis of this color polymorphism by testing whether the melanocortin-1 receptor (MC1R), a gene often involved in natural melanic polymorphism in birds, was associated with the observed plumage variation. Although we found three non-synonymous mutations, we detected no association between MC1R variants and color morphs, and the main amino-acid variant found in the Réunion grey white-eye was also present at high frequency in the Mauritius grey white-eye (Zosterops mauritianus), its sister species which shows no melanic polymorphism. In addition, neutrality tests and analysis of population structure did not reveal any obvious pattern of positive or balancing selection acting on MC1R. Altogether these results indicate that MC1R does not play a role in explaining the melanic variation observed in the Réunion grey white-eye. We propose that other genes such as POMC, Agouti or any other genes involved in pigment synthesis will need to be investigated in future studies if we are to understand how selection shapes complex patterns of melanin-based plumage pigmentation.
Trial Registration
All sequences submitted to Genbank. Accession number: JX914505 to JX914564.
Introduction
The genetic basis and origin of color polymorphism in natural populations is a classic theme in our understanding of ultimate and proximate causes of phenotypic variation and evolution [1]. In vertebrates, the study of melanic coloration has led to the characterization of important target genes that may underlie phenotypic variation and divergence in natural populations [2], [3]. One recurrent result emerging from most studies is the involvement of the melanocortin-1 receptor (MC1R) coding region in explaining variation in melanism, sometimes showing shared mutations due to convergent evolution between distantly related species [4]. In birds, associations between dark coloration and mutations in MC1R have been highlighted in a number of wild species (Table 1), including snow geese [5], fairy-wrens [6], bananaquits [7], swans [8], falcons [9], Acrocephalus warblers [10] and Monarcha flycatchers [11]. MC1R has been shown to play an important role in a variety of processes such as sexual selection [5], [11], [12], crypsis [13] and possibly immunity [9] although it is generally considered to have few pleiotropic effects [2]. While most studies have focused on functional substitutions in MC1R coding region in species displaying discrete color dimorphism, few have tried to examine amino acid variation in species with diverse melanin-based patterns of plumage pigmentation (but see [12], [14]). For instance, in studies of the blue-crowned manakin (Lepidothrix coronata) [15] which displays a gradation in melanic coloration according to geography, or the Old World leaf warblers (Phylloscopus sp.) in which there is interspecific variation in unmelanized plumage pattern elements [14], no association between the degree of melanism and nucleotide variation at MC1R could be found.
Table 1. Summary of major patterns of melanic variation in birds and their link with MC1R.
| Mutation | Phenotype | Species studied | References |
| Glu92→Lys92 | Extensive black (black plumage). Less marked in quail. Dominant. | Chicken (Gallus gallus) Japanese quail (Coturnix japonica) Bananaquit (Coereba flaveola) Tahiti Reed Warbler (Acrocephalus caffer) | [7], [10], [31], [39] |
| Ala16→ Thr16, Ile38→Asn38, Ile111→Val111, Gln157→Arg157, Val166→Ile166 | Ala16→ Thr16, Ile38→Asn38, Ile111→Val111, Gln157→Arg157, found associated with the mainland (blue) phenotype. Val166→Ile166 found associated with melanic phenotypeand is dominant. | White-winged Fairywren (Malurus leucopterus) | [6] |
| Val85→Met85 | Different amounts of grey or brown (heterozygous) to completely dark (homozygous). | Lesser snow goose (Chen c. caerulescens), Red-footed boobies (Sula sula) | [5], [40] |
| Glu100→Lys100 | Associated with neck melanism.Found withGlu92→Lys92. | Black-necked Swan (Cygnus melanocoryphus) | [8] |
| Deletion 114-117 | Dark plumage. Dominant. | Eleonora’s Falcon (Falco eleonorae) | [9] |
| Asp119→Asn119 | Black plumage. Dominant. | Chestnut-bellied Monarch from Ugi island (Monarcha castaneiventris) | [11] |
| His215→Pro215 | Alteration of light stripes on back and dorsal head. Associated with Glu92→Lys92. Recessive. | Chicken (Gallus gallus) | [31] |
| Arg230→His230 | Grey (heterozygous) to black (homozygous) plumages.No melanism in Coscoroba coscoroba. | Arctic skua (Stercorarius parasiticus), Black Swan (Cygnus atratus), Coscoroba Swan (Coscoroba coscoroba) | [5], [8] |
| Deletion 256 | Causes melanism. Wild allele is dominant. | Guinea fowl (Numida meleagris) | [41] |
| No mutation linked to phenotype | Black plumage. | Chestnut-bellied Monarch from Three Sisters Islands (Monarcha castaneiventris) | [11] |
| No mutation linked to phenotype | Geographically structured gradation from green to black plumage. | Blue-crowned manakin (Lepidothrix coronata) | [15] |
| No mutation linked to phenotype | Variation in melanization in wing bars, crown stripe and rump patches. | Old World leaf warblers (genus Phylloscopus) | [14] |
| No mutation linked to phenotype | Variation in the extent of phaeomelanin depositionacross the body. | Réunion Grey White-Eye (Zosterops borbonicus) | This study |
In this study, we assess whether MC1R could explain variation in melanistic patterns in the Réunion grey white-eye, Zosterops borbonicus, a species composed of four distinct plumage morphs on the topographically and ecologically complex island of Réunion (Mascarene archipelago). This species provides an excellent system because its prominent plumage color polymorphism stands in stark contrast to the single morph found in its sister species, Z. mauritianus [16], [17] and variation in plumage color among morphs, while conspicuous, is relatively complex in terms of melanin pigmentation patterns, with a completely brown morph, a completely grey morph, a grey-headed brown morph, and a grey-headed brown morph with a brown nape. The morphs occupy discrete geographic entities, with the exception of the brown and grey morphs that are completely sympatric at high altitudes (see [16], [17] for details). Hybrid zones arise where morphs come into contact, as happens between parapatric morphs. In contrast, there appears to be no assortative mating with regards to morph color in the area of sympatry between grey and brown morphs (unpublished data). Patterns of coloration among morphs are stable over time, with no sex effect [17]. Brown parts involve deposition of phaeomelanin in feather barbs and eumelanin deposition in barbules, while grey parts involve low deposition of phaeomelanin [17].
Although to date MC1R has not been associated with phaeomelanin variation in the presence of eumelanin, its central position in controlling the production of both eumelanin and phaeomelanin [18] makes it a relevant candidate in explaining at least partly this plumage color polymorphism.
The main aims of this study are to ask whether there is an association between mutations in MC1R and color variation in Z.borbonicus. First, we examined nucleotide variation in the coding region of MC1R and assessed whether mutations were associated with patterns of variation in melanin pigmentation. Secondly, we investigated whether natural selection could have shaped the pattern of nucleotide variation among morphs. Third, we asked whether sequence variation in MC1R coding region could be due to hitch-hiking to positively selected cis-regulatory mutations by examining whether color morph was associated with patterns of genetic differentiation.
Methods
Sampling
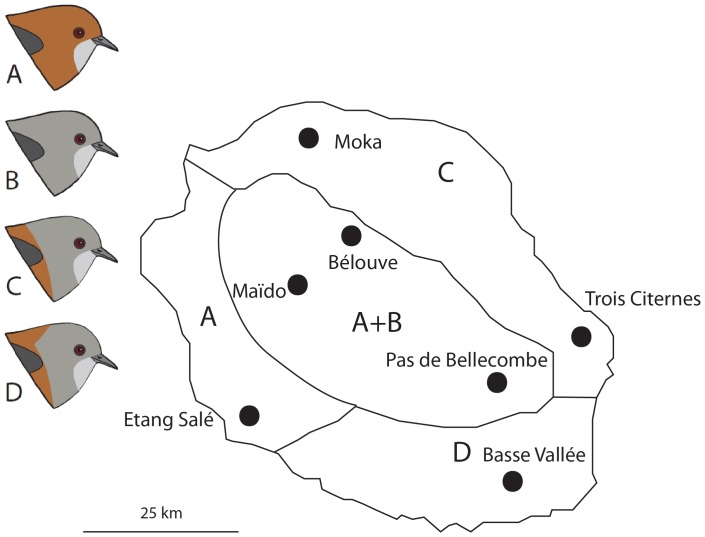
Blood samples used for DNA extraction were collected during field trips at different locations on the islands of Réunion (55°39′E; 21°00′S) and Mauritius (57°33′ E; 20°17′ S) between 2007 and 2009 (Figure 1, Table 2). Birds were captured using mistnets and approximately 10 µL of blood were collected from each bird. Blood was conserved in Queen’s lysis buffer [19] and stored at −20°C for long-term preservation. Morphs were identified by eye in the field, and visual assignments were further confirmed in the laboratory by using pictures taken in the field and on the basis of previous reflectance analysis [20]. With respect to the brown morph, reflectance studies suggested that highland (>1,500 meters high) and lowland forms are distinguishable in terms of coloration, so we analyzed these populations separately. We analyzed a total of 51 individuals from Réunion, including five brown individuals from lowland localities, 15 brown individuals from three highland localities, 13 grey individuals from two localities, eight grey-headed brown individuals from two localities and 10 grey-headed brown-naped brown individuals from one locality (Table 2). For comparison purposes, we also included nine individuals of Z. mauritianus in our analyses.
Figure 1. Map showing Z. borbonicus sampling localities, and distribution of the four morphs on Réunion.
Letters correspond to the different plumage morphs: A: Brown morph; B: Grey morph; C: Grey-headed brown morph; and D: Grey-headed brown-naped brown morph. For a more detailed description of pigmentation phenotypes, see [17]. Adapted from [18].
Table 2. Localities and number of birds sampled on the islands of Réunion and Mauritius.
| Locality/Morph | Sample size |
| Réunion | 51 |
| Brown | 20 |
| Bélouve | 5 |
| Maïdo | 4 |
| Pas de Bellecombe | 6 |
| Etang Salé | 5 |
| Grey | 13 |
| Maïdo | 4 |
| Pas de Bellecombe | 9 |
| Grey-headed brown | 8 |
| Moka | 5 |
| Forêt Mourouvin | 3 |
| Grey-headed brown-naped brown | 10 |
| Basse Vallée | 10 |
| Mauritius | 9 |
DNA Extraction and Amplification
DNA was extracted using a Qiagen® kit, following the manufacturer’s instructions for nucleated blood cells. We amplified a 817-bp fragment of the MC1R coding region, including all sites previously shown to be associated with plumage color change in birds, following [15] for conditions and primers.
Reactions were performed using: 5 µL of 5X buffer (Promega®), 0.5 µL 10 mM dNTPs, 0.125 µL of Taq (5 u/µL, Promega GoTaq® DNA polymerase), 1 µL of each primer (10 µM), 15.4 µL of sterile distilled water, and 2 µL of DNA (∼30 ng of template DNA), totaling 25 µL. The thermocycling profile was as follows: an initial denaturation at 94°C for 60 s, then 40 cycles consisting of a 45-s 94°C denaturation step, a 45-s 62°C annealing step, and a 60-s extension step at 72°C. A final elongation step at 72°C for 5 minutes ended the process. PCR products were visualized on 1% agarose gels. DNA was sequenced in both directions using a 96-well capillary sequencer 3730XL (Applied Biosystems ®) and the same primer pairs used for PCR reactions.
MC1R Sequence Analysis
Sequences were checked and aligned unambiguously by eye. MEGA 5 [21] was used to translate nucleotide sequences to amino-acid sequences. To guard against amplification of pseudogenes, the absence of misplaced stop codons and frame shift mutations was verified for all sequences. We aligned the sequences obtained with MC1R cDNA from chicken (Gallus gallus, Genbank accession number: AY220305) and Zebra finch (Taeniopygia guttata, Ensembl accession number: ENSTGUG00000008024) to detect potential variants at sites previously identified as being associated with melanic variation in other bird species. We noted double peaks at single sites that were approximately half the height of neighboring peaks. Individuals were considered as heterozygous if these double peaks were observed in both strands. To visualize the relationship among haplotypes we constructed a haplotype network using the Network software (http://www.fluxus-engineering.com/sharenet.htm).
Tests for Molecular Signatures of Selection
Despite having relatively similar effects on sequence polymorphism, demography and selection can be distinguished to varying degrees with five of the tests we employed: Tajima’s D [22], Fu’s Fs [23], Fay and Wu’s H [24] and Fu and Li’s D* and F* [25]. Fu and Li’s D* and F* focus on rare alleles and are useful in detecting positive selection in a context of low sequence diversity. Fu’s Fs and Tajima’s D are classical tests of selection focusing either on the distribution of haplotype frequencies relative to neutral expectations (Fu’s Fs) or on the difference between the number of segregating sites and the average number of nucleotide differences (Tajima’s D). We also calculated Fay and Wu’s H, which compares genealogies between and within species and is often presented as less sensitive to demographic events than other tests [24] but see [26]). In the case where positive selection acts on one or several morphs, negative values should be obtained for these tests, especially for tests supposedly more impacted by selection such as Tajima’s D or Fay and Wu’s H [24], [26], [27]. If balancing selection occurs, these tests should display significant positive values. The Japanese white-eye Zosterops japonicus (Genbank accession number JN635726) when necessary, and significance of the tests was assessed by 10,000 coalescent simulations on the basis of segregating sites using DNAsp version 5 [28].
Selection also acts on the ratio of non-synonymous to synonymous mutations. When a coding site is under positive selection, it can limit the appearance of other non-synonymous mutations. To identify putative functionally important sites, we performed the McDonald-Kreitmann test [29] using the Japanese white-eye as an outgroup.
We also examined whether significant differentiation occurred between morphs rather than between populations. This is expected if positive selection acts on a cis-regulatory mutation, as a selective sweep is likely to fix distinct haplotypes between morphs. We obtained differentiation indices using an analysis of molecular variance (AMOVA) with morphs as groups as implemented in Arlequin 3.5 [30]. P-values were obtained by performing 10,000 permutations.
Results
A total of 817 bp of the MC1R gene were successfully sequenced. Eight sites were variable, giving a total of nine different haplotypes. Mutations consisted of four non-synonymous and five synonymous substitutions. Non-synonymous substitutions were an Ala45→Val45, a Val172→ Ile172 and a Pro225→ Ser225 for Z. borbonicus and an Ala228→ Val228 for Z. mauritianus (Table 3).These amino acids all had a hydrophilic lateral chain except for Proline. Moreover, in chicken Ala45 is replaced by a Thr45, having a neutral lateral chain, suggesting this site is less constrained. These substitutions do not seem to modify greatly the chemical properties of the protein and are unlikely to have a large impact on the receptoŕs structure. This was supported by McDonald-Kreitman tests which failed to detect any sign of positive selection on amino acid-altering mutations at MC1R (Table 4).
Table 3. Amino-acids variants observed at the MC1R locus in 51 Z. borbonicus individuals representing the four Réunion morphs and nine Z. mauritianus individuals.
| Variant 1 | Variant 2 | Variant 3 | Variant 4 | |
| Position of the mutation | C134→T134 | G514→A514 | C673→T673 | C683→T683 |
| Amino-acid change | Ala45→Val45 | Val172→ Ile172 | Pro225→ Ser225 | Ala228→ Val228 |
| Brown (lowlands) | Not found | Heterozygous | Not found | Not found |
| Brown (highlands) | Not found | Heterozygous | Not found | Not found |
| Grey | Not found | Heterozygous | Not found | Not found |
| Grey-headed brown | Heterozygous | Not found | Not found | Not found |
| Grey-headed brown-naped brown | Not found | Heterozygous | Heterozygous | Not found |
| Mauritius | Not found | Not found | Not found | Heterozygous |
For each variant the corresponding nucleotide substitution is indicated, with its state (heterozygous or homozygous) in each morph and species studied here. Sequences were numbered in reference to the chicken genome (Genbank accession number: AY220305).
Table 4. Results for McDonald-Kreitman neutrality test.
| Non-synonymous mutations | Synonymous mutations | McDonald-Kreitmann test | |||
| Lowland brown | Fixed | 6 | 3 | NS | |
| Polymorphic | 1 | 1 | |||
| Highland brown | Fixed | 6 | 2 | NS | |
| Polymorphic | 1 | 3 | |||
| Brown (both lowland and highland) | Fixed | 6 | 2 | NS | |
| Polymorphic | 1 | 3 | |||
| Grey | Fixed | 6 | 2 | NS | |
| Polymorphic | 1 | 3 | |||
| Grey-headed brown | Fixed | 6 | 2 | NS | |
| Polymorphic | 1 | 2 | |||
| Grey-headed brown-naped brown | Fixed | 6 | 2 | NS | |
| Polymorphic | 2 | 3 | |||
| Zosterops borbonicus | Fixed | 6 | 2 | NS | |
| Polymorphic | 3 | 4 | |||
| Zosterops mauritianus | Fixed | 6 | 2 | NS | |
| Polymorphic | 1 | 3 | |||
Zosterops japonicus sequence was used as an outgroup. NS: non-significant.
No correlation between these substitutions and variation in pigmentation between Z. borbonicus morphs was detected (Figure 2). We found several shared mutations between Z. borbonicus morphs or between Z.borbonicus and Z. mauritianus, both synonymous and non-synonymous. Since the Mauritian species is monomorphic across its range, these mutations do not seem to be linked to color variation and might instead represent shared ancestral polymorphism.
Figure 2. MC1R non-synonymous variants network for 120 haplotypes from Z. borbonicus and Z. mauritianus.
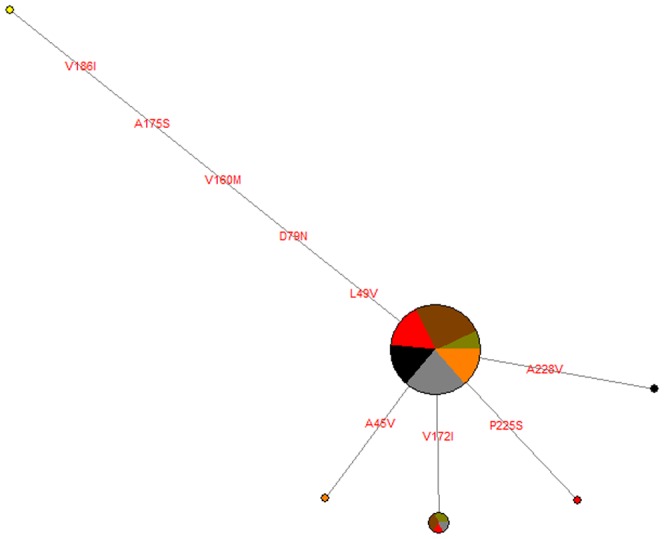
This is a median-joining network. Circles represent variants with areas proportional to their sample sizes. Each branch represents a single substitution with amino-acid position indicated. Proportions of individuals of each locality are indicated by pie charts for each haplotype (black: Z. mauritianus, light brown: lowland brown morph, dark brown: highland brown morph, grey: grey morph, orange: grey-headed brown morph, red: grey-headed brown-naped brown morph, yellow: outgroup, Zosterops japonicus).
Nucleotide diversity was relatively low (π = 0.00078 and 0.00167 for Z. borbonicus and Z. mauritianus respectively). All neutrality tests were skewed towards negative values in all Z. borbonicus morphs (Table 5). However, values were significantly less than zero only for Fs and H values in the brown morphs (both lowland and highland populations), the grey morph and the grey-headed brown morph. Negative Fs values suggest a role for demographic expansion, whereas negative Fay and Wu’s H could be consistent with long-term purifying selection in explaining patterns of variation at MC1R instead of positive selection associated to morphs.
Table 5. Diversity statistics and results from selection tests for Z. borbonicus morphs.
| Morph/Species | π | S | Tajima’s D | Fu and Li’s D* | Fu and Li’s F* | Fu’sFs | Fay and Wu’s H |
| Z.borbonicus | 0.00078 | 7 | −1.227 | −0.561 | −0.929 | −3.927 | −2.562 * |
| Brown | 0.00052 | 4 | −1.304 | −1.103 | −1.355 | −2.658 | −3.300 ** |
| Brown (lowland) | 0.00068 | 2 | −0.691 | −0.280 | −0.423 | −0.594 | −1.333 |
| Brown (highland) | 0.00048 | 4 | −1.574 | −0.968 | −1.329 | −3.219 * | −3.356 ** |
| Grey | 0.00082 | 4 | −0.962 | −0.897 | −1.060 | −1.845 | −2.499 * |
| Grey-headed brown | 0.00084 | 3 | −0.708 | −0.039 | −0.247 | −1.098 | −2.367 * |
| Grey-headed brown-naped brown | 0.00119 | 5 | −0.946 | −0.413 | −0.648 | −2.344 | −1.947 |
Π: nucleotide diversity. S: number of segregating sites. Significance levels:
p<0.05;
p<0.01.
The lack of positive selection associated to morphs was supported by the AMOVA analysis, which did not detect any significant morph effect (φct = 0.013, P>0.05). Since no variation in haplotype frequencies was associated to color morphs, no effect of a selective sweep linked to a putative cis-regulatory mutation could be detected.
Discussion
Despite its frequent involvement in pigmentation patterns in vertebrates, especially in birds (Table 1), MC1R does not seem to play a role in explaining variation in plumage pigmentation in Z. borbonicus. We found no relationship between plumage pigmentation and variation at the MC1R locus, for either synonymous or non-synonymous substitutions, and observed non-synonymous substitutions are unlikely to result in functional changes.
Since we could not sequence the first 23 and last 20 codons of MC1R we cannot exclude the possibility that functional modifications occurred in these regions. However, this seems unlikely since the region examined here contains all the sites previously described as important for MC1R function in birds [5], [7], [11], [31], [32]. We did not find any of the color-associated mutations already reported in previous studies on birds. Substitutions Val85→Met85, Glu92→Lys92 and Asp119→Asn119 [5], [7], [11] observed in bananaquits (Coereba flaveola), snow geese (Anser caerulescens) and the chestnut-bellied monarch (Monarcha castaneiventris) were not observed here. Similarly, other substitutions like Arg230→His230 observed in Arctic skuas (Stercorarius parasiticus) or Glu100→Lys100 reported in swans (Cygnus) were not found in our study [5], [8]. It is difficult to definitively rule out the possibility that MC1R cis-regulatory mutations underlie some pigmentation phenotypes in Z. borbonicus or other species. However, in our study, we found no indication for genetic hitchiking in MC1R coding sequences, as would be expected if they were linked to positively selected regions in nearby locations.
Our results are instead consistent with those obtained by [11], [14], [15]. Indeed, many studies having shown the involvement of MC1R focused on species displaying extreme dimorphism and rarely on variation in patterns of melanin deposition across the body (Table 1). This confirms that MC1R is not systematically involved in melanin-based pigmentation changes in birds, reinforcing the notion that understanding the evolution of plumage coloration in species with complex patterns of eumelanin/phaeomelanin deposition requires a wider exploration of other genes within the melanocortin pathway, as well as variation in other candidate genes. Indeed, several genetic and developmental mechanisms are likely to regulate the complex patterns of pigment deposition in feathers [33], [34] possibly interacting with MC1R regulatory variation, which also need to be characterized.
A potentially interesting candidate gene that may underlie such mechanisms is Agouti (ASIP), a paracrine signaling protein antagonist of MC1R involved in pigment patterning in domestic quail and chicken [35], [36] and in pocket mice [37]. In addition to MC1R, Agouti also interacts with MC3R and MC4R and has pleiotropic effects on food intake, energy expenditure or nociception [2]. Its antagonist, the pro-opiomelanocortin gene (POMC), is also a candidate since it interacts with the entire family of melanocortin receptors (MCRs), including MC1R, and may play a role in controlling many metabolic functions, such as stress resistance, reproductive investment or immunity [2] [38].
Since mutations in ASIP and POMC genes appear to be associated with many physiological, behavioral, and life-history traits, not just color, these two genes seem ideal candidates to understand the origin and evolution of complex melanin-based pigmentation polymorphisms. Yet adaptive changes in the pattern formation of eumelanin and phaeomelanin in Z. borbonicus and probably many other species are likely to involve a mixture of modifications in the structure and regulation of the genes underlying pigment production, suggesting that mechanisms of plumage color evolution may be more diverse than implied by recent studies of discrete melanic/non melanic polymorphisms.
Acknowledgments
Thomas Duval, Ben Warren, Guillaume Gélinaud, Dominique Strasberg, Juli Broggi, Magali Thierry, René-Claude Billot, Jean-Michel Probst, Isabelle Henry,Vincent Leconte, Marc Salamolard, Benoît Lequette, Vikash Tatayah, and field biologists and staff at the Mauritius Wildlife Foundation provided valuable help with fieldwork and logistics. We gratefully acknowledge the Mauritius National Parksand Conservation Services and the Réunion National Park for permission to conduct fieldwork. We also thank Patricia Jargeat and Emeline Lhuillier for much advice in the lab, Hopi Hoekstra and her lab members for kindly sharing their expertise in pigmentation genes and two anonymous reviewers for their useful comments.
Funding Statement
This work was supported by Institut Français de la Biodiversité (IFB) and ANR Biodiversity Program grants to CT, and the “Laboratoire d’Excellence” TULIP (ANR-10-LABX-41). YB was supported by a MESR (Ministère de l’Enseignement Supérieur et de la Recherche) PhD scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hoekstra HE, Coyne JA (2007) The locus of evolution: evo devo and the genetics of adaptation. Evolution 61: 995–1016. [DOI] [PubMed] [Google Scholar]
- 2. Ducrest A-L, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends in Ecology & Evolution 23: 502–510. [DOI] [PubMed] [Google Scholar]
- 3. Kopp A (2009) Metamodels and phylogenetic replication: a systematic approach to the evolution of developmental pathways. Evolution 63: 2771–2789. [DOI] [PubMed] [Google Scholar]
- 4. Römpler H, Rohland N, Lalueza-Fox C, Willerslev E, Kuznetsova T, et al. (2006) Nuclear gene indicates coat-color polymorphism in mammoths. Science 313: 62. [DOI] [PubMed] [Google Scholar]
- 5. Mundy NI, Badcock NS, Hart T, Scribner K, Janssen K, et al. (2004) Conserved genetic basis of a quantitative plumage trait involved in mate choice. Science 303: 1870–1873. [DOI] [PubMed] [Google Scholar]
- 6. Doucet SM, Shawkey MD, Rathburn MK, Mays HL, Montgomerie R (2004) Concordant evolution of plumage colour, feather microstructure and a melanocortin receptor gene between mainland and island populations of a fairy-wren. Proceedings of the Royal Society B: Biological Sciences 271: 1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theron E, Hawkins K, Bermingham E, Ricklefs RE, Mundy NI (2001) The molecular basis of an avian plumage polymorphism in the wild: a melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Current Biology 11: 550–557. [DOI] [PubMed] [Google Scholar]
- 8. Pointer MA, Mundy NI (2008) Testing whether macroevolution follows microevolution: are colour differences among swans (Cygnus) attributable to variation at the MCIR locus? BMC Evolutionary Biology 8: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangoso L, Grande JM, Ducrest A-L, Figuerola J, Bortolotti GR, et al. (2011) MC1R-dependent, melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora’s falcon. Journal of Evolutionary Biology 24: 2055–2063. [DOI] [PubMed] [Google Scholar]
- 10. Cibois A, Thibault J-C, Pasquet E (2012) The molecular basis of the plumage colour polymorphism in the Tahiti reed-warbler Acrocephalus Caffer. Journal of Avian Biology 43: 001–006. [Google Scholar]
- 11. Uy JAC, Moyle RG, Filardi CE, Cheviron ZA (2009) Difference in plumage color used in species recognition between incipient species is linked to a single amino acid substitution in the melanocortin-1 receptor. American Naturalist 174: 244–254. [DOI] [PubMed] [Google Scholar]
- 12. Nadeau NJ, Burke T, Mundy NI (2007) Evolution of an avian pigmentation gene correlates with a measure of sexual selection. Proceedings of the Royal Society B: Biological Sciences 274: 1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullen LM, Hoekstra HE (2008) Natural selection along an environmental gradient: a classic cline in mouse pigmentation. Evolution 62: 1555–1570. [DOI] [PubMed] [Google Scholar]
- 14. MacDougall-Shackleton EA, Blanchard L, Igdoura SA, Gibbs HL (2003) Unmelanized plumage patterns in Old World leaf warblers do not correspond to sequence variation at the melanocortin-1 receptor locus (MC1R). Molecular Biology and Evolution 20: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 15. Cheviron ZA, Hackett SJ, Brumfield RT (2006) Sequence variation in the coding region of the melanocortin-1 receptor gene (MC1R) is not associated with plumage variation in the blue-crowned manakin (Lepidothrix coronata). Proceedings of the Royal Society B: Biological Sciences 273: 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milá B, Warren BH, Heeb P, Thébaud C (2010) The geographic scale of diversification on islands: genetic and morphological divergence at a very small spatial scale in the Mascarene grey white-eye (Aves: Zosterops borbonicus). BMC Evolutionary Biology 10: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill FB (1973) Intra-island variation in the Mascarene White-eye Zosterops borbonica. Ornithological Monographs 12.
- 18. Hubbard JK, Uy JAC, Hauber ME, Hoekstra HE, Safran RJ (2010) Vertebrate pigmentation: from underlying genes to adaptive function. Trends in Genetics 26: 231–239. [DOI] [PubMed] [Google Scholar]
- 19. Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Canadian Journal of Zoology 69: 82–90. [Google Scholar]
- 20.Cornuault J (2008) Divergence due à la sélection dans une aire géographique restreinte: Etude de la variation phénotypique chez Zosterops borbonicus. Msc thesis, University Paul Sabatier, Toulouse, France.
- 21. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fay JC, Wu CI (2000) Hitchhiking under positive Darwinian selection. Genetics 155: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133: 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Przeworski M (2002) The signature of positive selection at randomly chosen loci. Genetics 160: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simonsen KL, Churchill GA, Aquadro CF (1995) Properties of statistical tests of neutrality for DNA polymorphism data. Genetics 141: 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 29. McDonald JH, Kreitman M (1991) Adaptive protein evolution at the Adh locus in Drosophila. Nature 351: 652–654. [DOI] [PubMed] [Google Scholar]
- 30. Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. [DOI] [PubMed] [Google Scholar]
- 31. Kerje S, Lind J, Schütz K, Jensen P, Andersson L (2003) Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Animal Genetics 34: 241–248. [DOI] [PubMed] [Google Scholar]
- 32. Mundy NI (2005) A window on the genetics of evolution: MC1R and plumage colouration in birds. Proceedings of the Royal Society B: Biological Sciences 272: 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badyaev AV (2006) Colorful phenotypes of colorless genotypes: toward a new evolutionary synthesis of color displays. Bird Coloration: Function and Evolution 2: 349–379. [Google Scholar]
- 34. Price T, Pavelka M (1996) Evolution of a colour pattern: history, development, and selection. Journal of Evolutionary Biology 9: 451–470. [Google Scholar]
- 35. Nadeau NJ, Minvielle F, Ito S, Inoue-Murayama M, Gourichon D, et al. (2008) Characterization of Japanese quail yellow as a genomic deletion upstream of the avian homolog of the mammalian ASIP (agouti) gene. Genetics 178: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hiragaki T, Inoue-Murayama M, Miwa M, Fujiwara A, Mizutani M, et al. (2008) Recessive black is allelic to the yellow plumage locus in Japanese quail and associated with a frameshift deletion in the ASIP gene. Genetics 178: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manceau M, Domingues VS, Mallarino R, Hoekstra HE (2011) The developmental role of Agouti in color pattern evolution. Science 331: 1062–1065. [DOI] [PubMed] [Google Scholar]
- 38. Roulin A, Ducrest A-L (2011) Association between melanism, physiology and behaviour: a role for the melanocortin system. European Journal of Pharmacology 660: 226–233. [DOI] [PubMed] [Google Scholar]
- 39. Nadeau NJ, Minvielle F, Mundy NI (2006) Association of a Glu92Lys substitution in MC1R with extended brown in Japanese quail (Coturnix japonica). Animal Genetics 37: 287–289. [DOI] [PubMed] [Google Scholar]
- 40. Baião PC, Schreiber E, Parker PG (2007) The genetic basis of the plumage polymorphism in red-footed boobies (Sula sula): a melanocortin-1 receptor (MC1R) analysis. Journal of Heredity 98: 287–292. [DOI] [PubMed] [Google Scholar]
- 41. Vidal O, Araguas RM, Fernández E, Heras S, Sanz N, et al. (2010) Melanism in guinea fowl (Numida meleagris) is associated with a deletion of Phenylalanine-256 in the MC1R gene. Animal Genetics 41: 656–658. [DOI] [PubMed] [Google Scholar]